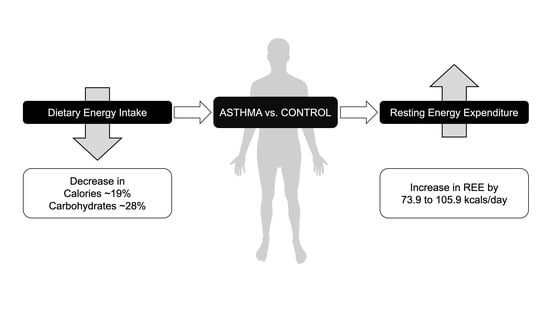
Resting Energy Expenditure Is Elevated in Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Clinical Assessments and Blood Chemistry
2.4. Body Composition
2.5. Resting Energy Expenditure (REE)
2.6. Dietary Analysis
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Body Composition
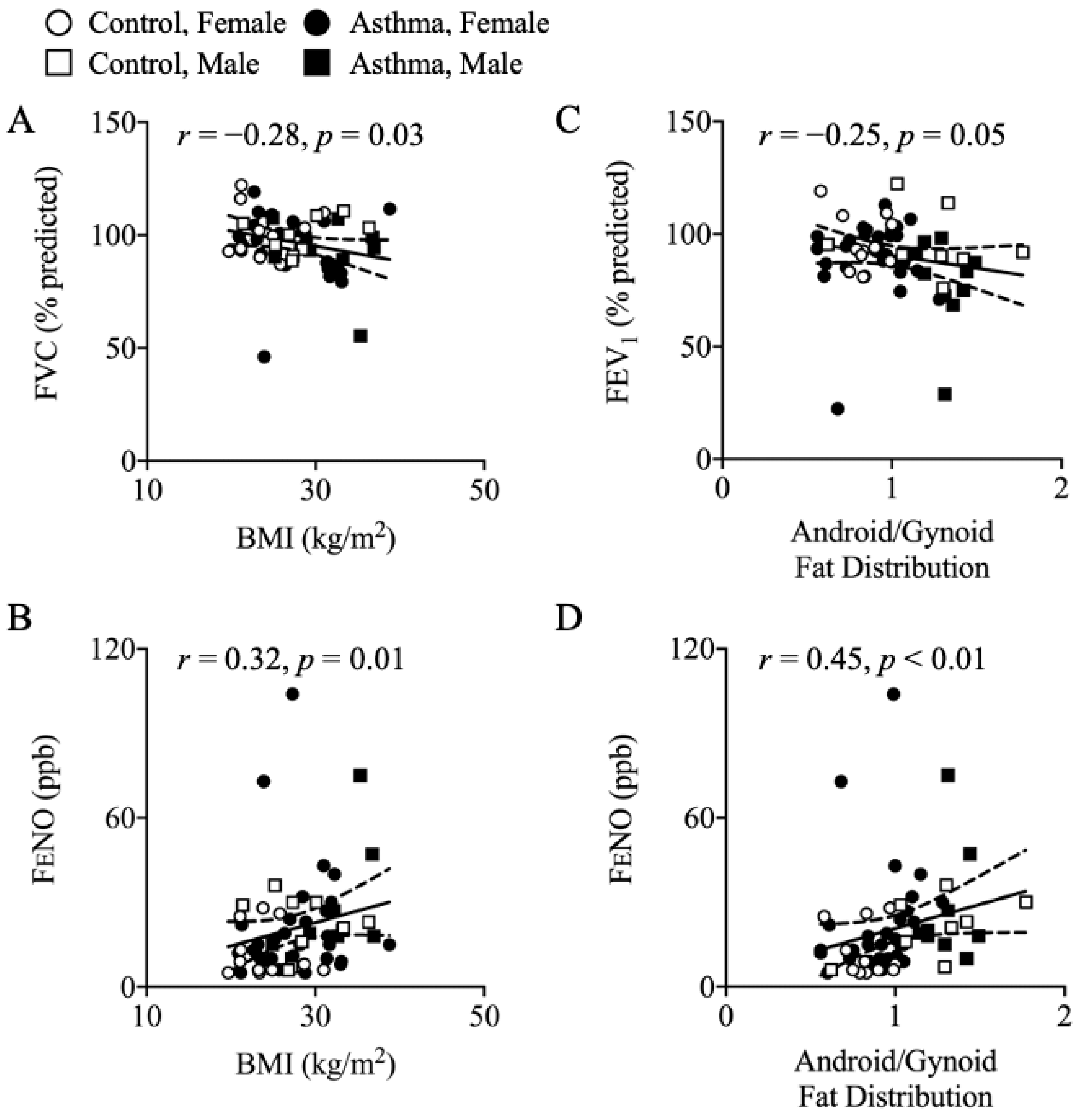
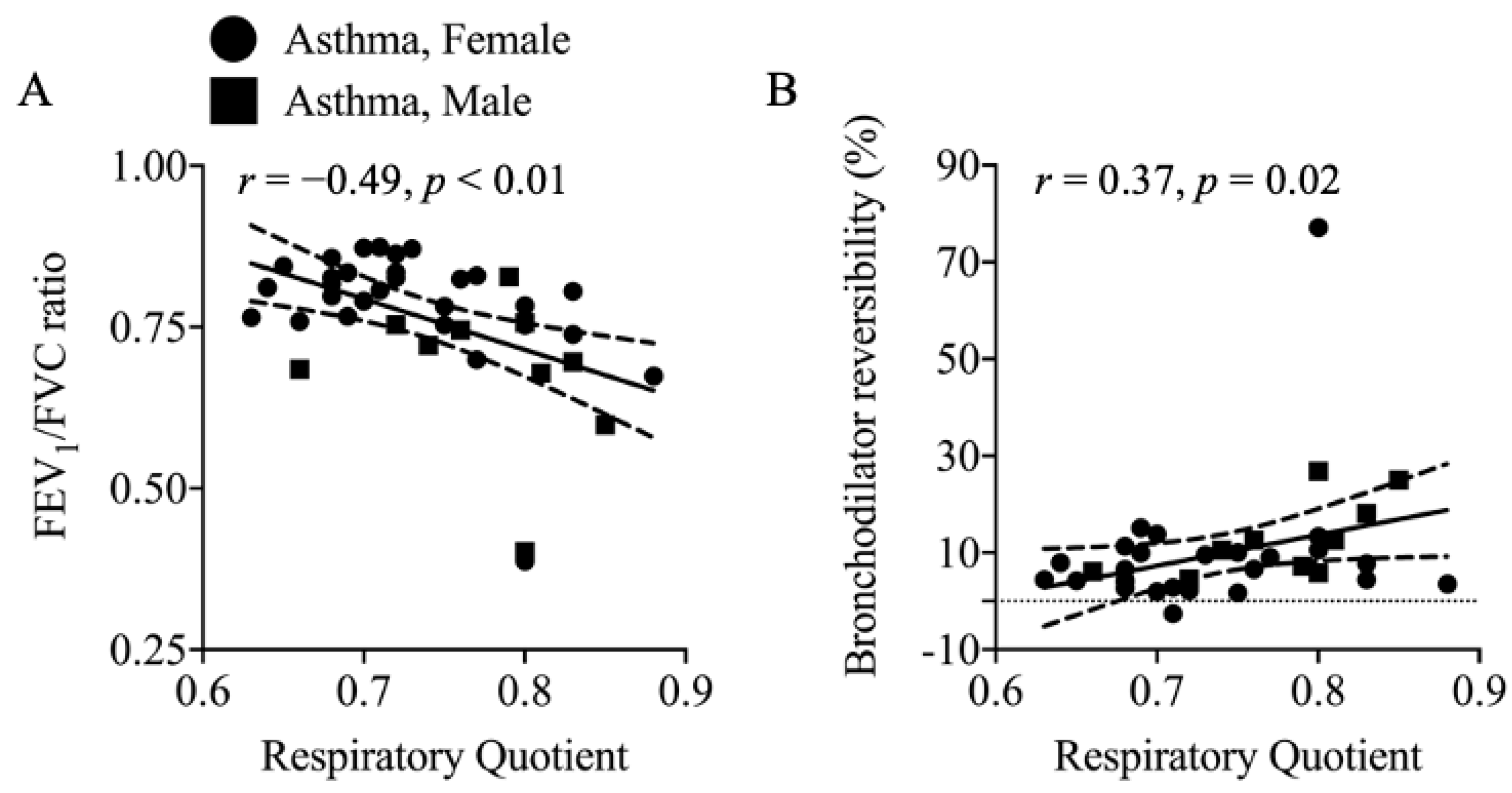
3.3. Resting Energy Expenditure (REE) and Respiratory Quotient (RQ)
3.4. Immune Cells and Inflammation
3.5. Habitual Dietary Intake
4. Discussion
4.1. Main Findings
4.2. Elevation of REE in Asthma
4.3. Body Composition
4.4. Elevated REE in Asthma: Contributing Factors
4.5. Dietary Intake
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| REE | Resting Energy Expenditure |
| DEXA | Dual-energy X-ray absorptiometry |
| FENO | Fractional Exhaled Nitric Oxide |
| REEIC | Resting Energy Expenditure measured by Indirect Calorimetry |
| REEMSJ | Resting Energy Expenditure predicted by Mifflin–St. Jeor equation |
| REEHB | Resting Energy Expenditure predicted by Harris–Benedict equation |
| REEWHO | Resting Energy Expenditure predicted by World Health Organization equation |
| REEOX | Resting Energy Expenditure predicted by Oxford equation |
| FVC | Forced Vital Capacity |
| FEV1 | Forced Expiratory Volume in 1 s |
| FNDDS | Food and Nutrient Database for Dietary Studies |
| USDA | United States Department of Agriculture |
| HEI | Healthy Eating Index |
| WBC | White Blood Cells |
| FFM | Fat Free Mass |
| Th | T-helper cells |
References
- Croisant, S. Epidemiology of Asthma: Prevalence and Burden of Disease. Adv. Exp. Med. Biol. 2014, 795, 17–29. [Google Scholar] [CrossRef]
- Beuther, D.A.; Sutherland, E.R. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef]
- Contreras, Z.A.; Chen, Z.; Roumeliotaki, T.; Annesi-Maesano, I.; Baïz, N.; Von Berg, A.; Bergström, A.; Crozier, S.; Duijts, L.; Ekström, S.; et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur. Respir. J. 2018, 52, 1800504. [Google Scholar] [CrossRef]
- Xu, W.; Ghosh, S.; Comhair, S.A.; Asosingh, K.; Janocha, A.J.; Mavrakis, D.A.; Bennett, C.D.; Gruca, L.L.; Graham, B.B.; Queisser, K.A.; et al. Increased mitochondrial arginine metabolism supports bioenergetics in asthma. J. Clin. Investig. 2016, 126, 2465–2481. [Google Scholar] [CrossRef]
- Winnica, D.; Corey, C.; Mullett, S.; Reynolds, M.; Hill, G.; Wendell, S.; Que, L.; Holguin, F.; Shiva, S. Bioenergetic Differences in the Airway Epithelium of Lean Versus Obese Asthmatics Are Driven by Nitric Oxide and Reflected in Circulating Platelets. Antioxid. Redox Signal. 2019, 31, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Mabalirajan, U.; Dinda, A.K.; Kumar, S.; Roshan, R.; Gupta, P.; Sharma, S.K.; Ghosh, B. Mitochondrial Structural Changes and Dysfunction Are Associated with Experimental Allergic Asthma. J. Immunol. 2008, 181, 3540–3548. [Google Scholar] [CrossRef]
- Aguilera-Aguirre, L.; Bacsi, A.; Saavedra-Molina, A.; Kurosky, A.; Sur, S.; Boldogh, I. Mitochondrial Dysfunction Increases Allergic Airway Inflammation. J. Immunol. 2009, 183, 5379–5387. [Google Scholar] [CrossRef]
- Song, X.; Li, B.; Wang, H.; Zou, X.; Gao, R.; Zhang, W.; Shu, T.; Zhao, H.; Liu, B.; Wang, J. Asthma alleviates obesity in males through regulating metabolism and energy expenditure. Biochim. Et Biophys. Acta Mol. Basis Dis. 2019, 1865, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.J.; Mocelin, H.T.; Bosa, V.L.; De Mello, E.D.; Fischer, G.B. Energy expenditure and estimated caloric intake in asthmatic adolescents with excess body weight. Nutrition 2010, 26, 952–957. [Google Scholar] [CrossRef]
- Maffeis, C.; Chiocca, E.; Zaffanello, M.; Golinelli, M.; Pinelli, L.; Boner, A. Energy intake and energy expenditure in prepubertal males with asthma. Eur. Respir. J. 1998, 12, 123–129. [Google Scholar] [CrossRef]
- Eliseeva, T.I.; Geppe, N.A.; Tush, E.V.; Khaletskaya, O.V.; Balabolkin, I.I.; Bulgakova, V.A.; Kubysheva, N.I.; Ignatov, S.K. Body Height of Children with Bronchial Asthma of Various Severities. Can. Respir. J. 2017, 2017, 8761404. [Google Scholar] [CrossRef]
- Movin, M.; Garden, F.L.; Protudjer, J.L.; Ullemar, V.; Svensdotter, F.; Andersson, D.; Kruse, A.; Cowell, C.T.; Toelle, B.G.; Marks, G.B.; et al. Impact of childhood asthma on growth trajectories in early adolescence: Findings from the Childhood Asthma Prevention Study (CAPS). Respirology 2016, 22, 460–465. [Google Scholar] [CrossRef]
- Sutherland, T.J.; McLachlan, C.R.; Sears, M.R.; Poulton, R.; Hancox, R.J. The relationship between body fat and respiratory function in young adults. Eur. Respir. J. 2016, 48, 734–747. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Kao, T.-W.; Fang, W.-H.; Wang, C.-C.; Chang, Y.-W.; Yang, H.-F.; Wu, C.-J.; Sun, Y.-S.; Chen, W.-L. Body Fat Percentage in Relation to Lung Function in Individuals with Normal Weight Obesity. Sci. Rep. 2019, 9, 3066. [Google Scholar] [CrossRef] [PubMed]
- Den Dekker, H.T.; Ros, K.P.I.; de Jongste, J.C.; Reiss, I.K.; Jaddoe, V.W.; Duijts, L. Body fat mass distribution and interrupter resistance, fractional exhaled nitric oxide, and asthma at school-age. J. Allergy Clin. Immunol. 2017, 139, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Madeira, F.B.; Hammad, H. Innate immune cells in asthma. Trends Immunol. 2013, 34, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Blundell, J.; Halford, J.; King, N.; Finlayson, G. The Regulation of Food Intake in Humans; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Eds.; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Szefler, S.J. Clinical need for a nebulized corticosteroid. J. Allergy Clin. Immunol. 1999, 104, 162–168. [Google Scholar] [CrossRef]
- Culver, B.H.; Graham, B.L.; Coates, A.L.; Wanger, J.; Berry, C.E.; Clarke, P.K.; Hallstrand, T.S.; Hankinson, J.L.; Kaminsky, D.A.; MacIntyre, N.R.; et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am. J. Respir. Crit. Care Med. 2017, 196, 1463–1472. [Google Scholar] [CrossRef]
- Mey, J.T.; Solomon, T.P.J.; Kirwan, J.P.; Haus, J.M. Skeletal muscle Nur77 and NOR1 insulin responsiveness is blunted in obesity and type 2 diabetes but improved after exercise training. Physiol. Rep. 2019, 7, e14042. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Marchetti, C.M.; Krishnan, R.K.; Vrobel, T.R.; Gonzalez, F.; Kirwan, J.P. Effects of Exercise and Caloric Restriction on Insulin Resistance and Cardiometabolic Risk Factors in Older Obese Adults--A Randomized Clinical Trial. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.; Butler, M.; Curran, M.; Illiano, P.; Sevick, M.; St-Jules, D. Evaluating steady-state resting energy expenditure using indirect calorimetry in adults with overweight and obesity. Clin. Nutr. 2020, 39, 2220–2226. [Google Scholar] [CrossRef]
- Mifflin, M.D.; Jeor, S.T.S.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization; WHO. United Nations University Energy and Protein Requirements; WHO: Geneva, Switzerland, 1985. [Google Scholar]
- Henry, C.J.K. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Knuth, N.D.; Huizenga, R.; Rood, J.C.; Ravussin, E.; Hall, K.D. Metabolic Slowing with Massive Weight Loss despite Preservation of Fat-Free Mass. J. Clin. Endocrinol. Metab. 2012, 97, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.H.; Speakman, J.R.; Arch, J.R.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Vink, N.M.; Postma, D.S.; Schouten, J.P.; Rosmalen, J.G.; Boezen, H.M. Gender differences in asthma development and remission during transition through puberty: The TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J. Allergy Clin. Immunol. 2010, 126, 498–504.e6. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Bogardus, C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am. J. Clin. Nutr. 1989, 49, 968–975. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Ravussin, E.; Speakman, J.R.; Galgani, J.E. Progress and challenges in analyzing rodent energy expenditure. Nat. Methods 2019, 16, 797–799. [Google Scholar] [CrossRef]
- Zeitlin, S.R.; Bond, S.; Wootton, S.; Gregson, R.K.; Radford, M. Increased resting energy expenditure in childhood asthma: Does this contribute towards growth failure? Arch. Dis. Child. 1992, 67, 1366–1369. [Google Scholar] [CrossRef]
- Allison, D.B.; Paultre, F.; Goran, M.I.; Poehlman, E.T.; Heymsfield, S.B. Statistical considerations regarding the use of ratios to adjust data. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 644–652. [Google Scholar]
- Daly, J.M.; Heymsfield, S.B.; Head, C.A.; Harvey, L.P.; Nixon, D.W.; Katzeff, H.; Grossman, G.D. Human energy requirements: Overestimation by widely used prediction equation. Am. J. Clin. Nutr. 1985, 42, 1170–1174. [Google Scholar] [CrossRef]
- Owen, E.O.; Kavle, E.; Owen, R.S.; Polansky, M.; Caprio, S.; Mozzoli, M.A.; Kendrick, Z.V.; Bushman, M.C.; Boden, G. A reappraisal of caloric requirements in healthy women. Am. J. Clin. Nutr. 1986, 44, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of Predictive Equations for Resting Metabolic Rate in Healthy Nonobese and Obese Adults: A Systematic Review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Creutzberg, E.; Schols, A.; Bothmer-Quaedvlieg, F.; Wouters, E. Prevalence of an elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur. J. Clin. Nutr. 1998, 52, 396–401. [Google Scholar] [CrossRef]
- Goldstein, S.; Askanazi, J.; Weissman, C.; Thomashow, B.; Kinney, J. Energy Expenditure in Patients with Chronic Obstructive Pulmonary Disease. Chest 1987, 91, 222–224. [Google Scholar] [CrossRef]
- Vahlkvist, S.; Pedersen, S. Fitness, daily activity and body composition in children with newly diagnosed, untreated asthma. Allergy 2009, 64, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Boot, A.M.; De Jongste, J.C.; Verberne, A.A.P.H.; Pols, H.A.P.; Keizer-Schrama, S.M.D.M. Bone mineral density and bone metabolism of prepubertal children with asthma after long-term treatment with inhaled corticosteroids. Pediatr. Pulmonol. 1997, 24, 379–384. [Google Scholar] [CrossRef]
- Mafort, T.T.; Rufino, R.; Costa, C.H.; Lopes, A.J. Obesity: Systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip. Respir. Med. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Bhardwaj, A. Physiology, Respiratory Quotient; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W. Inflammation in asthma: The cornerstone of the disease and target of therapy. J. Allergy Clin. Immunol. 1998, 102, 17–22. [Google Scholar] [CrossRef]
- Buttgereit, F.; Burmester, G.-R.; Brand, M.D. Bioenergetics of immune functions: Fundamental and therapeutic aspects. Immunol. Today 2000, 21, 194–199. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic Shifts in Immunity and Inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. How Metabolism Generates Signals during Innate Immunity and Inflammation. J. Biol. Chem. 2013, 288, 22893–22898. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]
- Freedman, A.R.; Lavietes, M.H. Energy requirements of the respiratory musculature in asthma. Am. J. Med. 1986, 80, 215–222. [Google Scholar] [CrossRef]
- Berthon, B.S.; MacDonald-Wicks, L.K.; Wood, L.G. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr. Res. 2014, 34, 179–190. [Google Scholar] [CrossRef]
- Thomas, J.; Enecio, C.E.; Chehreh, M.N.; Young, R.C. Oxygen cost of breathing III: Studies in asthmatic children. J. Natl. Med Assoc. 1976, 68, 374–377. [Google Scholar]
- Hoeks, J.; van Baak, M.A.; Hesselink, M.K.; Hul, G.B.; Vidal, H.; Saris, W.H.; Schrauwen, P. Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Oomen, J.M.; van Rossum, C.T.; Hoebee, B.; Saris, W.H.; van Baak, M.A. beta2-adrenergic receptor polymorphisms and salbutamol-stimulated energy expenditure. J. Clin. Endocrinol. Metab. 2005, 90, 2301–2307. [Google Scholar] [CrossRef]
- Creutzberg, E.; Schols, A.; Bothmer-Quaedvlieg, F.; Wesseling, G.; Wouters, E. Acute effects of nebulized salbutamol on resting energy expenditure in patients with chronic obstructive pulmonary disease and in healthy subjects. Respiration 1998, 65, 375–380. [Google Scholar] [CrossRef]
- Onslev, J.; Jacobson, G.; Narkowicz, C.; Backer, V.; Kalsen, A.; Kreiberg, M.; Jessen, S.; Bangsbo, J.; Hostrup, M. Beta2-adrenergic stimulation increases energy expenditure at rest, but not during submaximal exercise in active overweight men. Eur. J. Appl. Physiol. 2017, 117, 1907–1915. [Google Scholar] [CrossRef]
- Zein, J.G.; Erzurum, S.C. Asthma is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. What We Eat in America/National Health and Nutrition Examination Survey, 2015–2016. Health Eating Index-2015, Scores; U.S. Department of Agriculture, Center for Nutrition Policy and Promotion: Alexandria, VA, USA, 2015.
- Rolls, B.J. Do Chemosensory Changes Influence Food Intake in the Elderly? Physiol. Behav. 1999, 66, 193–197. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Graham, B.G. Taste and smell perception affect appetite and immunity in the elderly. Eur. J. Clin. Nutr. 2000, 54, S54–S63. [Google Scholar] [CrossRef]
- Mattes, R.D.; Cowart, B.J.; Schiavo, M.A.; Arnold, C.; Garrison, B.; Kare, M.R.; Lowry, L.D. Dietary evaluation of patients with smell and/or taste disorders. Am. J. Clin. Nutr. 1990, 51, 233–240. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.-S.; Hummel, T. The Influence of Olfactory Loss on Dietary Behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Arias-Guillen, C.J.; Prado, D.A.; Tuon, R.A.; Scudine, K.G.O.; Gavião, M.B.D.; Lamy, E.C.C.; Marquezin, M.C.S.; Castelo, P.M. Impact of asthma on children s gustatory sensitivity, masticatory and feeding behaviors. Physiol. Behav. 2020, 223, e112961. [Google Scholar] [CrossRef] [PubMed]
- Alobid, I.; Cardelus, S.; Benítez, P.; Guilemany, J.M.; Roca-Ferrer, J.; Picado, C.; Bernal-Sprekelsen, M.; Mullol, J. Persistent asthma has an accumulative impact on the loss of smell in patients with nasal polyposis. Rhinol. J. 2011, 49, 519–524. [Google Scholar]
- Cowart, B.J.; Flynn-Rodden, K.; McGeady, S.J.; Lowry, L.D. Hyposmia in allergic rhinitis. J. Allergy Clin. Immunol. 1993, 91, 747–751. [Google Scholar] [CrossRef]
- Guss, J.; Doghramji, L.; Reger, C.; Chiu, A.G. Olfactory Dysfunction in Allergic Rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 2009, 71, 268–272. [Google Scholar] [CrossRef]
- Bogdanov, V.; Herzog, M.; Kazopoulos, T.; Grafmans, D.; Zhu, Y.; Hummel, T. Bitter perception is altered in asthma and predicts its severity. J. Allergy Clin. Immunol. 2020, 146. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dakle, P.; Sinha, A.; Vishweswaraiah, S.; Nagori, A.; Salimath, S.; Prakash, Y.S.; Lodha, R.; Kabra, S.K.; Ghosh, B.; et al. Genetic variations in olfactory receptor gene OR2AG2 in a large multigenerational family with asthma. Sci. Rep. 2019, 9, 19029. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Shin, E.-S.; Park, S.Y.; Kim, S.; Kwon, H.-S.; Cho, Y.S.; Moon, H.-B.; Kim, T.-B. Association between Polymorphisms in Bitter Taste Receptor Genes and Clinical Features in Korean Asthmatics. Respiration 2016, 91, 141–150. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Wang, W.C.H.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.K.; Liggett, S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kawabata, Y.; Kawabata, F.; Nishimura, S.; Tabata, S. Expressions of multiple umami taste receptors in oral and gastrointestinal tissues, and umami taste synergism in chickens. Biochem. Biophys. Res. Commun. 2015, 466, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin Cells of the Human Gut: Sensors for Spices and Odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef]
- Aisenberg, W.H.; Huang, J.; Zhu, W.; Rajkumar, P.; Cruz, R.; Santhanam, L.; Natarajan, N.; Yong, H.M.; De Santiago, B.; Oh, J.J.; et al. Defining an olfactory receptor function in airway smooth muscle cells. Sci. Rep. 2016, 6, 38231. [Google Scholar] [CrossRef] [PubMed]

| Control | Asthma | p-Value | |
|---|---|---|---|
| n (sex: male, female) | 20 (9 m, 11 f) | 41 (10 m, 31 f) | - |
| Age (years) | 37.5 ± 11.3 | 38.9 ± 11.0 | 0.64 |
| SBP (mmHg) | 123.8 ± 15.0 | 120.4 ± 16.6 | 0.44 |
| DBP (mmHg) | 73.4 ± 8.8 | 74.8 ± 10.6 | 0.62 |
| Resting Heart Rate (bpm) | 70.1 ± 13.2 | 71.5 ± 11.3 | 0.68 |
| Temperature (°F) | 97.8 ± 0.5 | 97.9 ± 0.4 | 0.71 |
| O2 saturation (%) | 99.1 ± 1.1 | 98.9 ± 1.8 | 0.57 |
| FVC (L) | 4.4 ± 0.9 | 3.8 ± 1.1 | 0.03 |
| FVC (% predicted) | 102.0 ± 10.9 | 94.3 ± 13.8 | 0.05 |
| FEV1 (L) | 3.5 ± 0.7 | 2.9 ± 0.9 | 0.02 |
| FEV1 (% predicted) | 97.1 ± 12.9 | 87.8 ± 17.6 | 0.08 |
| FEV1/FVC ratio | 0.79 ± 0.07 | 0.76 ± 0.10 | 0.45 |
| FENO (ppb) | 16.8 ± 10.7 | 26.3 ± 30.2 | 0.26 |
| Control | Asthma | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Male n = 9 | Female n = 11 | Cohort | Male n= 10 | Female n= 31 | Cohort | Male | Female | |
| Height (cm) | 171.5 ± 9.9 | 179.2 ± 8.5 | 165.2 ± 5.5 | 168.6 ± 8.7 | 178.1 ± 8.6 | 165.6 ± 6.2 | 0.25 | 0.78 | 0.85 |
| Weight (kg) | 77.1 ± 17.7 | 91.3 ± 14.4 | 65.4 ± 10.0 | 81.0 ± 15.9 | 99.0 ± 12.1 | 75.2 ± 12.2 | 0.26 | 0.22 | 0.02 |
| BMI (kg/m2) | 26.0 ± 4.4 | 28.4 ± 4.4 | 24.0 ± 3.4 | 28.4 ± 4.7 | 31.3 ± 4.5 | 27.4 ± 4.4 | 0.06 | 0.17 | 0.02 |
| DEXA Data | Cohort | Male † n = 8 | Female n = 11 | Cohort | Male n= 10 | Female n= 31 | Cohort | Male | Female |
| BMD (g/cm2) | 1.21 ± 0.18 | 1.33 ± 0.10 | 1.13 ± 0.18 | 1.25 ± 0.11 | 1.36 ± 0.11 | 1.21 ± 0.09 | 0.36 | 0.53 | 0.05 |
| Fat mass (%) | 33.9±8.3 | 30.1±9.2 | 36.6±6.7 | 38.7±7.5 | 33.7±7.8 | 40.3 ± 6.7 | 0.03 | 0.39 | 0.12 |
| Fat mass (kg) | 24.6 ± 8.5 | 26.4 ± 10.1 | 23.3 ± 7.3 | 30.4 ± 9.5 | 32.5 ± 10.1 | 29.8 ± 9.3 | 0.02 | 0.22 | 0.04 |
| Fat-free mass (kg) | 48.2 ± 13 | 60.1 ± 11.0 | 39.5 ± 4.8 | 47.5 ± 10.2 | 62.8 ± 8.2 | 42.6 ± 4.1 | 0.83 | 0.55 | 0.05 |
| FFMI (kg/m2) | 16.3 ± 2.9 | 18.8 ± 2.6 | 14.5 ± 1.3 | 16.6 ± 2.6 | 19.9 ± 2.8 | 15.5 ± 1.4 | 0.67 | 0.41 | 0.03 |
| Gynoid fat (%) | 36.4 ± 9.4 | 29.6 ± 8.0 | 41.3 ± 7.0 | 41.8 ± 8.3 | 32.5 ± 7.3 | 44.8 ± 6.2 | 0.03 | 0.43 | 0.13 |
| Android fat (%) | 36.1 ± 12.0 | 37.6 ± 15.2 | 35.1 ± 9.7 | 41.5 ± 11.0 | 42.7 ± 10.3 | 41.1 ± 11.3 | 0.09 | 0.41 | 0.12 |
| A/G Ratio | 1.00 ± 0.3 | 1.2 ± 0.3 | 0.8 ± 0.1 | 1.0 ± 0.2 | 1.3 ± 0.1 | 0.9 ± 0.2 | 0.94 | 0.46 | 0.23 |
| Control | Asthma | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Indirect Calorimetry | Cohort | Male n = 9 | Female n = 11 | Cohort | Male n = 10 | Female n = 31 | Cohort | Male | Female |
| REE (kcals/day) | 1505 ± 342 | 1738 ± 289 | 1314 ± 258 | 1594 ± 337 | 1954 ± 398 | 1477 ± 216 | 0.34 | 0.20 | 0.05 |
| RQ | 0.77 ± 0.10 | 0.77 ± 0.08 | 0.78 ± 0.11 | 0.74 ± 0.06 | 0.78 ± 0.06 | 0.73 ± 0.06 | 0.18 | 0.88 | 0.13 |
| Prediction Equations | |||||||||
| MSJ (kcals/day) | 1572 ± 284 | 1838 ± 168 | 1355 ± 124 | 1552 ± 249 | 1905 ± 149 | 1438 ± 144 | 0.97 | 0.60 | 0.07 |
| Residuals | 67 ± 221 | 100 ± 231 | 40 ± 220 | −42 ± 236 | −49 ± 351 | −40 ± 194 | 0.02 | 0.09 | 0.10 |
| Residuals (%) | 7.0 ± 19.6 | 7.8 ± 17.5 | 6.4 ± 22.0 | −0.6 ± 16.5 | 0.8 ± 21.1 | −1.1 ± 15.0 | 0.01 | 0.09 | 0.09 |
| HB (kcals/day) | 1662 ± 306 | 1943 ± 216 | 1432 ± 105 | 1639 ± 270 | 2040 ± 192 | 1510 ± 126 | 0.80 | 0.45 | 0.04 |
| Residuals | 157 ± 229 | 205 ± 243 | 118 ± 221 | 46 ± 228 | 85 ± 333 | 33 ± 188 | 0.01 | 0.16 | 0.14 |
| Residuals (%) | 13.2 ± 21.3 | 13.8 ± 19.3 | 12.7 ± 23.8 | 4.9 ± 16.9 | 7.6 ± 21.7 | 4.0 ± 15.4 | 0.02 | 0.16 | 0.13 |
| WHO (kcals/day) | 1659 ± 302 | 1960 ± 153 | 1413 ± 82 | 1641 ± 296 | 2080 ± 168 | 1500 ± 154 | 0.81 | 0.24 | 0.03 |
| Residuals | 154 ± 238 | 222 ± 265 | 99 ± 208 | 48 ± 222 | 126 ± 290 | 22 ± 195 | 0.06 | 0.45 | 0.23 |
| Residuals (%) | 13.0 ± 21.2 | 15.3 ± 20.5 | 11.2 ± 22.5 | 4.7 ± 16.6 | 9.5 ± 19.2 | 3.2 ± 15.7 | <0.05 | 0.40 | 0.16 |
| OX (kcals/day) | 1594 ± 307 | 1890 ± 186 | 1352 ± 92 | 1586 ± 297 | 2021 ± 192 | 1445 ± 151 | 0.80 | 0.28 | 0.03 |
| Residuals | 89 ± 233 | 152 ± 261 | 38 ± 205 | -8 ± 220 | 66 ± 283 | −32 ± 195 | 0.07 | 0.50 | 0.22 |
| Residuals (%) | 8.4 ± 20.0 | 11.0 ± 19.6 | 6.3 ± 21.1 | 1.1 ± 16.1 | 6.2 ± 18.2 | −0.6 ± 15.3 | 0.06 | 0.50 | 0.18 |
| Model | Adjusted R2 | ANOVA p-Value | Coefficient | p-Value |
|---|---|---|---|---|
| MSJ | 0.511 | <0.01 | ||
| MSJ + Asthma | 0.526 | <0.01 | 107.8 | 0.10 |
| MSJ + FENO | 0.527 | <0.01 | 2.1 | 0.09 |
| HB | 0.529 | <0.01 | ||
| HB + Asthma | 0.545 | <0.01 | 108.8 | 0.09 |
| HB + FENO | 0.551 | <0.01 | 2.2 | 0.06 |
| WHO | 0.544 | <0.01 | ||
| WHO + Asthma | 0.558 | <0.01 | 103.8 | 0.10 |
| WHO + FENO | 0.550 | <0.01 | 1.6 | 0.18 |
| OX | 0.560 | <0.01 | ||
| OX + Asthma | 0.571 | <0.01 | 95.8 | 0.12 |
| OX + FENO | 0.569 | <0.01 | 1.7 | 0.14 |
| Model | Adjusted R2 | ANOVA p-Value | Coefficient | p-Value |
|---|---|---|---|---|
| Model 1 Age, Sex, Fat mass, and Fat-free Mass | 0.552 | <0.0001 | ||
| Model 2 Model 1 + Asthma | 0.554 | <0.0001 | 73.9 | 0.28 |
| Model 3 Model 1 + FENO (ppm) | 0.563 | <0.0001 | 1.9 | 0.13 |
| Model 4 Model 1 + White blood cell count (×106 cells) | 0.588 | <0.0001 | 45.3 | 0.02 |
| Model 5 | ||||
| Model 1 + Neutrophil count (×106 cells) | 0.580 | <0.0001 | 48.3 | 0.04 |
| Control n = 20 | Asthma n = 41 | p-Value | |
|---|---|---|---|
| WBC | 4.7 ± 1.1 | 5.9 ± 1.6 | <0.01 |
| RBC | 4.7 ± 0.4 | 4.8 ± 0.6 | 0.51 |
| Hemoglobin | 13.7 ± 1.4 | 13.9 ± 1.5 | 0.55 |
| Hematocrit | 41.7 ± 3.7 | 42.5 ± 4.2 | 0.47 |
| Platelets | 240 ± 43 | 242 ± 60 | 0.93 |
| MPV | 8.6 ± 0.7 | 8.5 ± 0.9 | 0.72 |
| Neutrophil (%) | 58.1 ± 9.0 | 60.6 ± 8.5 | 0.30 |
| Neutrophil (millions of cells) | 2.8 ± 0.9 | 3.6 ± 1.4 | 0.02 |
| Lymphocyte (%) | 31.2 ± 7.8 | 28.6 ± 7.2 | 0.21 |
| Lymphocyte (millions of cells) | 1.4 ± 0.4 | 1.6 ± 0.4 | 0.11 |
| Monocyte (%) | 5.2 ± 1.3 | 4.9 ± 1.2 | 0.33 |
| Monocyte (millions of cells) | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.10 |
| Eosinophil (%) | 2.5 ± 1.7 | 3.1 ± 3.0 | 0.36 |
| Eosinophil (millions of cells) | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.14 |
| Basophil (%) | 0.6 ± 0.2 | 0.7 ± 0.4 | 0.60 |
| Basophil (millions of cells) | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.16 |
| Control | Asthma | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Male n = 9 | Female n = 11 | Cohort | Male n = 10 | Female † n = 29 | Cohort | Male | Female | |
| Calorie Intake (kcals/day) | 1927 ± 751 | 2337 ± 901 | 1591 ± 381 | 1603 ± 411 | 1805 ± 474 | 1534 ± 371 | 0.10 | 0.20 | 0.60 |
| Calorie Intake (kcals/kg/day) | 25.1 ± 7.5 | 25.4 ± 8.4 | 24.9 ± 7.1 | 20.3 ± 6.0 | 18.8 ± 6.5 | 20.8 ± 5.9 | <0.01 | 0.07 | 0.07 |
| Protein (g/day) | 85 ± 47 | 110±61 | 65 ± 30 | 73 ± 31 | 87 ± 34 | 68 ± 29 | 0.20 | 0.28 | 0.68 |
| Protein (g/kg/day) | 1.1 ± 0.4 | 1.2 ± 0.6 | 1.0 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.07 | 0.24 | 0.23 |
| Fat (g/day) | 83 ± 42 | 105 ± 54 | 65 ± 17 | 70 ± 24 | 74 ± 18 | 69 ± 26 | 0.51 | 0.21 | 0.77 |
| Fat (g/kg/day) | 1.1 ± 0.4 | 1.1 ± 0.5 | 1.0 ± 0.3 | 0.9 ± 0.4 | 0.8 ± 0.2 | 0.9 ± 0.4 | 0.10 | 0.06 | 0.57 |
| Saturated fat (g/day) | 25 ± 10 | 31 ± 12 | 21 ± 5 | 23 ± 9 | 24 ± 8 | 23 ± 9 | 0.33 | 0.16 | 0.56 |
| Carbohydrates (g/day) | 208 ± 69 | 236 ± 62 | 184 ± 68 | 161 ± 53 | 191 ± 65 | 151 ± 46 | <0.01 | 0.14 | 0.08 |
| Carbohydrates (g/kg/day) | 2.8 ± 1.0 | 2.6 ± 0.6 | 2.9 ± 1.2 | 2.0 ± 0.7 | 2.0 ± 0.8 | 2.0 ± 0.7 | <0.01 | 0.09 | <0.01 |
| Sugar (g/day) | 83 ± 37 | 77 ± 38 | 87 ± 38 | 57 ± 28 | 69 ± 34 | 53 ± 24 | <0.01 | 0.63 | <0.01 |
| Fiber (g/day) | 16 ± 8 | 16 ± 4 | 16 ± 11 | 15 ± 7 | 16 ± 11 | 15 ± 5 | 0.64 | 0.37 | 0.93 |
| HEI (total score) | 57 ± 11 | 51 ± 9 | 62 ± 11 | 54 ± 12 | 48 ± 10 | 55 ± 12 | 0.29 | 0.61 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mey, J.T.; Matuska, B.; Peterson, L.; Wyszynski, P.; Koo, M.; Sharp, J.; Pennington, E.; McCarroll, S.; Micklewright, S.; Zhang, P.; et al. Resting Energy Expenditure Is Elevated in Asthma. Nutrients 2021, 13, 1065. https://doi.org/10.3390/nu13041065
Mey JT, Matuska B, Peterson L, Wyszynski P, Koo M, Sharp J, Pennington E, McCarroll S, Micklewright S, Zhang P, et al. Resting Energy Expenditure Is Elevated in Asthma. Nutrients. 2021; 13(4):1065. https://doi.org/10.3390/nu13041065
Chicago/Turabian StyleMey, Jacob T., Brittany Matuska, Laura Peterson, Patrick Wyszynski, Michelle Koo, Jacqueline Sharp, Emily Pennington, Stephanie McCarroll, Sarah Micklewright, Peng Zhang, and et al. 2021. "Resting Energy Expenditure Is Elevated in Asthma" Nutrients 13, no. 4: 1065. https://doi.org/10.3390/nu13041065
APA StyleMey, J. T., Matuska, B., Peterson, L., Wyszynski, P., Koo, M., Sharp, J., Pennington, E., McCarroll, S., Micklewright, S., Zhang, P., Aronica, M., Hoddy, K. K., Champagne, C. M., Heymsfield, S. B., Comhair, S. A. A., Kirwan, J. P., Erzurum, S. C., & Mulya, A. (2021). Resting Energy Expenditure Is Elevated in Asthma. Nutrients, 13(4), 1065. https://doi.org/10.3390/nu13041065