Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Nutritional Biomarkers and Polyphenols
2.3. Markers of Inflammation and Bacterial Translocation
2.4. T-Lymphocyte Subsets
2.5. Microbiota Composition
2.6. Bioinformatics and Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Adherence to the SMD Stratified by Randomisation Group
3.3. Metabolic, Microbial Translocation, Inflammatory, and Immunological Parameters
3.4. Gut Microbiota Diversity and Richness
4. Sub-Study with MSM Individuals Stratified by the MEDAS Score
4.1. Metabolic, Microbial Translocation, Inflammatory, and Immunological Parameters Stratified by the MEDAS Scores
4.2. Metabolic, Microbial Translocation, Inflammatory, and Immunological Parameters in MSM Stratified by Their MEDAS Scores
4.3. Gut Microbial Diversity and Richness
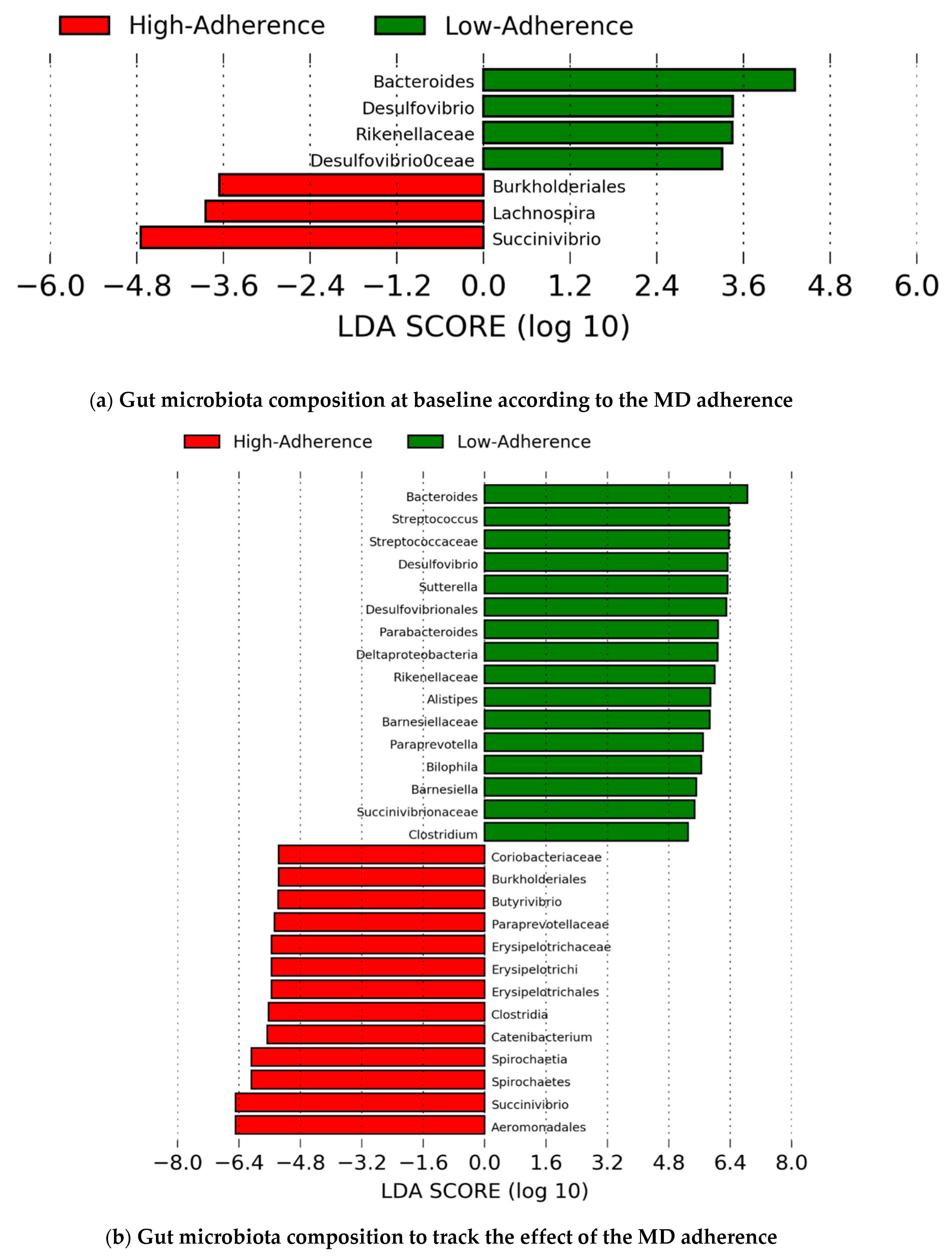
4.4. Gut Microbiota Composition
4.5. Association between Changes in the Gut Microbiota Composition and Changes in the Metabolic, Microbial Translocation, Inflammatory, and Immunological Parameters
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Danforth, K.; Granich, R.; Wiedeman, D.; Baxi, S.; Padian, N.; Holmes, K.K.; Bertozzi, S.; Bloom, B.R.; Jha, P. Global Mortality and Morbidity of HIV/AIDS. Dis. Control. Priorities 2017, 6, 29–44. [Google Scholar]
- Martínez, E.; Milinkovic, A.; Buira, E.; De Lazzari, E.; Leon, A.; Larrousse, M.; Lonca, M.; Laguno, M.; Blanco, J.; Mallolas, J.; et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007, 8, 251–258. [Google Scholar] [CrossRef]
- Phillips, A.N.; Neaton, J.; Lundgren, J.D. The role of HIV in serious diseases other than AIDS. AIDS 2008, 22, 2409–2418. [Google Scholar] [CrossRef] [Green Version]
- The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US) Committee on Social Security HIV Disability Criteria. HIV and Disability: Updating the Social Security Listings. In HIV-Associated Conditions With Listings Elsewhere; The National Academic Press: Washington, DC, USA, 2010. [Google Scholar]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Brenchley, J.M.; A Price, D.; Schacker, T.W.; E Asher, T.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.M.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Hatano, H. Immune activation and HIV persistence. Curr. Opin. HIV AIDS 2013, 8, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Stephanie, M.; Dillon, D.N.F.; Wilson, C.C. The Gut Microbiome and HIV-1 Pathogenesis: A Two Way Street. AIDS Res. Hum. Retrovir. 2016, 30, 2737–2751. [Google Scholar]
- Noguera-Julian, M.; Rocafort, M.; Guillén, Y.; Rivera, J.; Casadellà, M.; Nowak, P.; Hildebrand, F.; Zeller, G.; Parera, M.; Bellido, R.; et al. Gut Microbiota Linked to Sexual Preference and HIV Infection. EBioMedicine 2016, 5, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet–microbe–host interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lăcătușu, C.M.; Grigorescu, E.D.; Floria, M.; Onofriescu, A.; Mihai, B.M. The mediterranean diet: From an environment-driven food culture to an emerging medical prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharm. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; De La Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline. JAMA Intern. Med. 2015, 175, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Storelli, F.V.; Doménech, M.; Salas-Salvadó, J.; Martín, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary polyphenol intake is associated with HDL-cholesterol and a better profile of other components of the metabolic syndrome: A PREDIMED-plus sub-study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Rivera-Pinto, J.; Egozcue, J.J.; Pawlowsky-Glahn, V.; Paredes, R.; Noguera-Julian, M.; Calle, M.L. Balances: A New Perspective for Microbiome Analysis. mSystems 2018, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Willig, A.L.; Overton, E.T.; Saag, M.S. The Silent Epidemic-Frailty and Aging with HIV. Total Patient Care HIV HCV 2016, 1, 6–17. [Google Scholar]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Heal. Dis. 2015, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Prioritites for CVD, diabetes and obesity-A comprehensive RV. Circulation 2017, 133, 187–225. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar, R.H.; Gupta, A.; Rosaria, L.M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Agli, M.; Buscialà, A.; Bosisio, E. Vascular effects of wine polyphenols. Cardiovasc. Res. Engl. 2004, 63, 593–602. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stradling, C.; Thomas, G.N.; Hemming, K.; Taylor, S.; Taheri, S. Randomized parallel-group pilot trial (Best foods for your heart) comparing the effects of a Mediterranean Portfolio diet with a low saturated fat diet on HIV dyslipidemia. Clin. Nutr. 2021, 40, 860–869. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; Da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef] [Green Version]
- Sehrawat, S.; Rouse, B.T. Interplay of regulatory T cell and Th17 cells during infectious diseases in humans and animals. Front. Immunol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Blaschitz, C.; Raffatellu, M. Th17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 2010, 30, 196–203. [Google Scholar] [CrossRef] [Green Version]
- ElHed, A.; Unutmaz, D. Th17 cells and HIV infection. Curr. Opin. HIV AIDS 2010, 5, 146–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, F.; Duque, A.L.R.F.; Saad, S.M.I.; Sivieri, K. Gut microbiome approaches to treat obesity in humans. Appl. Microbiol. Biotechnol. 2019, 103, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Mrázek, J.; Tepšič, K.; Avguštin, G.; Kopečný, J. Diet-dependent shifts in ruminal butyrate-producing bacteria. Folia Microbiol. (Praha.) 2006, 51, 294–298. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Mao, G.; Wu, D.; Yu, C.; Cheng, H.; Xiao, H.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Highly Branched RG-I Domain Enrichment Is Indispensable for Pectin Mitigating against High-Fat Diet-Induced Obesity. J. Agric. Food Chem. 2020, 68, 8688–8701. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sha, L.; Li, Y.; Zhu, L.; Wang, Z.; Li, K.; Lu, H.; Bao, T.; Guo, L.; Zhang, X.; et al. Dietary α-Linolenic Acid-Rich Flaxseed Oil Exerts Beneficial Effects on Polycystic Ovary Syndrome Through Sex Steroid Hormones—Microbiota—Inflammation Axis in Rats. Front. Endocrinol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Vesterbacka, J.; Rivera, J.; Noyan, K.; Parera, M.; Neogi, U.; Calle, M.; Paredes, R.; Sönnerborg, A.; Noguera-Julian, M.; Nowak, P. Richer gut microbiota with distinct metabolic profile in HIV infected Elite Controllers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Tsigalou, C.; Paraschaki, A.; Karvelas, A.; Kantartzi, K.; Gagali, K.; Tsairidis, D.; Bezirtzoglou, E. Gut microbiome and Mediterranean diet in the context of obesity. Current knowledge, perspectives and potential therapeutic targets. Metab. Open 2021, 9, 100081. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gurav, A.; Sivaprakasam, S.; Bhutia, Y.D.; Boettger, T.; Singh, N.; Ganapathy, V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J. 2015, 469, 267–278. [Google Scholar] [CrossRef] [PubMed]







| Subject Selection | MEDAS Group | Basal (Week 0) | End of Study (Week 12) |
|---|---|---|---|
| All Individuals (n = 74) | |||
| Control group | High-Adherence | 6 (16%) | 6 (16%) |
| (n = 37) | (MEDAS ≥ 10) | ||
| Medium-Adherence | 20 (54%) | 20 (54%) | |
| (7 ≤ MEDAS < 10) | |||
| Low-Adherence | 11 (30%) | 11 (30%) | |
| (MEDAS < 7) | |||
| SMD group | High-Adherence | 3 (8%) | 31 (89%) * |
| (n = 37) | (MEDAS ≥ 10) | ||
| Medium-Adherence | 17 (46%) | 4 (11%) ** | |
| (7 ≤ MEDAS < 10) | |||
| Low-Adherence | 17 (46%) | 0 ** | |
| (MEDAS < 7) | |||
| MSM (n = 60) | |||
| Control group | High-Adherence | 6 (19%) | 6 (19%) |
| (n = 32) | (MEDAS ≥ 10) | ||
| Medium-Adherence | 18 (56%) | 18 (56%) | |
| (7 ≤ MEDAS < 10) | |||
| Low-Adherence | 8 (25%) | 8 (25%) | |
| (MEDAS < 7) | |||
| SMD group | High-Adherence | 3 (11%) | 24 (92%) * |
| (n = 28) | (MEDAS ≥10) | ||
| Medium-Adherence | 14 (50%) | 2 (8%) ** | |
| (7 ≤ MEDAS < 10) | |||
| Low-Adherence | 11 (39%) | 0 ** | |
| (MEDAS < 7) | |||
| Delta (Δ) MEDAS group | Number of subjects | ||
| MSM (n = 60) | |||
| Control group | Best improvement, Δ ≥ 4 | 0 | |
| (n = 32) | Δ = 1–3 | 1 (3%) | |
| No improvement, Δ = 0 | 31 (97%) | ||
| SMD group | Best improvement, Δ ≥ 4 | 17 (61%) | |
| (n = 28) | Δ = 1–3 | 9 (32%) | |
| No improvement, Δ = 0 | 2 (7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor-Ibáñez, R.; Blanco-Heredia, J.; Etcheverry, F.; Sánchez-Palomino, S.; Díez-Fuertes, F.; Casas, R.; Navarrete-Muñoz, M.Á.; Castro-Barquero, S.; Lucero, C.; Fernández, I.; et al. Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals. Nutrients 2021, 13, 1141. https://doi.org/10.3390/nu13041141
Pastor-Ibáñez R, Blanco-Heredia J, Etcheverry F, Sánchez-Palomino S, Díez-Fuertes F, Casas R, Navarrete-Muñoz MÁ, Castro-Barquero S, Lucero C, Fernández I, et al. Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals. Nutrients. 2021; 13(4):1141. https://doi.org/10.3390/nu13041141
Chicago/Turabian StylePastor-Ibáñez, Roque, Juan Blanco-Heredia, Florencia Etcheverry, Sonsoles Sánchez-Palomino, Francisco Díez-Fuertes, Rosa Casas, María Ángeles Navarrete-Muñoz, Sara Castro-Barquero, Constanza Lucero, Irene Fernández, and et al. 2021. "Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals" Nutrients 13, no. 4: 1141. https://doi.org/10.3390/nu13041141
APA StylePastor-Ibáñez, R., Blanco-Heredia, J., Etcheverry, F., Sánchez-Palomino, S., Díez-Fuertes, F., Casas, R., Navarrete-Muñoz, M. Á., Castro-Barquero, S., Lucero, C., Fernández, I., Leal, L., Benito, J. M., Noguera-Julian, M., Paredes, R., Rallón, N., Estruch, R., Torrents, D., & García, F. (2021). Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals. Nutrients, 13(4), 1141. https://doi.org/10.3390/nu13041141








