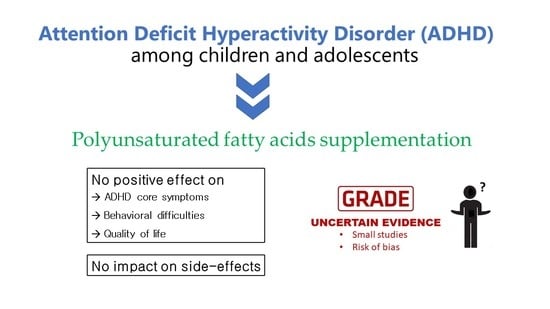
Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Extraction of Individual Randomized Controlled Studies
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
3.1. Literature Search
3.2. Review of the Evidence
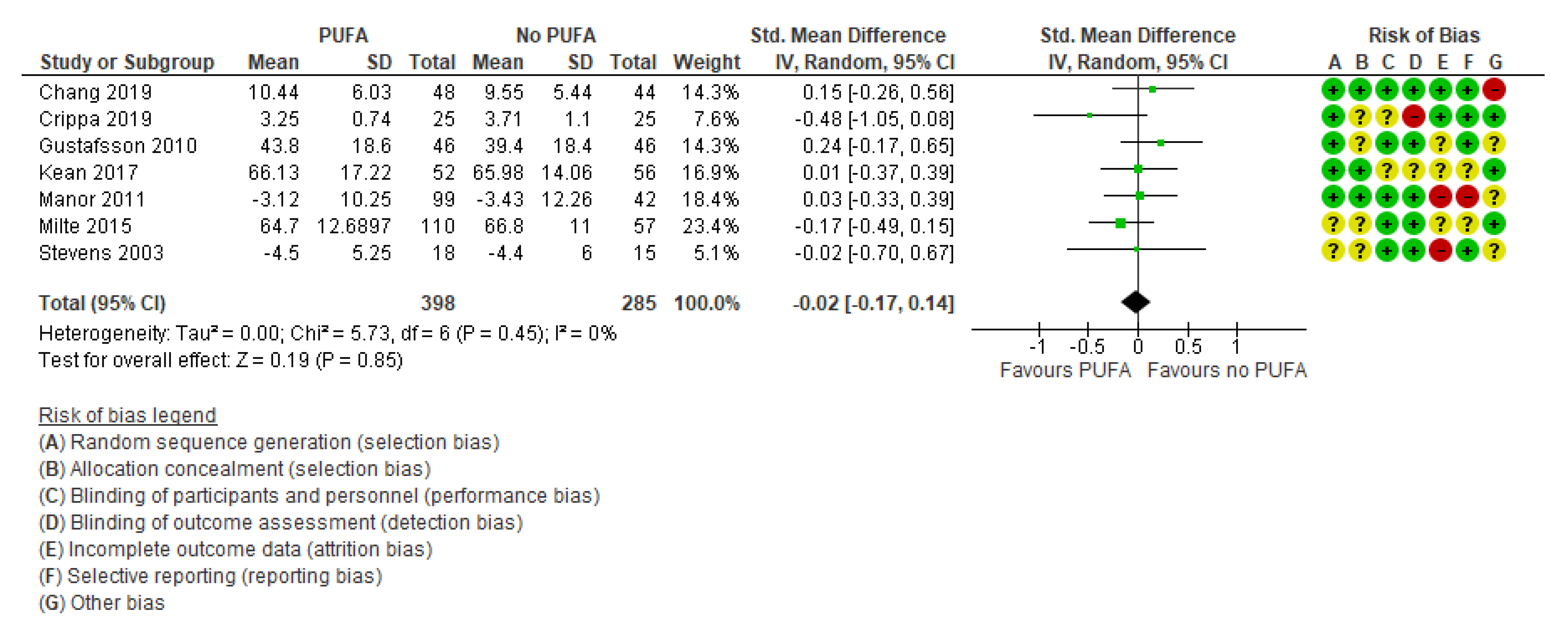
3.2.1. Primary Outcomes
3.2.2. Secondary Outcomes
3.3. Certainty of Evidence (GRADE)
4. Discussion
Strengths and Limitations of the Current Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef]
- Barkley, R.A.; Fischer, M. Hyperactive Child Syndrome and Estimated Life Expectancy at Young Adult Follow-Up: The Role of ADHD Persistence and Other Potential Predictors. J. Atten. Disord. 2019, 23, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Mohr-Jensen, C.; Steinhausen, H.C. A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations. Clin. Psychol. Rev. 2016, 48, 32–42. [Google Scholar] [CrossRef]
- Dalsgaard, S.; Leckman, J.F.; Mortensen, P.B.; Nielsen, H.S.; Simonsen, M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: A prospective cohort study. Lancet Psychiatry 2015, 2, 702–709. [Google Scholar] [CrossRef]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef]
- Galera, C.; Melchior, M.; Chastang, J.F.; Bouvard, M.P.; Fombonne, E. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: The GAZEL Youth study. Psychol. Med. 2009, 39, 1895–1906. [Google Scholar] [CrossRef] [Green Version]
- Erskine, H.E.; Norman, R.E.; Ferrari, A.J.; Chan, G.C.; Copeland, W.E.; Whiteford, H.A.; Scott, J.G. Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 841–850. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Brandeis, D.; Cortese, S.; Daley, D.; Ferrin, M.; Holtmann, M.; Stevenson, J.; Danckaerts, M.; Van der Oord, S.; Döpfner, M.; et al. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am. J. Psychiatry 2013, 170, 275–289. [Google Scholar] [CrossRef]
- De, C.F.; Cortese, S.; Adamo, N.; Janiri, L. Pharmacological and non-pharmacological treatment of adults with ADHD: A meta-review. Evid. Based Ment. Health 2017, 20, 4–11. [Google Scholar]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015, 351, h5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.P.-C.; Jingling, L.; Huang, Y.-T.; Lu, Y.-J.; Su, K.-P. Delay Aversion, Temporal Processing, and N-3 Fatty Acids Intake in Children with Attention-Deficit/Hyperactivity Disorder (ADHD). Clin. Psychol. Sci. 2016, 4, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. Fatty acids and immune function: New insights into mechanisms. Br. J. Nutr. 2007, 98 (Suppl. 1), S41–S45. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Essential fatty acids in health and chronic diseases. Forum Nutr. 2003, 56, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. Assessing risk of bias in cross-over trials. In Cochrane Handbook of Systematic Reviews of Interventions; The Cochrane Collaboration Version 5.1.0; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Schunemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Atkins, D.; Brozek, J.; Vist, G.; Alderson, P.; Glasziou, P.; Falck-Ytter, Y.; Schünemann, H.J. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 2011, 64, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Guanfacine Hydrochloride Extended Release (Intuniv XR) Tablets: For the Treatment of Attention-Deficit/Hyperactivity Disorder [Internet]: Appendix 5: Validity of Outcome Measures; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2015. [Google Scholar]
- Zhang, S.; Faries, D.E.; Vowles, M.; Michelson, D. ADHD Rating Scale IV: Psychometric properties from a multinational study as a clinician-administered instrument. Int. J. Methods Psychiatr. Res. 2005, 14, 186–201. [Google Scholar] [CrossRef]
- Buitelaar, J.K.; Montgomery, S.A.; van Zwieten-Boot, B.J. Attention deficit hyperactivity disorder: Guidelines for investigating efficacy of pharmacological intervention. Eur. Neuropsychopharmacol. 2003, 13, 297–304. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.G.; Mitchell, E.A.; Turbott, S.H. The effects of essential fatty acid supplementation by Efamol in hyperactive children. J. Abnorm. Child Psychol. 1987, 15, 75–90. [Google Scholar] [CrossRef]
- Arnold, L.E.; Kleykamp, D.; Votolato, N.A.; Taylor, W.A.; Kontras, S.B.; Tobin, K. Gamma-linolenic acid for attention-deficit hyperactivity disorder: Placebo-controlled comparison to D-amphetamine. Biol. Psychiatry 1989, 25, 222–228. [Google Scholar] [CrossRef]
- Bélanger, S.A.; Vanasse, M.; Spahis, S.; Sylvestre, M.P.; Lippé, S.; l’Heureux, F.; Ghadirian, P.; Vanasse, C.M.; Levy, E. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr. Child Health 2009, 14, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, P.A.; Birberg-Thornberg, U.; Duchén, K.; Landgren, M.; Malmberg, K.; Pelling, H.; Strandvik, B.; Karlsson, T. EPA supplementationn improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010, 99, 1540–1549. [Google Scholar] [CrossRef]
- Johnson, M.; Ostlund, S.; Fransson, G.; Kadesjo, B.; Gillberg, C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: A randomized placebo-controlled trial in children and adolescents. J. Atten. Disord. 2009, 12, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, S.; Hamazaki, T.; Terasawa, K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder—a placebo-controlled double-blind study. Eur. J. Clin. Nutr. 2004, 58, 467–473. [Google Scholar] [CrossRef]
- Manor, I.; Magen, A.; Keidar, D.; Rosen, S.; Tasker, H.; Cohen, T.; Richter, Y.; Zaaroor-Regev, D.; Manor, Y.; Weizman, A. The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: A double-blind placebo-controlled trial, followed by an open-label extension. Eur. Psychiatry 2012, 27, 335–342. [Google Scholar] [CrossRef]
- Raz, R.; Carasso, R.L.; Yehuda, S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: A double-blind placebo-controlled study. J. Child Adolesc. Psychopharmacol. 2009, 19, 167–177. [Google Scholar] [CrossRef]
- Sinn, N.; Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J. Dev. Behav. Pediatr. 2007, 28, 82–91. [Google Scholar] [CrossRef]
- Stevens, L.; Zhang, W.; Peck, L.; Kuczek, T.; Grevstad, N.; Mahon, A.; Zentall, S.S.; Eugene Arnold, L.; Burgess, J.R. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 2003, 38, 1007–1021. [Google Scholar] [CrossRef]
- Voigt, R.G.; Llorente, A.M.; Jensen, C.L.; Fraley, J.K.; Berretta, M.C.; Heird, W.C. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2001, 139, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, N.; Kaysar, N.; Zaruk-Adasha, Y.; Pelled, D.; Brichon, G.; Zwingelstein, G.; Bodennec, J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n-3 fatty acids containing phospholipids. Am. J. Clin. Nutr. 2008, 87, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Assareh, M.; Davari Ashtiani, R.; Khademi, M.; Jazayeri, S.; Rai, A.; Nikoo, M. Efficacy of Polyunsaturated Fatty Acids (PUFA) in the Treatment of Attention Deficit Hyperactivity Disorder. J. Atten. Disord. 2017, 21, 78–85. [Google Scholar] [CrossRef]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.; Demmelmair, H.; Koletzko, B.; Eilander, A.; Hoeksma, M.; Durston, S. Reduced Symptoms of Inattention after Dietary Omega-3 Fatty Acid Supplementation in Boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef] [Green Version]
- Dashti, N.; Hekmat, H.; Soltani, H.R.; Rahimdel, A.; Javaherchian, M. Comparison of therapeutic effects of omega-3 and methylphenidate (ritalin®) in treating children with attention deficit hyperactivity disorder. Iran. J. Psychiatry Behav. Sci. 2014, 8, 7. [Google Scholar]
- Dubnov-Raz, G.; Khoury, Z.; Wright, I.; Raz, R.; Berger, I. The effect of alpha-linolenic acid supplementation on ADHD symptoms in children: A randomized controlled double-blind study. Front. Hum. Neurosci. 2014, 8, 780. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, S.; Terasawa, K.; Rabeler, R.; Hirayama, T.; Inoue, T.; Tatsumi, Y.; Purpura, M.; Jäger, R. The effect of phosphatidylserine administration on memory and symptoms of attention-deficit hyperactivity disorder: A randomised, double-blind, placebo-controlled clinical trial. J. Hum. Nutr. Diet. 2014, 27, 284–291. [Google Scholar] [CrossRef]
- Manor, I.; Magen, A.; Keidar, D.; Rosen, S.; Tasker, H.; Cohen, T.; Richter, Y.; Zaaroor-Regev, D.; Manor, Y.; Weizman, A. Safety of phosphatidylserine containing omega3 fatty acids in ADHD children: A double-blind placebo-controlled trial followed by an open-label extension. Eur. Psychiatry 2013, 28, 386–391. [Google Scholar] [CrossRef]
- Milte, C.M.; Parletta, N.; Buckley, J.D.; Coates, A.M.; Young, R.M.; Howe, P.R.C. Increased Erythrocyte Eicosapentaenoic Acid and Docosahexaenoic Acid Are Associated with Improved Attention and Behavior in Children With ADHD in a Randomized Controlled Three-Way Crossover Trial. J. Atten. Disord. 2015, 19, 954–964. [Google Scholar] [CrossRef]
- Moghaddam, M.F.; Shamekhi, M.; Rakhshani, T. Effectiveness of methylphenidate and PUFA for the treatment of patients with ADHD: A double-blinded randomized clinical trial. Electron. Physician 2017, 9, 4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Mohammadbeigi, A.; Sheykholeslam, H.; Moshiri, E.; Dorreh, F. Omega-3 and Zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J. Res. Pharm. Pract. 2016, 5, 22. [Google Scholar]
- Widenhorn-Muller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Perera, H.; Jeewandara, K.C.; Seneviratne, S.; Guruge, C. Combined omega3 and omega6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: A double-blind, placebo-controlled study. J. Child Neurol. 2012, 27, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Barragan, E.; Breuer, D.; Dopfner, M. Efficacy and Safety of Omega-3/6 Fatty Acids, Methylphenidate, and a Combined Treatment in Children with ADHD. J. Atten. Disord. 2017, 21, 433–441. [Google Scholar] [CrossRef]
- Chang, J.P.C.; Su, K.P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.T.; Chiang, Y.J.; Chen, H.T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cornu, C.; Mercier, C.; Ginhoux, T.; Masson, S.; Mouchet, J.; Nony, P.; Kassai, B.; Laudy, V.; Berquin, P.; Franc, N.; et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur. Child Adolesc. Psychiatry 2018, 27, 377–384. [Google Scholar] [CrossRef]
- Crippa, A.; Tesei, A.; Sangiorgio, F.; Salandi, A.; Trabattoni, S.; Grazioli, S.; Agostoni, C.; Molteni, M.; Nobile, M. Behavioral and cognitive effects of docosahexaenoic acid in drug-naive children with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Eur. Child Adolesc. Psychiatry 2019, 28, 571–583. [Google Scholar] [CrossRef]
- Dopfner, M.; Dose, C.; Breuer, D.; Heintz, S.; Schiffhauer, S.; Banaschewski, T. Efficacy of Omega-3/Omega-6 Fatty Acids in Preschool Children at Risk of ADHD: A Randomized Placebo-Controlled Trial. J. Atten. Disord. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.D.; Sarris, J.; Scholey, A.; Silberstein, R.; Downey, L.A.; Stough, C. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524 R) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomised, double-blind, placebo-controlled trial. Psychopharmacology 2017, 234, 403–420. [Google Scholar] [PubMed] [Green Version]
- Mohammadzadeh, S.; Baghi, N.; Yousefi, F.; Yousefzamani, B. Effect of omega-3 plus methylphenidate as an alternative therapy to reduce attention deficit-hyperactivity disorder in children. Korean J. Pediatr. 2019, 62, 360. [Google Scholar] [CrossRef]
- Rodriguez, C.; Garcia, T.; Areces, D.; Fernandez, E.; Garcia-Noriega, M.; Domingo, J.C. Supplementation with high-content docosahexaenoic acid triglyceride in attention-deficit hyperactivity disorder: A randomized double-blind placebo-controlled trial. Neuropsychiatr. Dis. Treat. 2019, 15, 1193. [Google Scholar] [CrossRef] [Green Version]
- Tesei, A.; Crippa, A.; Ceccarelli, S.B.; Mauri, M.; Molteni, M.; Agostoni, C.; Nobile, M. The potential relevance of docosahexaenoic acid and eicosapentaenoic acid to the etiopathogenesis of childhood neuropsychiatric disorders. Eur. Child Adolesc. Psychiatry 2017, 26, 1011–1030. [Google Scholar] [CrossRef]
- Gillies, D.; Sinn, J.K.; Lad, S.S.; Leach, M.J.; Ross, M.J. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. 2012, 10, CD007986. [Google Scholar] [CrossRef]
- van der Wurff, I.S.M.; Meyer, B.J.; de Groot, R.H.M. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (n-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients 2020, 12, 3115. [Google Scholar] [CrossRef]
- Schachter, H.; Kourad, K.; Meraki, Z.; Lumb, A.; Tran, K.; Miguelez, M. Effects of Omega-3 FattyAcids on Mental Health. Evidence Report/TechnologyAssessment No 116 (Publication No 05-E022-2); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005. [Google Scholar]
- Pelsser, L.M.; Frankena, K.; Toorman, J.; Rodrigues, P.R. Diet and ADHD, Reviewing the Evidence: A Systematic Review of Meta-Analyses of Double-Blind Placebo-Controlled Trials Evaluating the Efficacy of Diet Interventions on the Behavior of Children with ADHD. PLoS ONE 2017, 12, e0169277. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Lange, K.M.; Makulska-Gertruda, E.; Nakamura, Y.; Reissmann, A.; Sakaue, Y.; Takano, T.; Takeuchi, Y. The Role of Nutritional Supplements in the Treatment of ADHD: What the Evidence Says. Curr. Psychiatry Rep. 2017, 19, 8. [Google Scholar] [CrossRef]
- Catalá-López, F.; Hutton, B.; Núñez-Beltrán, A.; Page, M.J.; Ridao, M.; Macías Saint-Gerons, D.; Catalá, M.A.; Tabarés-Seisdedos, R.; Moher, D. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS ONE 2017, 12, e0180355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banaschewski, T.; Belsham, B.; Bloch, M.H.; Ferrin, M.; Johnson, M.; Kustow, J.; Robinson, S.; Zuddas, A. Supplementation with polyunsaturated fatty acids (PUFAs) in the management of attention deficit hyperactivity disorder (ADHD). Nutr. Health 2018, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Perez Carmona, M.P. Complementary/alternative medicine in adolescents with attention deficit hyperactivity disorder and mood disorders. Rev. Chil. Pediatr. 2017, 88, 294–299. [Google Scholar]
- Derbyshire, E. Do Omega-3/6 Fatty Acids Have a Therapeutic Role in Children and Young People with ADHD? J. Lipids 2017, 2017, 6285218. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.P.; Su, K.P. Nutritional Neuroscience as Mainstream of Psychiatry: The Evidence- Based Treatment Guidelines for Using Omega-3 Fatty Acids as a New Treatment for Psychiatric Disorders in Children and Adolescents. Clin. Psychopharmacol. Neurosci. 2020, 18, 469–483. [Google Scholar] [CrossRef]
- Chang, J.P.; Su, K.P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilskov Rytter, M.J.; Andersen, L.B.B.; Houmann, T.; Bilenberg, N.; Hvolby, A.; Mølgaard, C.; Michaelsen, K.F.; Lauritzen, L. Diet in the treatment of ADHD in children—a systematic review of the literature. Nord. J. Psychiatry 2015, 69, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, MR000030. [Google Scholar] [CrossRef]







| Population | Children and adolescents between the age of 6 and 18 years (≥6 and ≤18), diagnosed with ADHD in accordance with ICD-10 or DSM criteria (both 4 and 5) for ADHD. |
| Intervention | Supplementation of polyunsaturated fatty acids (PUFAs). We included studies investigating both omega 3 and 6 fatty acids. |
| Comparison | No treatment—placebo and/or regular diet. |
| Outcome, primary | ADHD core symptoms, parent rated ADHD core symptoms, teacher rated Timing and effect measures ADHD core symptoms, both parent and teacher rated, were investigated at end of treatment. Minimal clinically important difference (MCID) 30% mean total score change difference between treatment groups, which is equivalent to between-treatment difference of 5.2 to 7.7 points [19,20,21]. |
| Outcome, secondary | Behavioral difficulties, parent rated Behavioral difficulties, teacher rated Quality of life Diarrhea Gastrointestinal discomfort Nausea Timing and effect measures Behavioral difficulties both parent and teacher rated was investigated at end of treatment. Quality of life was investigated at the longest follow-up time (minimum 3 months after end of treatment). In our published protocol, we initially planned to assess diarrhea, gastrointestinal discomfort, and nausea at longest follow-up. This was later changed to end of treatment, as the identified studies did not provide any follow-up data on these outcomes. |
| Study design | All randomized controlled studies, with interventions matching the defined research question. |
| # | Searches |
|---|---|
| 1 | exp unsaturated fatty acid/ |
| 2 | Diet therapy/ or diet supplementation/ |
| 3 | exp Fish oil/ |
| 4 | exp Carnitine/ |
| 5 | ((fatty adj1 acid*) or ((Polyunsaturated or poly-unsaturated or unsaturated) adj1 (fat or fatty)) or omega-3 or omega3 or omega 3 or omega-6 or omega6 or omega 6 or (docosahexaenoic adj acid*) or (eicosapentaenoic adj acid*) or (arachidonic adj acid)).ti,ab,kw. |
| 6 | ((fish adj1 oil*) or cod liver oil* or lax oil* or tuna oil* or carnitine or Levocarnitine or “L Carnitine” or L-carnitine or bicarnitine).ti,ab,kw. |
| 7 | ((diet* or food or nutrition) adj1 (therapy or supplement*)).ti,ab,kw. |
| 8 | or/1–7 |
| 9 | exp Attention Deficit Disorder/ |
| 10 | (ADHD or (hyperkinetic adj1 disorder*) or (Attention adj1 Deficit adj1 Disorder) or (attention-deficit adj1 disorder)).ti,ab,kw. |
| 11 | 9 or 10 |
| 12 | 8 and 11 |
| 13 | limit 12 to (randomized controlled trial or controlled clinical trial) |
| 14 | (((random* or cluster-random* or quasi-random* or control?ed or crossover or cross-over or blind* or mask*) adj4 (trial*1 or study or studies or analy*)) or rct).ti,ab,kw. |
| 15 | (placebo* or single-blind* or double-blind* or triple-blind*).ti,ab,kw. |
| 16 | ((single or double or triple) adj2 (blind* or mask*)).ti,ab,kw. |
| 17 | ((patient* or person* or participant* or population* or allocat* or assign*) adj3 random*).ti,ab,kw. |
| 18 | 14 or 15 or 16 or 17 |
| 19 | 12 and 18 |
| 20 | 13 or 19 |
| 21 | limit 20 to (yr = “2017–2020” and (english or danish or german or norwegian or swedish)) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Händel, M.N.; Rohde, J.F.; Rimestad, M.L.; Bandak, E.; Birkefoss, K.; Tendal, B.; Lemcke, S.; Callesen, H.E. Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2021, 13, 1226. https://doi.org/10.3390/nu13041226
Händel MN, Rohde JF, Rimestad ML, Bandak E, Birkefoss K, Tendal B, Lemcke S, Callesen HE. Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients. 2021; 13(4):1226. https://doi.org/10.3390/nu13041226
Chicago/Turabian StyleHändel, Mina Nicole, Jeanett Friis Rohde, Marie Louise Rimestad, Elisabeth Bandak, Kirsten Birkefoss, Britta Tendal, Sanne Lemcke, and Henriette Edemann Callesen. 2021. "Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials" Nutrients 13, no. 4: 1226. https://doi.org/10.3390/nu13041226
APA StyleHändel, M. N., Rohde, J. F., Rimestad, M. L., Bandak, E., Birkefoss, K., Tendal, B., Lemcke, S., & Callesen, H. E. (2021). Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients, 13(4), 1226. https://doi.org/10.3390/nu13041226