Blood Pressure-Lowering Effect of Wine Lees: Dose-Response Study, Effect of Dealcoholization and Possible Mechanisms of Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
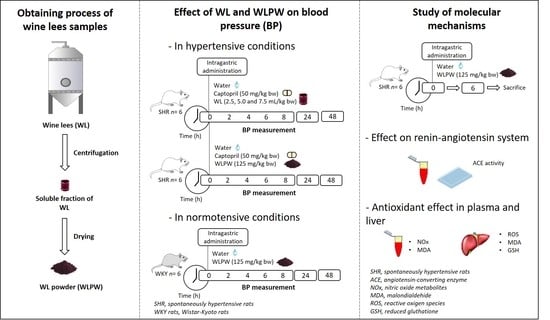
2.2. Obtaining and Characterisation of the Wine Lees Samples
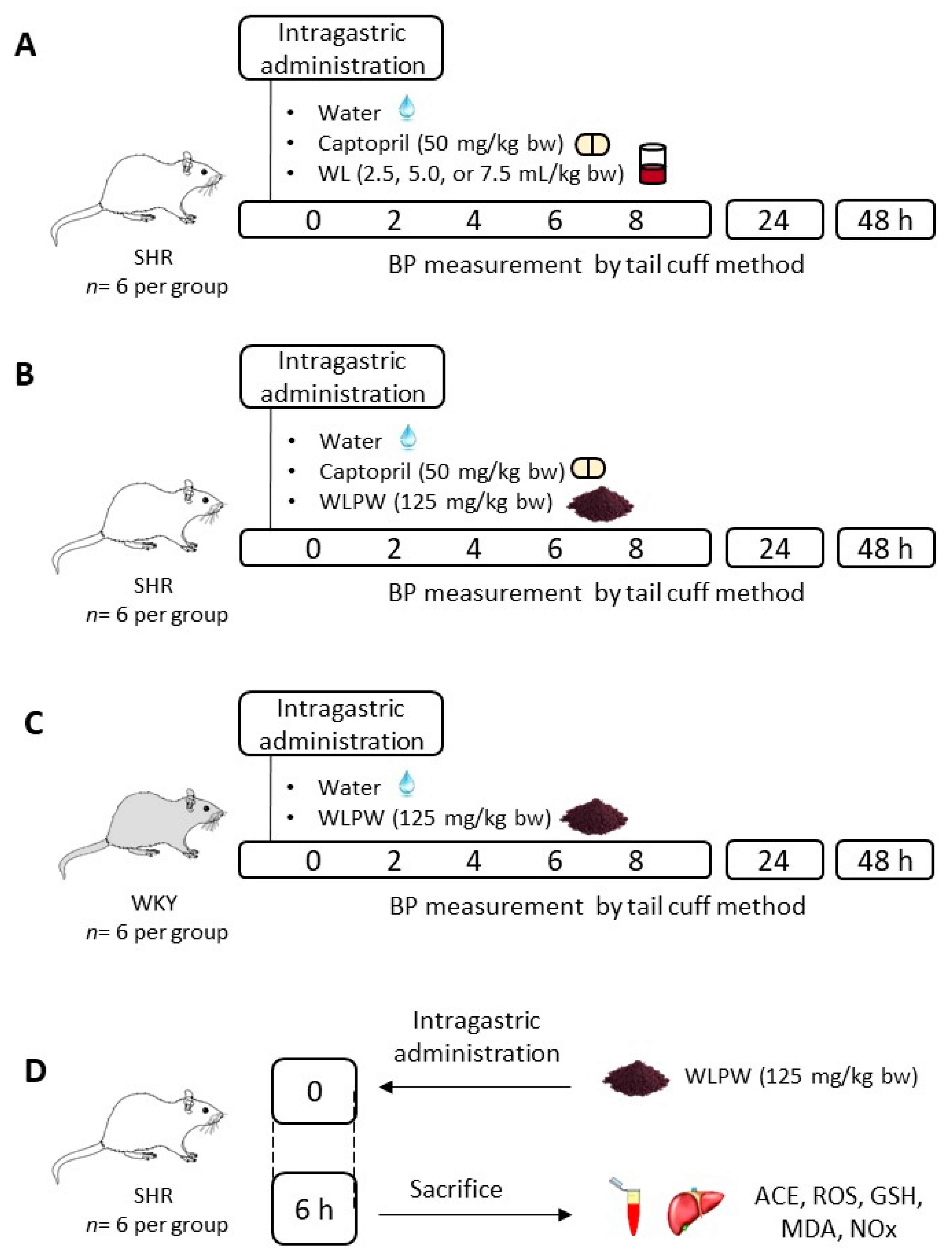
2.3. Experimental Procedure in Rats
2.4. Determination of Plasma ACE Activity
2.5. Reduced Glutathione Assay
2.6. Malondialdehyde Production
2.7. Reactive Oxygen Species
2.8. Nitric Oxide Metabolites in Plasma
2.9. Statistical Analysis
3. Results
3.1. Effect of Different Doses of Wine Lees on Blood Pressure in Hypertensive Rats
3.2. Effect of Dried Wine Lees on Blood Pressure in Hypertensive and Normotensive Rats
3.3. Mechanisms Involved in the Antihypertensive Effect of the Wine Lees Extract
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 29 January 2021).
- Nishiyama, A.; Kobori, H. Independent Regulation of Renin–Angiotensin–Aldosterone System in the Kidney. Clin. Exp. Nephrol. 2018, 22, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Te Riet, L.; van Esch, J.H.M.; Roks, A.J.M.; Van Den Meiracker, A.H.; Danser, A.H.J. Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Brown, N.J.; Vaughan, D.E. Angiotensin-Converting Enzyme Inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Mao, C.; Xu, Z.; Zhang, L. Angiotensin-Converting Enzymes and Drug Discovery in Cardiovascular Diseases. Drug Discov. Today 2010, 15, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Girgih, A.T.; Nwachukwu, I.D.; Hasan, F.; Fagbemi, T.N.; Gill, T.; Aluko, R.E. Kinetics of the Inhibition of Renin and Angiotensin I-Converting Enzyme by Cod (Gadus Morhua) Protein Hydrolysates and Their Antihypertensive Effects in Spontaneously Hypertensive Rats. Food Nutr. Res. 2015, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, P.; Pandey, A.; Azad, C.S.; Tia, N.; Singh, M.; Gambhir, I.S. Association of Oxidative Stress and Endothelial Dysfunction in Hypertension. Anal. Biochem. 2020, 590, 113535. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive Oxygen Species: Key Regulators in Vascular Health and Diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural Antioxidants and Hypertension: Promise and Challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High Value-Added Compounds from Fruit and Vegetable by-Products-Characterization, Bioactivities, and Application in the Development of Novel Food Products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Wightman, J.D.; Heuberger, R.A. Effect of Grape and Other Berries on Cardiovascular Health. J. Sci. Food Agric. 2015, 95, 1584–1597. [Google Scholar] [CrossRef]
- Levantesi, G.; Marfisi, R.; Mozaffarian, D.; Franzosi, M.G.; Maggioni, A.; Nicolosi, G.L.; Schweiger, C.; Silletta, M.; Tavazzi, L.; Tognoni, G.; et al. Wine Consumption and Risk of Cardiovascular Events after Myocardial Infarction: Results from the GISSI-Prevenzione Trial. Int. J. Cardiol. 2013, 163, 282–287. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-Molecular Procyanidin Rich Grape Seed Extract Exerts Antihypertensive Effect in Males Spontaneously Hypertensive Rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Pons, Z.; Guerrero, L.; Margalef, M.; Arola, L.; Arola-Arnal, A.; Muguerza, B. Effect of Low Molecular Grape Seed Proanthocyanidins on Blood Pressure and Lipid Homeostasis in Cafeteria Diet-Fed Rats. J. Physiol. Biochem. 2014, 70, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Acute Administration of Single Oral Dose of Grape Seed Polyphenols Restores Blood Pressure in a Rat Model of Metabolic Syndrome: Role of Nitric Oxide and Prostacyclin. Eur. J. Nutr. 2015, 55, 749–758. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef] [Green Version]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. Changes in Arterial Blood Pressure Caused by Long-Term Administration of Grape Seed Proanthocyanidins in Rats with Established Hypertension. Food Funct. 2020, 11, 8735–8742. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Fernández-Vallinas, S.; Pons, Z.; Arola, L.; Aleixandre, A.; Muguerza, B. Involvement of Nitric Oxide and Prostacyclin in the Antihypertensive Effect of Low-Molecular-Weight Procyanidin Rich Grape Seed Extract in Male Spontaneously Hypertensive Rats. J. Funct. Foods 2014, 6, 419–427. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of Lees in Wine Production: A Review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Margalef, M.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile. Nutrients 2021, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef]
- OIV. Código Prácticas Enológicas; OIV: Paris, France, 2016; Volume 33. [Google Scholar]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunniff, P. Official Method 969.33 of AOAC International. In Official Methods of Analysis of AOAC Internationa; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Quiñones, M.; Miguel, M.; Muguerza, B.; Aleixandre, A. Effect of a Cocoa Polyphenol Extract in Spontaneously Hypertensive Rats. Food Funct. 2011, 2, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Pons, Z.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients 2018, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Kamencic, H.; Lyon, A.; Paterson, P.G.; Juurlink, B.H.J. Monochlorobimane Fluorometric Method to Measure Tissue Glutathione. Anal. Biochem. 2000, 286, 35–37. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suarez, M.; Muguerza, B.; Bravo, F.I. Long-Term Administration of Protein Hydrolysate from Chicken Feet Induces Antihypertensive Effect and Confers Vasoprotective Pattern in Diet-Induced Hypertensive Rats. J. Funct. Foods 2019, 55. [Google Scholar] [CrossRef]
- Gabbia, D.; Pozzo, L.; Zigiotto, G.; Roverso, M.; Sacchi, D.; Pozza, A.D.; Carrara, M.; Bogialli, S.; Floreani, A.; Guido, M.; et al. Dexamethasone Counteracts Hepatic Inflammation and Oxidative Stress in Cholestatic Rats via CAR Activation. PLoS ONE 2018, 13, e0204336. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Gautreaux, M.D.; Berg, R.D. Measurement of Nitrate and Nitrite in Extracellular Fluids: A Window to Systemic Nitric Oxide Metabolism. Methods 1995, 7, 84–90. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.A.; Yuan Yuan, D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant Therapy for Management of Oxidative Stress Induced Hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Trovato-Salinaro, A.; Cambria, M.; Locascio, M.; Rienzo, L.; Condorelli, D.; Mancuso, C.; De Lorenzo, A.; Calabrese, E. The Hormetic Role of Dietary Antioxidants in Free Radical-Related Diseases. Curr. Pharm. Des. 2010, 16, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. NeuroMol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phyther. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Margalef, M.; Arola-Arnal, A.; Muguerza, B.; Miguel, M.; Aleixandre, A. The Blood Pressure Effect and Related Plasma Levels of Flavan-3-Ols in Spontaneously Hypertensive Rats. Food Funct. 2015, 6, 3479–3489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cienfuegos-Jovellanos, E.; del Mar Quiñones, M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive Effect of a Polyphenol-Rich Cocoa Powder Industrially Processed to Preserve the Original Flavonoids of the Cocoa Beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martínez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized Red Wine Decreases Systolic and Diastolic Blood Pressure and Increases Plasma Nitric Oxide: Short Communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pino-García, R.; Rivero-Pérez, M.D.; González-Sanjosé, M.L.; Croft, K.D.; Muñiz, P. Antihypertensive and Antioxidant Effects of Supplementation with Red Wine Pomace in Spontaneously Hypertensive Rats. Food Funct. 2017, 8, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin Improves Vascular Endothelial Function through Promotion of Autophagy in Hypertensive Rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alañón, M.E.; Castle, S.M.; Serra, G.; Lévèques, A.; Poquet, L.; Actis-Goretta, L.; Spencer, J.P.E. Acute Study of Dose-Dependent Effects of (−)-Epicatechin on Vascular Function in Healthy Male Volunteers: A Randomized Controlled Trial. Clin. Nutr. 2020, 39, 746–754. [Google Scholar] [CrossRef]
- Rodríguez-Mateos, A.; Weber, T.; Skene, S.S.; Ottaviani, J.I.; Crozier, A.; Kelm, M.; Schroeter, H.; Heiss, C. Assessing the Respective Contributions of Dietary Flavanol Monomers and Procyanidins in Mediating Cardiovascular Effects in Humans: Randomized, Controlled, Double-Masked Intervention Trial. Am. J. Clin. Nutr. 2018, 108, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Grape Seed Flavanols Decrease Blood Pressure via Sirt-1 and Confer a Vasoprotective Pattern in Rats. J. Funct. Foods 2016, 24, 164–172. [Google Scholar] [CrossRef]
- Calfío, C.; Huidobro-Toro, J.P. Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis Microphylla) with Nutraceutical Potential. Molecules 2019, 24, 2700. [Google Scholar] [CrossRef] [Green Version]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a Quercetin-Rich Onion Skin Extract on 24 h Ambulatory Blood Pressure and Endothelial Function in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomised Double-Blinded Placebo-Controlled Cross-over Trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzun, H.; Karter, Y.; Aydin, S.; Çurgunlu, A.; Şimşek, G.; Yücel, R.; Vehiyd, S.; Ertürk, N.; Kutlu, A.; Benian, A.; et al. Oxidative Stress in White Coat Hypertension; Role of Paraoxonase. J. Hum. Hypertens. 2004, 18, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Guichard, C.; Bächler, J.P. Relationship between Oxidative Stress and Essential Hypertension. Hypertens. Res. 2007, 30, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Tanito, M.; Nakamura, H.; Kwon, Y.W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced Oxidative Stress and Impaired Thioredoxin Expression in Spontaneously Hypertensive Rats. Antioxid. Redox Signal. 2004, 6, 89–97. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide Anions and Hyperoxia Inactivate Endothelium-Derived Relaxing Factor. Am. J. Physiol. Circ. Physiol. 1986, 250, H822–H827. [Google Scholar] [CrossRef]
- Crowley, S.D. The Cooperative Roles of Inflammation and Oxidative Stress in the Pathogenesis of Hypertension. Antioxid. Redox Signal. 2014, 20, 102–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurcevic, I.L.; Dora, M.; Guberovic, I.; Petras, M.; Brncic, S.R.; Dikic, D. Polyphenols from Wine Lees as a Novel Functional Bioactive Compound in the Protection against Oxidative Stress and Hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J.L. 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase Inhibitors Protect against Oxidized Low-Density Lipoprotein-Induced Endothelial Dysfunction. Endothel. J. Endothel. Cell Res. 2003, 10, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, H.F.; Reid, R.G.; Yousef, S.; Boyle, S.P. A Specific, Accurate, and Sensitive Measure of Total Plasma Malondialdehyde by HPLC. J. Lipid Res. 2013, 54, 852–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Parameters | WLPW |
|---|---|
| Moisture | 7.85 ± 1.49% |
| Total protein content a | 24.08 ± 6.20% |
| Total phenolic content a | 82.40 ± 0.80 mg GAE/g |
| ACEi activity (IC50) a | 13.38 ± 0.91 µg/mL |
| ACEi activity (IC50) | 3.23 ± 0.25 µg prot/mL |
| Antioxidant activity (EC50) a | 5.50 ± 0.58 µg/mL |
| Compound | Quantity (µg/g) |
|---|---|
| Flavanols | |
| Catechin | 3905.20 ± 19.20 |
| Catechin gallate a | 32.00 ± 0.40 |
| Epicatechin | 1739.20 ± 6.12 |
| (Epi)catechin O-glucoside iso1 b | 20.00 ± 0.01 |
| (Epi)catechin O-glucoside iso2 b | 13.20 ± 0.00 |
| (Epi)catechin O-glucoside iso3 b | 58.80 ± 1.17 |
| Procyanidin dimer B2 | 1384.00 ± 0.40 |
| Procyanidin dimer iso1 c | 2568.40 ± 6.12 |
| Procyanidin dimer iso2 c | 570.40 ± 2.50 |
| Procyanidin dimer iso3 c | 118.40 ± 0.83 |
| Procyanidin dimer iso4 c | 512.00 ± 4.25 |
| Procyanidin dimer iso5 c | 172.80 ± 0.89 |
| Procyanidin trimer iso1 c | 651.20 ± 4.12 |
| Procyanidin trimer iso2 c | 574.00 ± 10.60 |
| Procyanidin trimer iso3 c | 244.40 ± 2.32 |
| Procyanidin trimer iso4 c | 128.80 ± 4.23 |
| Procyanidin trimer iso5 c | 551.60 ± 3.81 |
| Flavonols | |
| Quercetin | 1471.20 ± 4.85 |
| Quercetin-3-O-glucoside d | 65.20 ± 0.42 |
| Quercetin-3-O-glucuronide d | 96.80 ± 0.82 |
| Kaempferol d | 206.00 ± 1.63 |
| Kaempferol-3-O-glucuronide d | 19.20 ± 0.38 |
| Isorhamnetin d | 446.40 ± 2.51 |
| Phenolic Acids | |
| Gallic acid | 4834.80 ± 96.60 |
| Caffeic acid | 130.80 ± 0.84 |
| Caffeic acid O-glucoside iso1 e | 22.00 ± 0.80 |
| Caffeic acid O-glucoside iso2 e | 26.40 ± 1.20 |
| p-Coumaric acid | 137.60 ± 0.74 |
| 4-Hydroxybenzoic acid | 66.80 ± 2.32 |
| Ferulic acid | 30.00 ± 0.47 |
| Vanillic acid | 93.20 ± 2.61 |
| Stilbenes | |
| trans-Resveratrol f | 184.00 ± 0.80 |
| Resveratrol iso1 f | 118.00 ± 0.40 |
| Resveratrol O-glucoside iso1 f | 10.80 ± 0.40 |
| Resveratrol O-glucoside iso2 f | 54.00 ± 1.60 |
| Piceatannol f | 168.00 ± 2.17 |
| Piceatannol 3-O-glucoside iso1 f | 8.80 ± 0.00 |
| Piceatannol 3-O-glucoside iso2 f | 2.40 ± 0.00 |
| Viniferin-iso1 f | 10.80 ± 0.00 |
| Viniferin-iso2 f | 32.40 ± 0.45 |
| Anthocyanins | Quantity (µg/g) |
|---|---|
| Gallocatechin-Malvidin-3-glucoside dimer a | 9.86 ± 0.04 |
| Malvidin-3-glucoside-(epi)catechin a | 44.43 ± 0.13 |
| Delphinidin-3-glucoside b | 147.58 ± 1.82 |
| Cyanidin-3-glucoside b | 9.05 ± 0.81 |
| Petunidin-3-glucoside c | 201.21 ± 2.26 |
| Petunidin-3-glucoside-pyruvic acid c | 3.56 ± 0.04 |
| Peonidin-3-glucoside c | 108.84 ± 2.50 |
| Malvidin-3-glucoside a | 2426.95 ± 20.01 |
| Peonidin-3-glucoside-pyruvic acid c | 1.63 ± 0.03 |
| Delphinidin-(6-acetyl)-3-glucoside b | 36.33 ± 0.90 |
| Visitin A (malvidin-3-glucoside-pyruvic acid) a | 49.07 ± 0.13 |
| Visitin B (malvidin-3-glucoside-acetaldehyde) a | 122.52 ± 0.59 |
| Malvidin-3-glucoside-ethyl-(epi)catechin a | 14.61 ± 0.03 |
| Cyanidin-(6-acetyl)-3-glucoside b | 8.14 ± 0.23 |
| Acetylvisitin A a | 31.41 ± 0.44 |
| Malvidin-3-glucoside-ethyl-(epi)catechin a | 55.06 ± 0.24 |
| Petunidin-(6-acetyl)-3-glucoside c | 51.55 ± 1.87 |
| Malvidin-3-glucoside-ethyl-(epi)catechin a | 81.77 ± 0.73 |
| Acetylvisitin B a | 66.45 ± 0.44 |
| Peonidin-(6-acetyl)-3-glucoside c | 52.79 ± 1.24 |
| Delphinidin-(6-coumaroyl)-3-glucoside b | 17.47 ± 0.27 |
| Malvidin-(6-acetyl)-3-glucoside a | 1135.64 ± 0.84 |
| Coumaroylvisitin A a | 8.01 ± 0.07 |
| Malvidin-(6-caffeoyl)-3-glucoside a | 14.56 ± 0.27 |
| Cyanidin-(6-coumaroyl)-3-glucoside b | 3.96 ± 0.16 |
| Catechin-ethyl-Malvidin-3-acetylglucoside dimer a | 35.09 ± 0.31 |
| Petunidin-(6-coumaroyl)-3-glucoside c | 29.79 ± 0.36 |
| Pinotin A (malvidin-3-glucoside-vinylcatechol) a | 33.59 ± 0.51 |
| Malvidin-glucoside-vinyl-catechin a | 6.12 ± 0.03 |
| Coumaroylvisitin B a | 36.48 ± 0.28 |
| Malvidin-3-glucoside-vinylguaiacol a | 23.78 ± 0.20 |
| Catechin-ethyl-malvidin-3-coumaroylglucoside dimer a | 27.39 ± 0.11 |
| Catechin-ethyl-malvidin-3-acetylglucoside dimer a | 5.78 ± 0.06 |
| Peonidin-(6-coumaroyl)-3-glucoside c | 37.78 ± 1.08 |
| Malvidin-(6-coumaroyl)-3-glucoside a | 430.71 ± 0.60 |
| Malvidin-glucoside-vinyl-catechin a | 6.5 ± 0.02 |
| Acetyl-pinotin A a | 0.28 ± 0.00 |
| Malvidin 3-O-glucoside 4-vinylphenol (Pigment A) a | 25.58 ± 0.08 |
| Catechin-ethyl-malvidin-3-coumaroylglucoside dimer a | 4.74 ± 0.01 |
| Malvidin acetyl 3-O-glucoside 4-vinylphenol (Acetyl-pigment A) a | 15.32 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Suárez, M.; Mulero, M.; Arola, L.; Bravo, F.I.; Muguerza, B. Blood Pressure-Lowering Effect of Wine Lees: Dose-Response Study, Effect of Dealcoholization and Possible Mechanisms of Action. Nutrients 2021, 13, 1142. https://doi.org/10.3390/nu13041142
López-Fernández-Sobrino R, Soliz-Rueda JR, Suárez M, Mulero M, Arola L, Bravo FI, Muguerza B. Blood Pressure-Lowering Effect of Wine Lees: Dose-Response Study, Effect of Dealcoholization and Possible Mechanisms of Action. Nutrients. 2021; 13(4):1142. https://doi.org/10.3390/nu13041142
Chicago/Turabian StyleLópez-Fernández-Sobrino, Raúl, Jorge R. Soliz-Rueda, Manuel Suárez, Miquel Mulero, Lluís Arola, Francisca Isabel Bravo, and Begoña Muguerza. 2021. "Blood Pressure-Lowering Effect of Wine Lees: Dose-Response Study, Effect of Dealcoholization and Possible Mechanisms of Action" Nutrients 13, no. 4: 1142. https://doi.org/10.3390/nu13041142
APA StyleLópez-Fernández-Sobrino, R., Soliz-Rueda, J. R., Suárez, M., Mulero, M., Arola, L., Bravo, F. I., & Muguerza, B. (2021). Blood Pressure-Lowering Effect of Wine Lees: Dose-Response Study, Effect of Dealcoholization and Possible Mechanisms of Action. Nutrients, 13(4), 1142. https://doi.org/10.3390/nu13041142