Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outcomes
2.1.1. Primary Outcomes
2.1.2. Secondary Outcomes
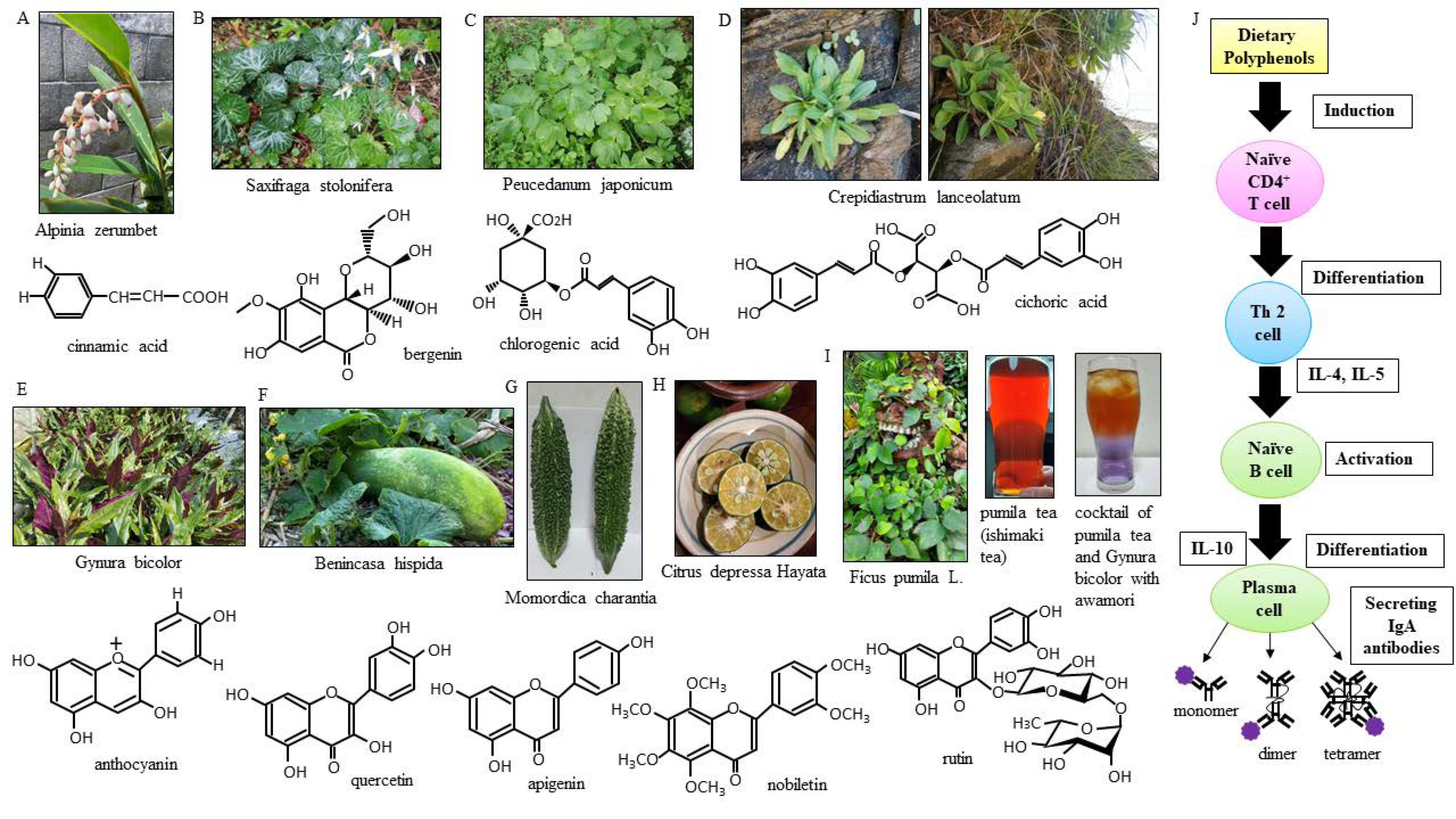
2.2. The Procedure for Extracting Phenolic Compounds from Vegetables of the Island
2.3. Statistical Analysis
3. Results
3.1. Study Design
3.2. Patients Who Ate Island Vegetables vs. Those Who Did Not
3.3. Uninfected Patients Who Did Not Eat Island Vegetables
3.4. IgA and IgG Levels Were Correlated with sIL-2R Levels
4. Discussion
4.1. IgA and Viruses
4.2. IgA, Polyphenols, and Immunity
4.3. Why Okinawan Vegetables Are High in Polyphenols
4.4. Okinawan Vegetables High in Polyphenols
4.5. Multimerized IgA Antibodies
4.6. IgA, IgG, and sIL-2R
4.7. The Role of Polyphenols in Defense against Coronaviruses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okinawa Prefectural Government. Welcome to Okinawa. Naha, Okinawa. 1972. Available online: https://www.pref.okinawa.jp/site/chijiko/kohokoryu/foreign/english/welcome.html (accessed on 20 May 2021).
- Matsunami, K.; Otsuka, H. Okinawan Subtropical Plants as a Promising Resource for Novel Chemical Treasury. Chem Pharm Bull. 2018, 66, 519–526. [Google Scholar] [CrossRef] [PubMed]
- MEIm, X.D.; Cao, Y.F.; Che, Y.Y.; Li, J.; Shang, Z.P.; Zhao, W.J.; Qiao, Y.J.; Zhang, J.Y. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar] [CrossRef]
- Aniya, Y. Development of bioresources in Okinawa: Understanding the multiple targeted actions of antioxidant phytochemicals. J. Toxicol. Pathol. 2018, 31, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.H.; Khan, T.; Khurshid, R.; Nadeem, M.; Drouet, S.; Hano, C. UV-C mediated accumulation of pharmacologically significant phytochemicals under light regimes in in vitro culture of Fagonia indica (L.). Sci. Rep. 2021, 12, 679. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [Green Version]
- Somerville, V.S.; Braakhuis, A.J.; Hopkins, W.G. Effect of Flavonoids on Upper Respiratory Tract Infections and Immune Function: A Systematic Review and Meta-Analysis. Adv. Nutr. 2016, 7, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [Green Version]
- Taira, T.; Yamaguchi, S.; Takahashi, A.; Okazaki, Y.; Yamaguchi, A.; Sakaguchi, H.; Chiji, H. Dietary polyphenols increase fecal mucin and immunoglobulin A and ameliorate the disturbance in gut microbiota caused by a high fat diet. J. Clin. Biochem. Nutr. 2015, 57, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Gould, V.M.W.; Francis, J.N.; Anderson, K.J.; Georges, B.; Cope, A.V.; Tregoning, J.S. Nasal IgA Provides Protection against Human Influenza Challenge in Volunteers with Low Serum Influenza Antibody Titre. Front. Microbiol. 2017, 8, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, K.N.; Leung, J.C.; Lai, F.M.; Tam, J.S. T-lymphocyte activation in IgA nephropathy: Serum-soluble interleukin 2 receptor level, interleukin 2 production, and interleukin 2 receptor expression by cultured lymphocytes. J. Clin. Immunol. 1989, 9, 485–492. [Google Scholar] [CrossRef]
- Parera, M.; Rivera, F.; Egido, J.; Campos, A. The role of interleukin 2 (IL-2) and serum-soluble IL-2 receptor cells in idiopathic IgA nephropathy. Clin. Immunol. Immunopathol. 1992, 63, 196–199. [Google Scholar] [CrossRef]
- Kuraoka, M.; Hashiguchi, M.; Hachimura, S.; Kaminogawa, S. CD4(-) c-kit(-) CD3epsilon(-)IL-2Ralpha(+) Peyer’s patch cells are a novel cell subset which secrete IL-5 in response to IL-2: Implications for their role in IgA production. Eur. J. Immunol. 2004, 34, 1920–1929. [Google Scholar] [CrossRef]
- Folin, O.; Karsner, H.T.; Denis, W. Nitrogen retention in the blood in experimental acute nephritis of the cat. J. Exp. Med. 1912, 16, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Nagata, J.; Matsuzoe, T.; Akamine, Y.; Maeda, G. Inhibitory Effects of Traditional Okinawan Vegetable Methanol Extracts and Their Primary Constituents on Histamine Release from Human Basophilic KU812 cells. Food Sci. Technol. Res. 2018, 24, 321–327. [Google Scholar] [CrossRef]
- Tonegawa, S. Somatic generation of antibody diversity. Nature 1983, 302, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Candore, G.; Caruso, C.; Jirillo, E.; Covelli, V. Polyphenols from red wine modulate immune responsiveness: Biological and clinical significance. Curr. Pharm. Des. 2008, 14, 2733–2748. [Google Scholar] [CrossRef]
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential use of dietary natural products, especially polyphenols, for improving type-1 allergic symptoms. Curr. Pharm. Des. 2014, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.H.; Lin, J.Y. Acorus gramineusand and Euodia ruticarpa Steam Distilled Essential Oils Exert Anti-Inflammatory Effects Through Decreasing Th1/Th2 and Pro-/Anti-Inflammatory Cytokine Secretion Ratios In Vitro. Biomolecules 2020, 10, 338. [Google Scholar] [CrossRef] [Green Version]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, C.; Inoue, M.; Kusakabe, M. A snapshot of a coral “holobiont”: A transcriptome assembly of the scleractinian coral, porites, captures a wide variety of genes from both the host and symbiotic zooxanthellae. PLoS ONE 2014, 9, e85182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Taguchi, C.; Kishimoto, Y.; Fukushima, Y.; Kondo, K.; Yamakawa, M.; Wada, K.; Nagata, C. Dietary intake of total polyphenols and the risk of all-cause and specific-cause mortality in Japanese adults: The Takayama study. Eur. J. Nutr. 2020, 59, 1263–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghareeb, M.A.; Sobeh, M.; Rezq, S.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo. Molecules 2018, 23, 3238. [Google Scholar] [CrossRef] [Green Version]
- Teschke, R.; Xuan, T.D. Viewpoint: A Contributory Role of Shell Ginger (Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm) for Human Longevity in Okinawa, Japan? Nutrients. 2018, 10, 166. [Google Scholar]
- Upadhyay, A.; Chompoo, J.; Kishimoto, W.; Makise, T.; Tawata, S. HIV-1 integrase and neuraminidase inhibitors from Alpinia zerumbet. J. Agric. Food Chem. 2011, 59, 2857–2862. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.M.; Yang, S.; Song, B.A.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Liu, F.; Xue, W.; et al. Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L) Meeb. Bioorg. Med. Chem. 2008, 16, 1337–1344. [Google Scholar] [CrossRef]
- Taneyama, M.; Yoshida, S. Studies on C-glycosides in higher plants. I. Occurrence of bergenin in Saxifragaceae. Bot. Mag. 1978, 91, 109–112. [Google Scholar] [CrossRef]
- Suwa, R.; Tajima, H.; Gima, S.; Uehara, N.; Watanabe, K.; Yabuta, S.; Tominaga, J.; Kawamitsu, Y. Polyphenol Production in Peucedanum japonicum Thunb. varies with Soil Type and Growth Stage. Hortic. J. Preview 2018, 87, 382–388. Available online: https://www.jstage.jst.go.jp/article/hortj/advpub/0/advpub_OKD-069/_pdf (accessed on 20 May 2021). [CrossRef] [Green Version]
- Maeda, G.; Hirose, N.; Yonaha, M.; Fujino, T.; Takaesu, Y. Comparison of polyphenol content between wild strain and cultivated strain of Hosobawadan (Crepidiastrum Lanceolatum (Houtt.) Nakaki). Bull. Okinawa Prefect. Agric. Res. Cent. 2010, 4, 47–51. Available online: https://agriknowledge.affrc.go.jp/RN/2010793027.pdf (accessed on 20 May 2021).
- Chao, C.Y.; Liu, W.H.; Wu, J.J.; Yin, M.C. Phytochemical profile, antioxidative and anti-inflammatory potentials of Gynura bicolor DC. J. Sci. Food Agric. 2015, 95, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Anshul, S.; Sushil, K.C.; Hans, R.B.; Neelutpal, G.; Surajit, K.G. Rapid High-Performance Thin-Layer Chromatographic Method to Estimate Quercetin in Benincasa hispida (Thunb.) Cogn. Fruit Pulp. J. Planar Chromatogr. 2019, 32, 467–474. [Google Scholar]
- Busuioc, A.C.; Botezatu, A.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef] [PubMed]
- Sing, P.T.; Sophie, E.P.; Costas, E.S.; Paul, D.R. Extraction of Flavonoids from Bitter Melon. Food Nutr. Sci. 2014, 5, 458–465. [Google Scholar]
- Ana, P.L.; Marília, B.G.; Maria, E.P.; Jean, H.O.; Edmilson, A.C.; Vanessa, V.A.S.; Jesui, V.V. Quantification of phenolic compounds in ripe and unripe bitter melons (Momordica charantia) and evaluation of the distribution of phenolic compounds in different parts of the fruit by UPLC–MS/MS. Chem. Pap. 2020, 74, 2613–2625. [Google Scholar]
- Wada, K. Shiikuwasha (Citrus depressa Hayata): Biological function and aroma. Seibutsu Shiryou Bunseki 2017, 40, 271–278. Available online: http://j-jabs.umin.jp/40/40.271.pdf (accessed on 20 May 2021).
- Delaney, B.; Phillips, K.; Buswell, D.; Mowry, B.; Nickels, D.; Cox, D.; Wang, H.B.; Manthey, J. Immunotoxicity of a standardized citrus polymethoxylated flavone extract. Food Chem. Toxicol. 2001, 39, 1087–1094. [Google Scholar] [CrossRef]
- Suzuki, K.; Gonda, K.; Kishimoto, Y.; Katsumoto, Y.; Takenoshita, S. Potential curing and beneficial effects of Ooitabi (Ficus pumila L.) on hypertension and dyslipidaemia in Okinawa. J. Hum. Nutr. Diet 2021, 34, 395–401. [Google Scholar] [CrossRef]
- Gonda, K.; Suzuki, K.; Sunabe, Y.; Kono, K.; Takenoshita, S. Ficus pumila L. improves the prognosis of patients infected with HTLV-1, an RNA virus. Nutr. J. 2021, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kawaguchi, A.; Ainai, A.; Tamura, S.; Ito, R.; Multihartina, P.; Setiawaty, V.; Pangesti, K.N.; Odagiri, T.; Tashiro, M.; et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc. Natl. Acad. Sci. USA 2015, 112, 7809–7814. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Sano, K.; Suzuki, T.; Ainai, A.; Taga, Y.; Ueno, T.; Tabata, K.; Saito, K.; Wada, Y.; Ohara, Y.; et al. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog. 2019, 15, e1007427. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Tanaka, A.; Fukuda, Y.; Miyata, Y.; Murata, Y.; Kishino-Oki, Y.; Homma, T.; Ohnishi, T.; Sagara, H. Successful Treatment of Seasonal Influenza A (H3N2) infection-related Hemophagocytic Lymphocytosis in an Elderly Man. Kansenshogaku Zasshi 2016, 90, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Riordan, S.M.; McIver, C.J.; Wakefield, D.; Thomas, M.C.; Duncombe, V.M.; Bolin, T.D. Serum immunoglobulin and soluble IL-2 receptor levels in small intestinal overgrowth with indigenous gut flora. Dig. Dis. Sci. 1999, 44, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Uji, M.; Matsushita, H.; Iwata, S. Microscopic polyangiitis after influenza vaccination. Intern. Med. 2005, 44, 892–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.K.; Yeh, C.H.; Iwamoto, T.; Satsu, H.; Shimizu, M.; Totsuka, M. Dietary Flavonoid Naringenin Induces Regulatory T Cells via an Aryl Hydrocarbon Receptor Mediated Pathway. J. Agric. Food Chem. 2012, 60, 2171–2178. [Google Scholar] [CrossRef]
- Annunziata, G.; Sanduzzi, Z.M.; Santoro, C.; Ciampaglia, R.; Stornaiuolo, M.; Tenore, G.C.; Sanduzzi, A.; Novellino, E. May Polyphenols Have a Role Against Coronavirus Infection? An Overview of in vitro Evidence. Front. Med. 2020, 7, 240. [Google Scholar] [CrossRef]


| Okinawan vegetables | Polyphenols (Flavonoids) | Content (mg/100 g FW) 1 | Daily intake (mg/day) |
|---|---|---|---|
| Alpinia zerumbet | phenolic acids (benzoic and cinnamic acid) | 150 (Leaf) | 10–20 |
| Saxifraga stolonifera | bergenin | 10–100 (Leaf) | 25–50 |
| Peucedanum japonicum Thunb | chlorogenic acid | 120–300 (Leaf) | 10–180 |
| 100–200 (Stem) | |||
| 700–1300 (Flower) | |||
| rutin | 70–200 (Leaf) | ||
| Crepidiastrum lanceolatum | chicoric acid | 144 (Leaf) | 3.0–10 |
| Gynura bicolor | phenolic acid | 1428–1569 (Leaf) | 70–200 |
| carotenoid | 921–1007 (Leaf) | ||
| anthocyanin | 2135–2407 (Leaf) | ||
| Benincasa hispida | quercetin | 4 (Fruit) | 3.0–4.0 |
| rutin | 12 (Fruit) | ||
| Momordica charantia | phenolic acids (apigenin and chrysin) | 964 (Ripe Fruit) | 500–900 |
| Citrus depressa Hayata | nobiletin | 129–170 (dry-Peel) | 10–20 |
| Ficus pumila L. | rutin | 1.0 (Leaf) | 2.4 |
| apigenin | 0.7(Leaf) | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonda, K.; Kanazawa, H.; Maeda, G.; Matayoshi, C.; Hirose, N.; Katsumoto, Y.; Kono, K.; Takenoshita, S. Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses. Nutrients 2021, 13, 1773. https://doi.org/10.3390/nu13061773
Gonda K, Kanazawa H, Maeda G, Matayoshi C, Hirose N, Katsumoto Y, Kono K, Takenoshita S. Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses. Nutrients. 2021; 13(6):1773. https://doi.org/10.3390/nu13061773
Chicago/Turabian StyleGonda, Kenji, Hideto Kanazawa, Goki Maeda, Chisa Matayoshi, Naoto Hirose, Yukiteru Katsumoto, Koji Kono, and Seiichi Takenoshita. 2021. "Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses" Nutrients 13, no. 6: 1773. https://doi.org/10.3390/nu13061773
APA StyleGonda, K., Kanazawa, H., Maeda, G., Matayoshi, C., Hirose, N., Katsumoto, Y., Kono, K., & Takenoshita, S. (2021). Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses. Nutrients, 13(6), 1773. https://doi.org/10.3390/nu13061773






