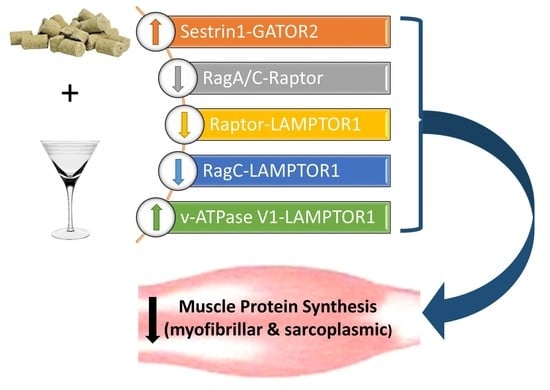
Alcohol Acutely Antagonizes Refeeding-Induced Alterations in the Rag GTPase-Ragulator Complex in Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Protocols
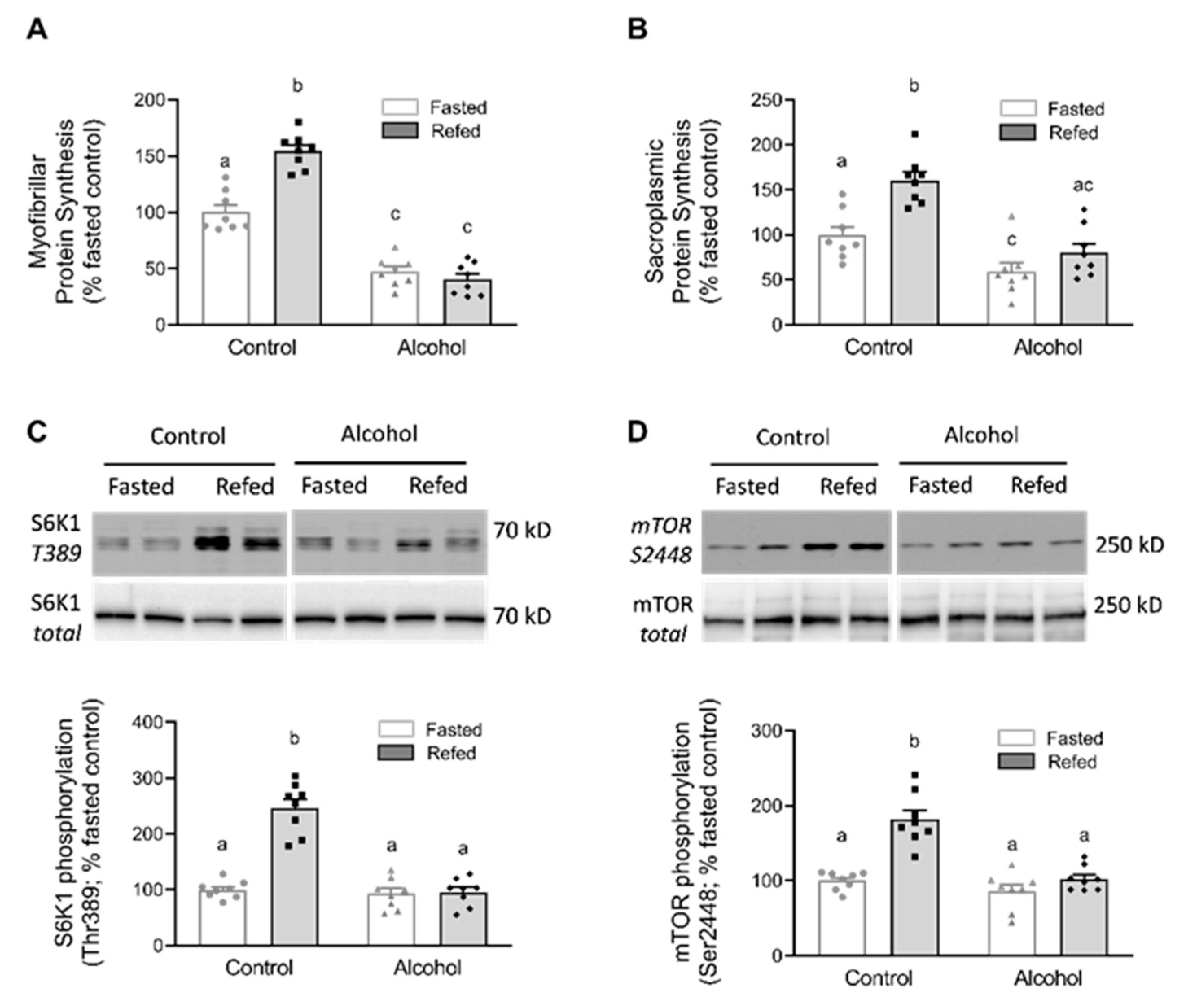
2.2. Protein Synthesis in Myofibrillar and Sarcoplasmic Pools
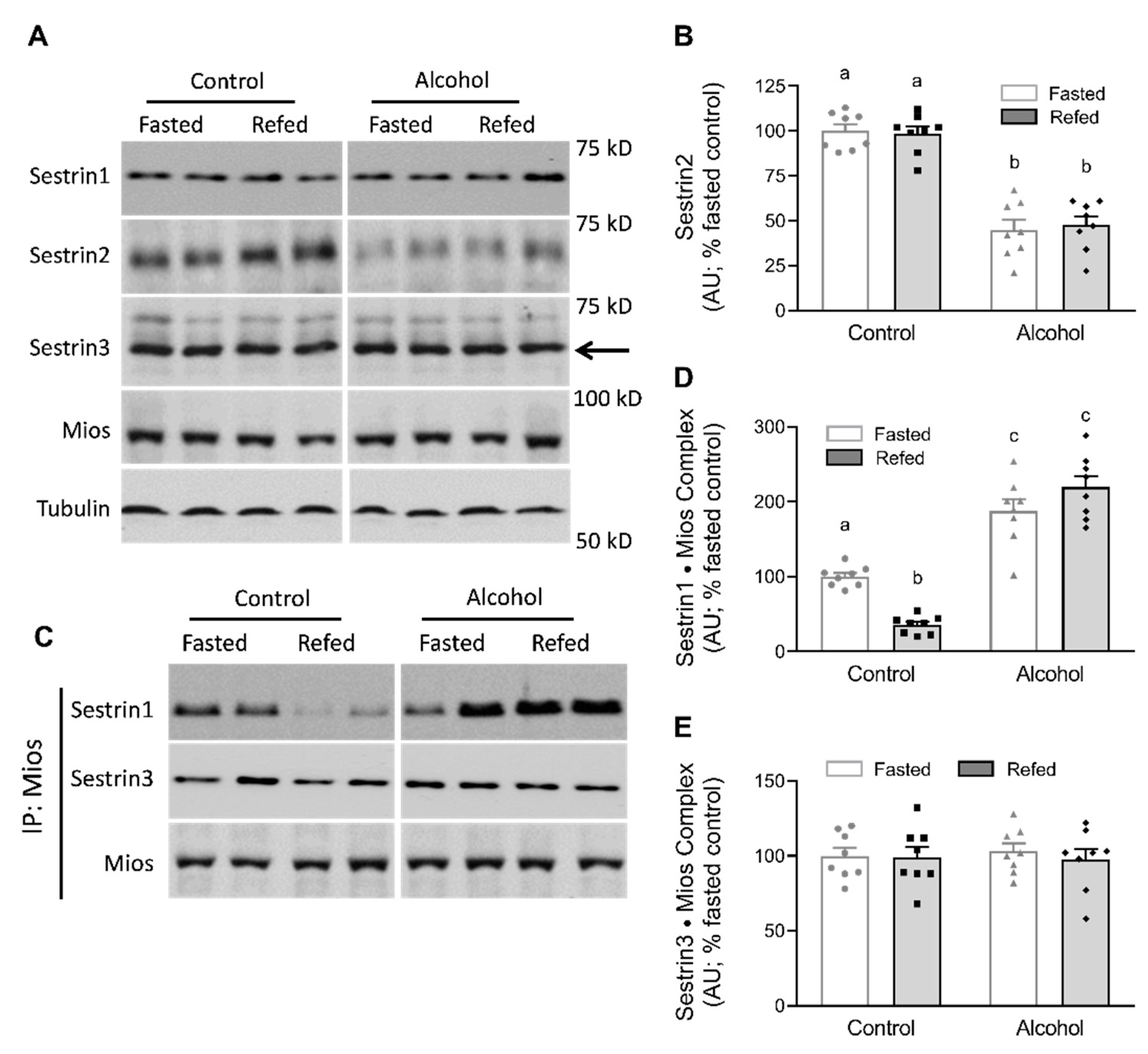
2.3. Western Blot and Immunoprecipitation
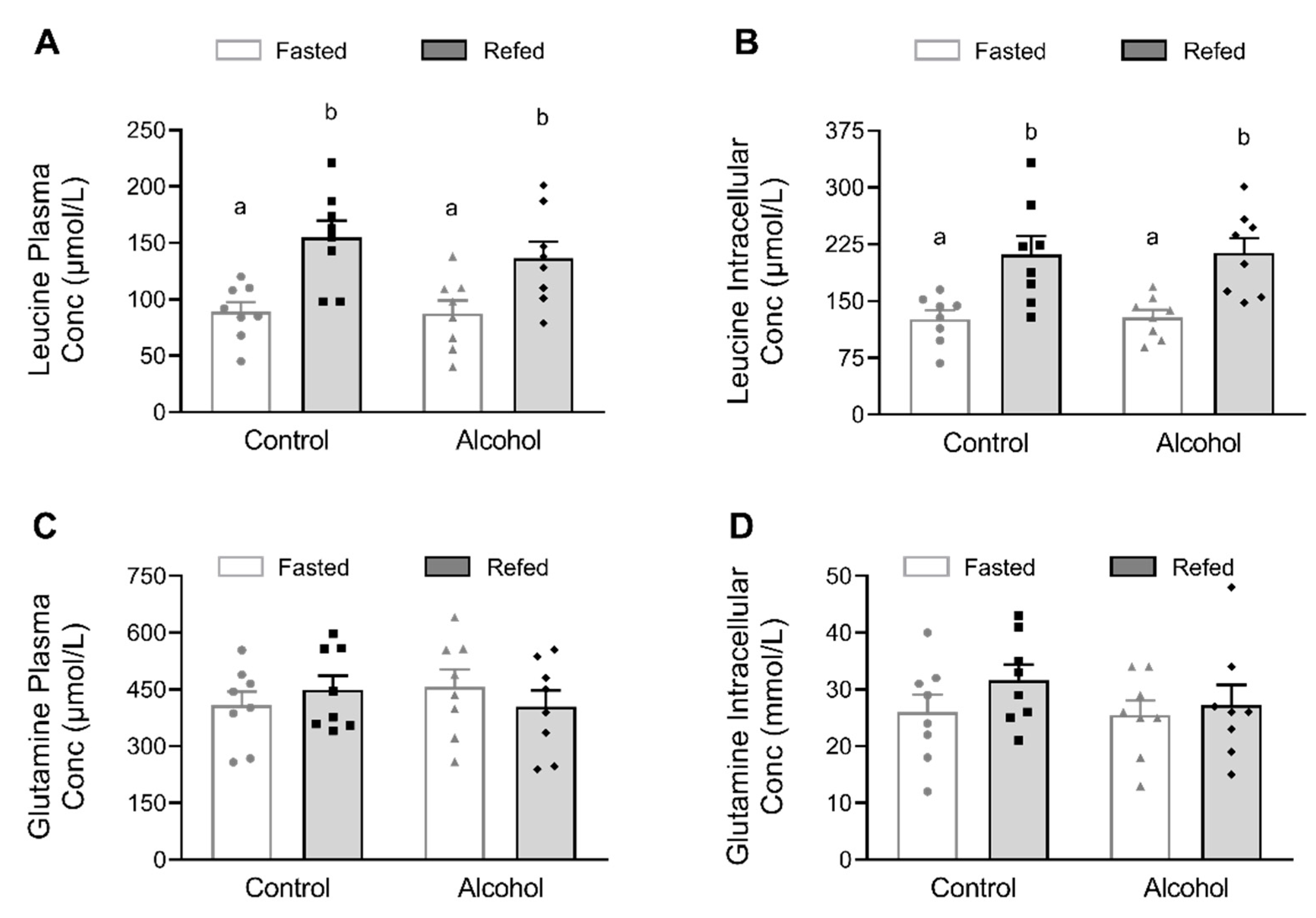
2.4. Leucine, Glutamine, Insulin and Alcohol Concentrations
2.5. RNA Extraction and Real-Time Quantitative PCR
2.6. Statistics
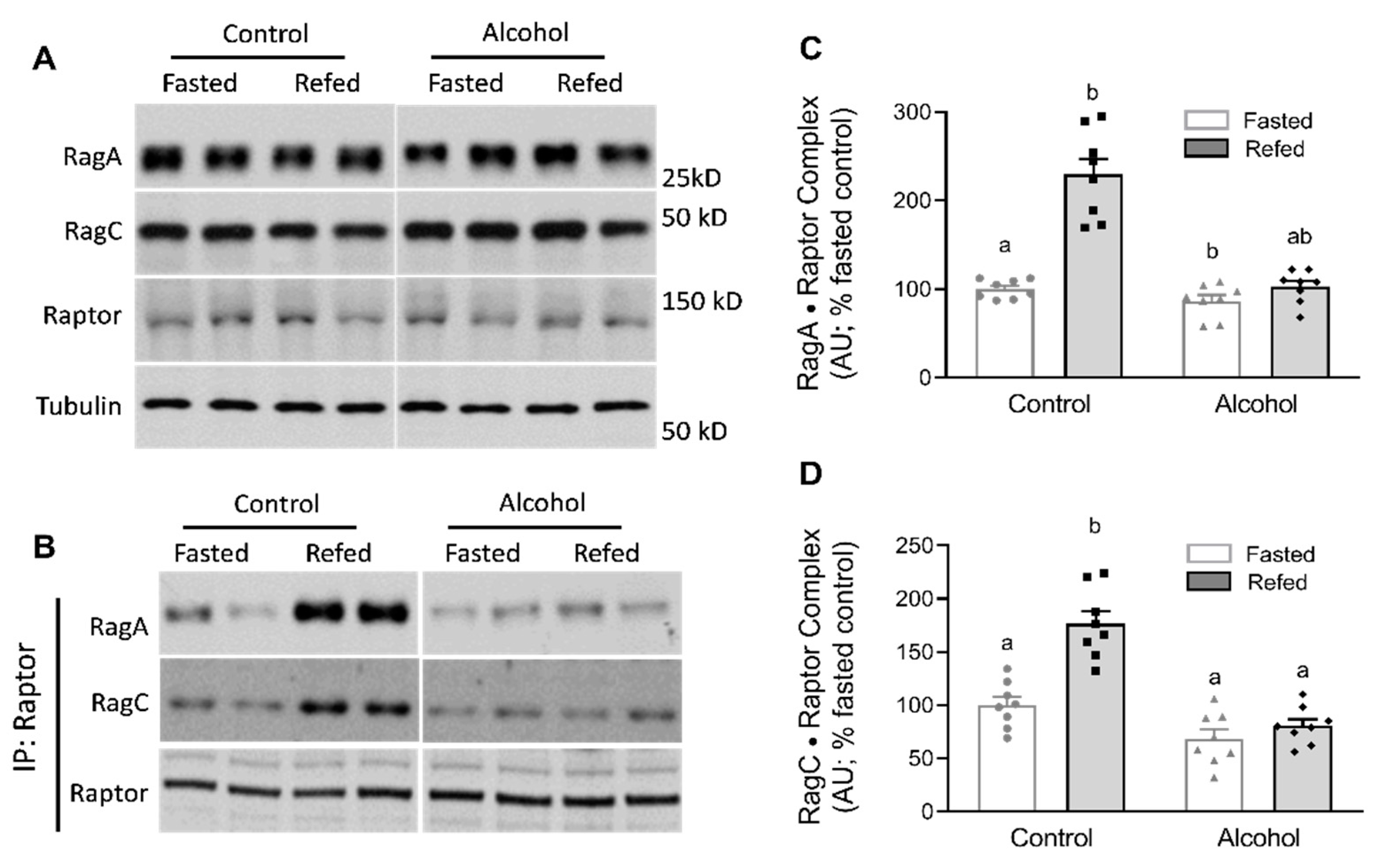
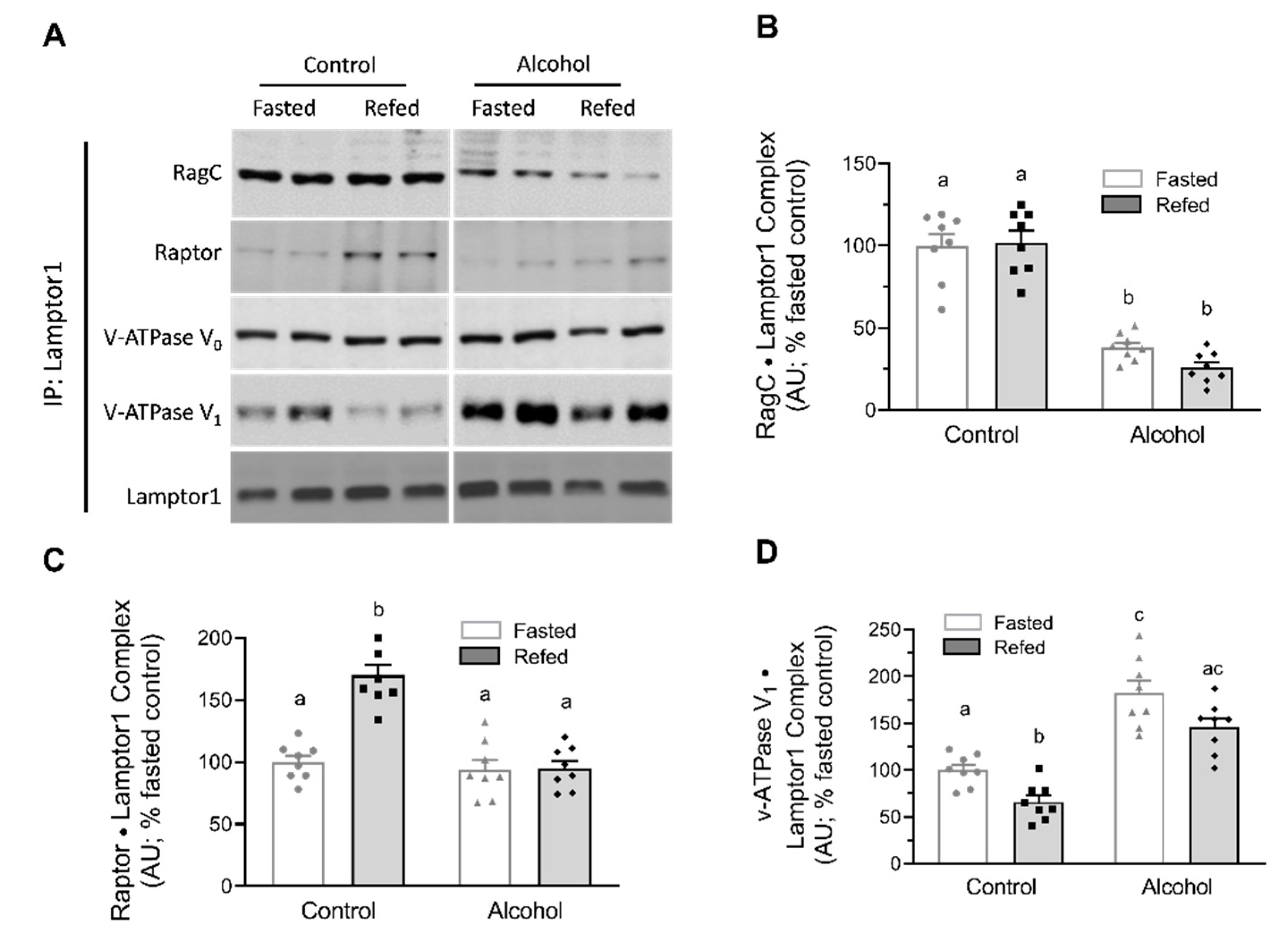
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kimball, S.R.; Lang, C.H. Mechanisms Underlying Muscle Protein Imbalance Induced by Alcohol. Annu. Rev. Nutr. 2018, 38, 197–217. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Dysregulation of skeletal muscle protein metabolism by alcohol. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E699–E712. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.H.; Frost, R.A.; Kumar, V.; Wu, D.; Vary, T.C. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol. Clin. Exp. Res. 2000, 24, 322–331. [Google Scholar] [CrossRef]
- Kumar, V.; Frost, R.A.; Lang, C.H. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E917–E928. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Deshpande, N.; Kumar, V.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1205–E1215. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Lynch, C.J.; Vary, T.C. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J. Nutr. 2010, 140, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, A.A.; Koll, M.; Wallace, M.C.; Jones, J.; Miell, J.P.; Garlick, P.J.; Preedy, V.R. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E874–E882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, J.L.; Lang, C.H. Ethanol acutely antagonizes the refeeding-induced increase in mTOR-dependent protein synthesis and decrease in autophagy in skeletal muscle. Mol. Cell. Biochem. 2019, 456, 41–51. [Google Scholar] [CrossRef]
- Kimball, S.R. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am. J. Clin. Nutr. 2014, 99, 237S–242S. [Google Scholar] [CrossRef] [Green Version]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [Green Version]
- Parmigiani, A.; Nourbakhsh, A.; Ding, B.; Wang, W.; Kim, Y.C.; Akopiants, K.; Guan, K.L.; Karin, M.; Budanov, A.V. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014, 9, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Chantranupong, L.; Wolfson, R.L.; Orozco, J.M.; Saxton, R.A.; Scaria, S.M.; Bar-Peled, L.; Spooner, E.; Isasa, M.; Gygi, S.P.; Sabatini, D.M. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef] [Green Version]
- Hong-Brown, L.Q.; Brown, C.R.; Kazi, A.A.; Navaratnarajah, M.; Lang, C.H. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am. J. Physiol. Cell Physiol. 2012, 302, C1557–C1565. [Google Scholar] [CrossRef] [Green Version]
- Mekheal, M.; Steiner, J.L.; Lang, C.H. Acute alcohol prevents the refeeding-induced decrease in autophagy but does not alter the increased protein synthetic response in heart. Alcohol 2018, 73, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A.; Hornberger, T.A. Measuring protein synthesis with SUnSET: A valid alternative to traditional techniques? Exerc. Sport Sci. Rev. 2013, 41, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, J.L.; Kimball, S.R.; Lang, C.H. Acute Alcohol-Induced Decrease in Muscle Protein Synthesis in Female Mice Is REDD-1 and mTOR-Independent. Alcohol Alcohol. 2016, 51, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vary, T.C.; Lang, C.H. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol. Biol. 2008, 447, 343–355. [Google Scholar] [CrossRef]
- Laufenberg, L.J.; Pruznak, A.M.; Navaratnarajah, M.; Lang, C.H. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 2014, 46, 2787–2798. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.L. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Orellana, R.A.; Kimball, S.R.; Suryawan, A.; Escobar, J.; Nguyen, H.V.; Jefferson, L.S.; Davis, T.A. Insulin stimulates muscle protein synthesis in neonates during endotoxemia despite repression of translation initiation. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E629–E636. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M. Amino acid transporters: Eminences grises of nutrient signalling mechanisms? Biochem. Soc. Trans. 2009, 37, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, J.H.; Yoon, I.; Lee, C.; Fallahi Sichani, M.; Kang, J.S.; Kang, J.; Guo, M.; Lee, K.Y.; Han, G.; et al. Coordination of the leucine-sensing Rag GTPase cycle by leucyl-tRNA synthetase in the mTORC1 signaling pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E5279–E5288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimball, S.R.; Farrell, P.A.; Jefferson, L.S. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J. Appl. Physiol. 2002, 93, 1168–1180. [Google Scholar] [CrossRef]
- Lang, C.H.; Pruznak, A.M.; Deshpande, N.; Palopoli, M.M.; Frost, R.A.; Vary, T.C. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol. Clin. Exp. Res. 2004, 28, 1758–1767. [Google Scholar] [CrossRef]
- Bevington, A.; Brown, J.; Butler, H.; Govindji, S.; M-Khalid, K.; Sheridan, K.; Walls, J. Impaired system a amino acid transport mimics the catabolic effects of acid in L6 cells. Eur. J. Clin. Investig. 2002, 32, 590–602. [Google Scholar] [CrossRef]
- Peyrollier, K.; Hajduch, E.; Blair, A.S.; Hyde, R.; Hundal, H.S. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: Evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system a amino acid transport. Biochem. J. 2000, 350, 361–368. [Google Scholar]
- Liu, X.M.; Reyna, S.V.; Ensenat, D.; Peyton, K.J.; Wang, H.; Schafer, A.I.; Durante, W. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: Role in cell growth. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 768–770. [Google Scholar] [CrossRef]
- Baird, F.E.; Bett, K.J.; MacLean, C.; Tee, A.R.; Hundal, H.S.; Taylor, P.M. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E822–E829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryawan, A.; Davis, T.A. Amino Acid- and Insulin-Induced Activation of mTORC1 in Neonatal Piglet Skeletal Muscle Involves Sestin2-GATOR2, Rag A/C-mTOR, and RHEB-mTOR Complex Formation. J. Nutr. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Rudar, M.; Fiorotto, M.L.; Davis, T.A. Differential regulation of mTORC1 activation by leucine and beta-hydroxy-beta-methylbutyrate in skeletal muscle of neonatal pigs. J. Appl. Physiol. 2020, 128, 286–295. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef] [Green Version]
- Sun-Wada, G.H.; Wada, Y. Role of vacuolar-type proton ATPase in signal transduction. Biochim. Biophys. Acta 2015, 1847, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Shimkus, K.L.; Lacko, H.A.; Kutzler, L.; Jefferson, L.S.; Kimball, S.R. Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E817–E828. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Wang, H.; Melick, C.H.; Navlani, R.; Frank, A.R.; Jewell, J.L. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 2020, 295, 2890–2899. [Google Scholar] [CrossRef] [Green Version]

| Control | Alcohol | |||
|---|---|---|---|---|
| Fasted | Refed | Fasted | Refed | |
| LAT1 (slc7a5) | 1.00 ± 0.11 | 1.25 ± 0.11 | 0.92 ± 0.05 | 1.11 ± 0.24 |
| LAT2 (slca2) | 1.00 ± 0.07 | 0.99 ± 0.09 | 1.11 ± 0.18 | 0.94 ± 0.14 |
| SNAT2 (slc38a2) | 1.00 ± 0.05 | 1.04 ± 0.15 | 1.06 ± 0.12 | 1.03 ± 0.08 |
| CAT1 (slc7a1) | 1.00 ± 0.11 | 0.89 ± 0.24 | 0.98 ± 0.12 | 1.05 ± 0.12 |
| PAT-1 (slc36a1) | 1.00 ± 0.07 | 0.93 ± 0.09 | 0.97 ± 0.08 | 0.92 ± 0.09 |
| PAT-2 (slc36a2) | 1.00 ± 0.12 | 1.08 ± 0.12 | 1.13 ± 0.21 | 1.08 ± 0.11 |
| PAT-4 (slc36a4) | 1.00 ± 0.08 | 0.97 ± 0.10 | 0.94 ± 0.11 | 1.04 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laufenberg, L.J.; Crowell, K.T.; Lang, C.H. Alcohol Acutely Antagonizes Refeeding-Induced Alterations in the Rag GTPase-Ragulator Complex in Skeletal Muscle. Nutrients 2021, 13, 1236. https://doi.org/10.3390/nu13041236
Laufenberg LJ, Crowell KT, Lang CH. Alcohol Acutely Antagonizes Refeeding-Induced Alterations in the Rag GTPase-Ragulator Complex in Skeletal Muscle. Nutrients. 2021; 13(4):1236. https://doi.org/10.3390/nu13041236
Chicago/Turabian StyleLaufenberg, Lacee J., Kristen T. Crowell, and Charles H. Lang. 2021. "Alcohol Acutely Antagonizes Refeeding-Induced Alterations in the Rag GTPase-Ragulator Complex in Skeletal Muscle" Nutrients 13, no. 4: 1236. https://doi.org/10.3390/nu13041236
APA StyleLaufenberg, L. J., Crowell, K. T., & Lang, C. H. (2021). Alcohol Acutely Antagonizes Refeeding-Induced Alterations in the Rag GTPase-Ragulator Complex in Skeletal Muscle. Nutrients, 13(4), 1236. https://doi.org/10.3390/nu13041236