Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations Among Overweight Metabolically Healthy Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Intervention
2.3. Anthropometric Measurements
2.4. Biochemical Measurements
2.5. Data Management and Statistical Analysis
2.6. Ethical Consideration
3. Results
3.1. Study Population Characteristics and Changes in Anthropometry and Biochemical Parameters
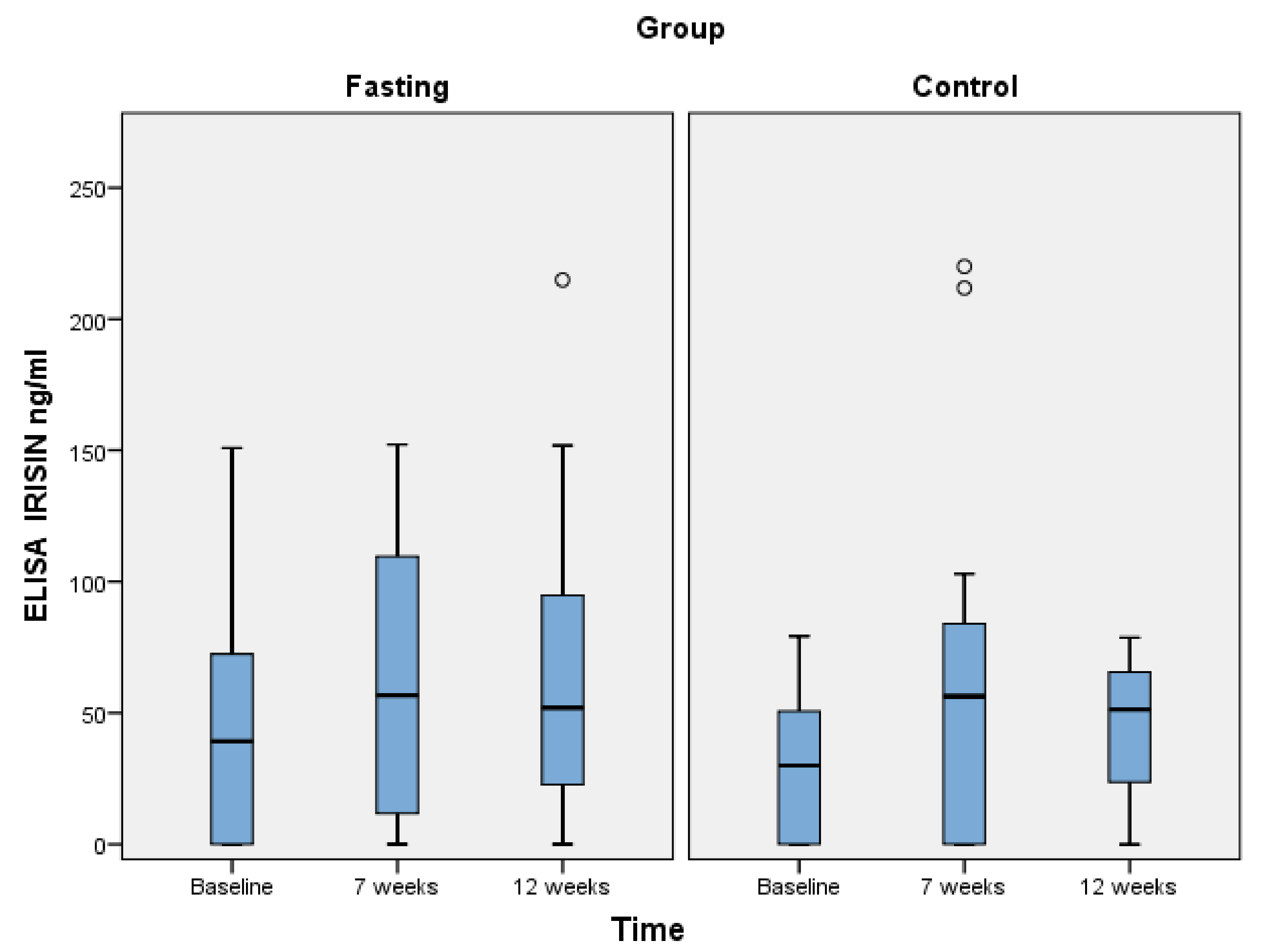
3.2. Changes in Irisin Concentrations
3.3. Effects of Various Parameters on Irisin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Persynaki, A.; Karras, S.; Pichard, C. Unraveling the metabolic health benefits of fasting related to religious beliefs: A narrative review. Nutrition 2017, 35, 14–20. [Google Scholar] [CrossRef]
- Koufakis, T.; Karras, S.N.; Antonopoulou, V.; Angeloudi, E.; Zebekakis, P.; Kotsa, K. Effects of Orthodox religious fasting on human health: A systematic review. Eur. J. Nutr. 2017, 56, 2439–2455. [Google Scholar] [CrossRef]
- Karras, S.; Persynaki, A.; Petroczi, A.; Barkans, E.; Mulrooney, H.; Kypraiou, M.; Tzotzas, T.; Tziomalos, K.; Kotsa, K.; Tsioudas, A.A.; et al. Health benefits and consequences of the Eastern Orthodox fasting in monks of Mount Athos: A cross-sectional study. Eur. J. Clin. Nutr. 2017, 71, 743–749. [Google Scholar] [CrossRef]
- Koufakis, T.; Karras, S.N.; Zebekakis, P.; Kotsa, K. Orthodox Religious Fasting as a Medical Nutrition Therapy for Dyslipidemia: Where do we stand and how far can we go? Eur. J. Clin. Nutr. 2018, 72, 474–479. [Google Scholar] [CrossRef]
- Karras, S.; Koufakis, T.; Petroczi, A.; Folkerts, D.; Kypraiou, M.; Mulrooney, H.; Naughton, D.P.; Persynaki, A.; Zebekakis, P.; Skoutas, D.; et al. Christian Orthodox Fasting in Practice: A comparative evaluation between Greek Orthodox general population fasters and Athonian monks. Nutrition 2019, 59, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Turek, F.W. Timing of Meals: When Is as Critical as What and How Much. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E369–E380. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of Intermittent Fasting on Body Composition and Clinical Health Markers in Humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Antonopoulou, V.; Karalazou, P.; Thisiadou, K.; Mitrofanova, E.; Mulrooney, H.; Petróczi, A.; Zebekakis, P.; et al. Effects of Orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int. J. Food Sci. Nutr. 2021, 72, 82–92. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Polyzos, S.A.; Karalazou, P.; Thisiadou, K.; Zebekakis, P.; Makedou, K.; Kotsa, K. Similar late effects of a 7-week orthodox religious fasting and a time restricted eating pattern on anthropometric and metabolic profiles of overweight adults. Int. J. Food Sci. Nutr. 2020, 72, 248–258. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belen Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Zügel, M.; Sun, Z.; Steinacker, J.M.; Schumann, U. Association between circulating irisin and insulin resistance in non-diabetic adults: A meta-analysis. Metabolism 2016, 65, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin. Endocrinol. 2015, 82, 615–619. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Greek National Dietary Guidelines for Adults. 2014. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/greece/en/ (accessed on 2 February 2019).
- Food Processor Analysis Software. 2018. Available online: https://www.esha.com/products/food-processor/ (accessed on 2 February 2019).
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating Irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Park, K.H.; Shin, S.; Zaichenko, L.; Davis, C.R.; Crowell, J.A.; Joung, H.; Mantzoros, C.S. Diet quality and diet patterns in relation to circulating cardiometabolic biomarkers. Clin. Nutr. 2016, 35, 484–490. [Google Scholar] [CrossRef]
- Schlögl, M.; Piaggi, P.; Votruba, S.B.; Walter, M.; Krakoff, J.; Thearle, M.S. Increased 24-hour ad libitum food intake is associated with lower plasma Irisin concentrations the following morning in adult humans. Appetite 2015, 90, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Alzoughool, F.; Al Hourani, H.; Atoum, M.; Abdelgader, R.; Alanagreh, L. Irisin, leptin and adiponectin levels are reduced significantly during fasting. Mediterr. J. Nutr. Metab. 2019, 4, 389–396. [Google Scholar] [CrossRef]
- De la Iglesia, R.; Lopez-Legarrea, P.; Crujeiras, A.B.; Pardo, M.; Casanueva, F.F.; Zulet, M.A.; Martinez, J.A. Plasma Irisin depletion under energy restriction is associated with improvements in lipid profile in metabolic syndrome patients. Clin. Endocrinol. 2014, 81, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Osella, A.R.; Colaianni, G.; Correale, M.; Pesole, P.L.; Bruno, I.; Buongiorno, C.; Deflorio, V.; Leone, C.M.; Colucci, S.C.; Grano, M.; et al. Irisin Serum Levels in Metabolic Syndrome Patients Treated with Three Different Diets: A Post-Hoc Analysis from a Randomized Controlled Clinical Trial. Nutrients 2018, 10, 844. [Google Scholar] [CrossRef]
- Blüher, S.; Panagiotou, G.; Petroff, D.; Markert, J.; Wagner, A.; Klemm, T.; Filippaios, A.; Keller, A.; Mantzoros, C.S. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring) 2014, 22, 1701–1718. [Google Scholar] [CrossRef]
- Morelli, C.; Avolio, E.; Galluccio, A.; Caparello, G.; Manes, E.; Ferraro, S.; De Rose, D.; Santoro, M.; Barone, I.; Catalano, S.; et al. Impact of Vigorous-Intensity Physical Activity on Body Composition Parameters, Lipid Profile Markers, and Irisin Levels in Adolescents: A Cross-Sectional Study. Nutrients 2020, 12, 742. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; So, B.; Son, J.S.; Yoon, D.; Song, W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: A pilot study. Physiol. Res. 2016, 65, 271–279. [Google Scholar] [CrossRef]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Winn, N.C.; Grunewald, Z.I.; Liu, Y.; Heden, T.D.; Nyhoff, L.M.; Kanaley, J. A Plasma Irisin Modestly Increases during Moderate and High-Intensity Afternoon Exercise in Obese Females. PLoS ONE 2017, 12, e0170690. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A novel interplay between irisin and PTH: From basic studies to clinical evidence in hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child. 2005, 91, 612–617. [Google Scholar] [CrossRef]
- El Chliaoutakis, J.; Drakou, I.; Gnardellis, C.; Galariotou, S.; Carra, H.; Chliaoutaki, M. Greek Christian Orthodox Ecclesiastical lifestyle: Could it become a pattern of health-related behavior? Prev. Med. 2002, 34, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Spanaki, C.; Rodopaios, N.E.; Koulouri, A.; Pliakas, T.; Papadopoulou, S.K.; Vasara, E.; Skepastianos, P.; Serafeim, T.; Boura, I.; Dermitzakis, E.; et al. The Christian Orthodox Church Fasting Diet Is Associated with Lower Levels of Depression and Anxiety and a Better Cognitive Performance in Middle Life. Nutrients 2021, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, T.; Karras, S.N.; Zebekakis, P.; Kotsa, K. Orthodox religious fasting: A vital subset of the Mediterranean diet. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2020; pp. 73–77. [Google Scholar]
- Gemming, L.; Jiang, Y.; Swinburn, B.; Utter, J.; Mhurchu, C.N. Under-reporting remains a key limitation of self-reported dietary intake: An analysis of the 2008/09 New Zealand Adult Nutrition Survey. Eur. J. Clin. Nutr. 2014, 68, 59–64. [Google Scholar] [CrossRef] [PubMed]
| Parameter | TRE Group | OF Group | p-Value |
|---|---|---|---|
| Participants; Women [n (%)] | 14; (71.4%) | 29; (75.9%) | 0.75 |
| Age (years) | 46.3 ± 8.7 | 49.9 ± 8.9 | 0.21 |
| Weight (kg) | 77.4 ± 20.2 | 77.6 ± 17.1 | 0.97 |
| BMI (kg/m2) | 28.3 ± 6.7 | 29.0 ± 6.0 | 0.75 |
| Waist circumference (cm) | 92.6 ± 16.4 | 92.4 ± 15.0 | 0.98 |
| Body fat (%) | 32.5 ± 7.3 | 35.4 ± 9.1 | 0.29 |
| Lean body mass (kg) | 51.6 ± 12.7 | 47.5 ± 9.9 | 0.26 |
| TC (mg/dL) | 191.0 ± 25.0 | 189.0 ± 36.0 | 0.81 |
| HDL-C (mg/dL) | 58.8 ± 17.8 | 51.2 ± 11.1 | 0.09 |
| HDL-C (mg/dL) | 117.0 ± 19.6 | 117.0 ± 30.9 | 0.99 |
| TG (mg/dL) | 78.2 ± 21.1 | 103.4 ± 46.8 | 0.63 |
| FPG (mg/dL) | 90.1 ± 11.7 | 83.0 ± 8.5 | 0.07 |
| FPI (μIU/mL) | 19.4 ± 28.2 | 10.6 ± 8.4 | 0.27 |
| Irisin (ng/mL) | p-Value | ||||
|---|---|---|---|---|---|
| Group | Baseline | 7 Weeks | 12 Weeks | ||
| OF (n = 29) | Mean (SD) | 43.6 (42.2) | 68.4 (51.5) | 64.3 (54.4) | Baseline vs. 7 w: 0.21 Baseline vs. 12 w: 0.01 7 w vs. 12 w: 0.65 |
| TRE (n = 14) | Mean (SD) | 29.3 (27.2) | 65.6 (72.4) | 44.2 (26.6) | Baseline vs. 7 w: 0.10 Baseline vs. 12 w: 0.73 7 w vs. 12 w: 0.24 |
| p-value | 0.28 | 0.99 | 0.04 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karras, S.N.; Koufakis, T.; Adamidou, L.; Dimakopoulos, G.; Karalazou, P.; Thisiadou, K.; Makedou, K.; Kotsa, K. Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations Among Overweight Metabolically Healthy Individuals. Nutrients 2021, 13, 1071. https://doi.org/10.3390/nu13041071
Karras SN, Koufakis T, Adamidou L, Dimakopoulos G, Karalazou P, Thisiadou K, Makedou K, Kotsa K. Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations Among Overweight Metabolically Healthy Individuals. Nutrients. 2021; 13(4):1071. https://doi.org/10.3390/nu13041071
Chicago/Turabian StyleKarras, Spyridon N., Theocharis Koufakis, Lilian Adamidou, Georgios Dimakopoulos, Paraskevi Karalazou, Katerina Thisiadou, Kali Makedou, and Kalliopi Kotsa. 2021. "Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations Among Overweight Metabolically Healthy Individuals" Nutrients 13, no. 4: 1071. https://doi.org/10.3390/nu13041071
APA StyleKarras, S. N., Koufakis, T., Adamidou, L., Dimakopoulos, G., Karalazou, P., Thisiadou, K., Makedou, K., & Kotsa, K. (2021). Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations Among Overweight Metabolically Healthy Individuals. Nutrients, 13(4), 1071. https://doi.org/10.3390/nu13041071







