Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Gardenia jasminoides (GJ)
2.2. Chromatography Analysis of GJ
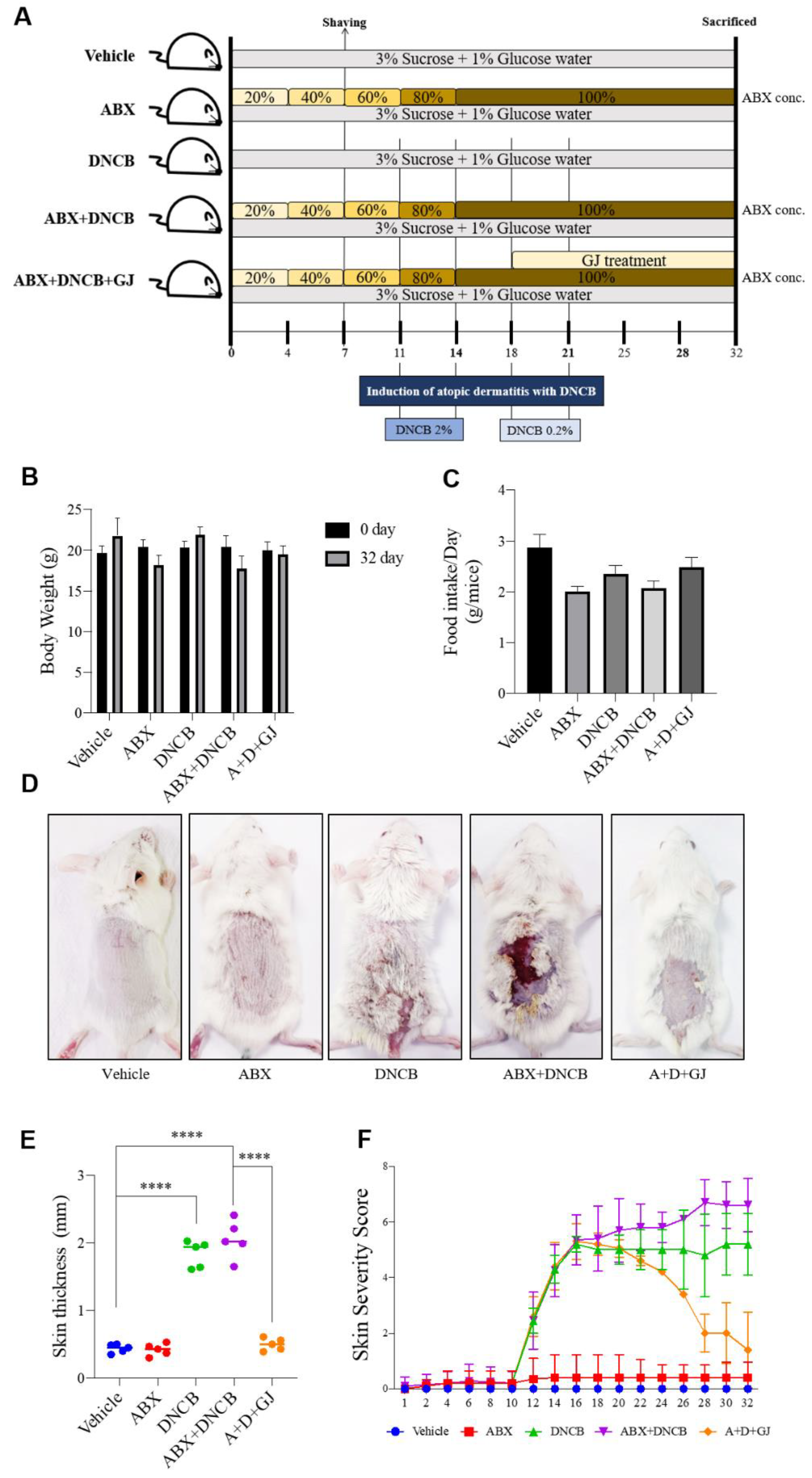
2.3. Animal Experiments
2.4. Enzyme-Linked Immune Sorbent Assay (ELISA)
2.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.6. Protein Extraction and Immunoblotting Assay
2.7. Hematoxylin and Eosin (H&E) and Toluidine Blue Staining
2.8. Immunohistochemistry (IHC) Staining
2.9. Whole Blood Immune Cell Count
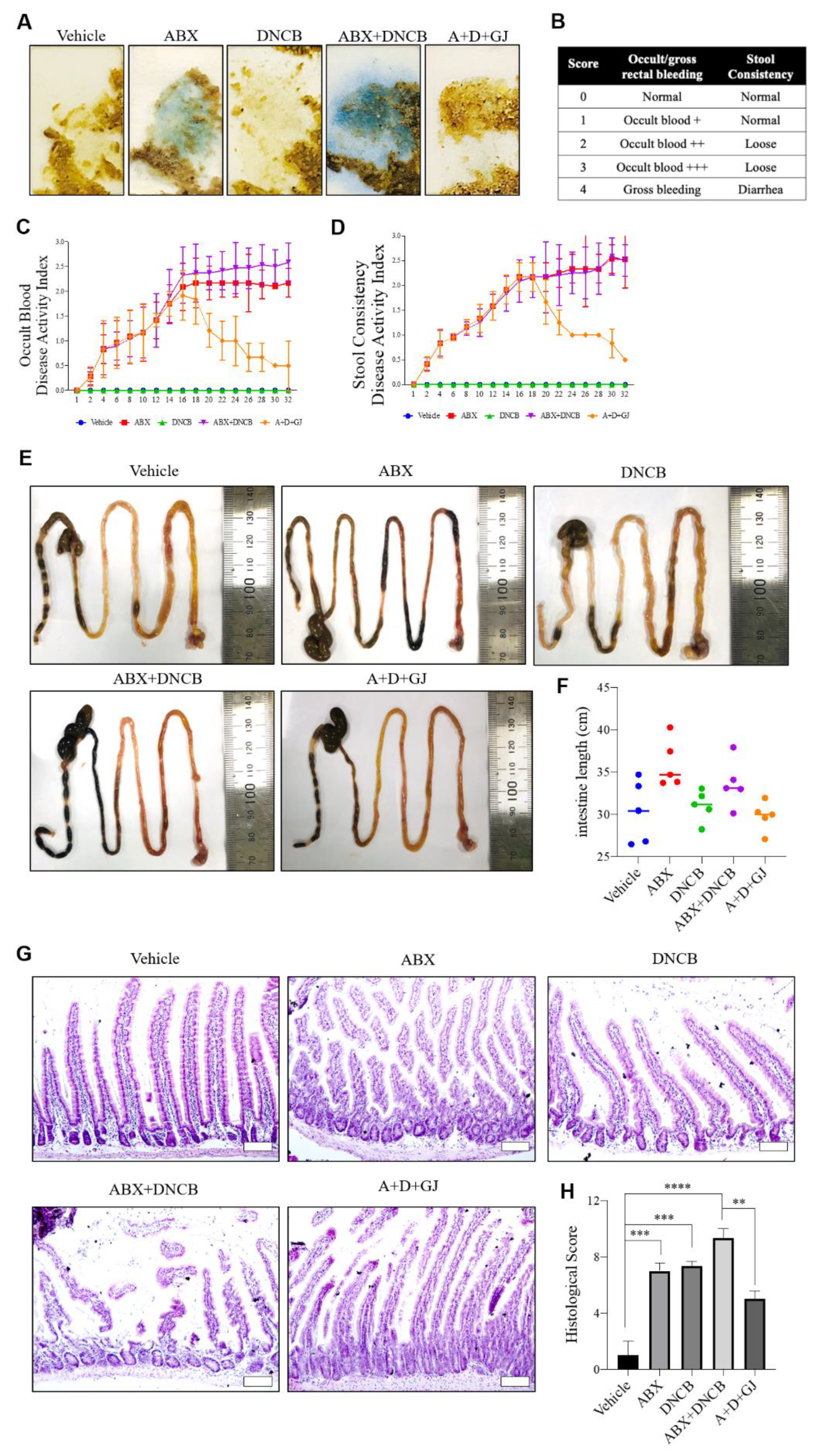
2.10. Fecal Occult Blood Test
2.11. Intestinal Microbiota Analysis
2.11.1. Metagenome (16s rRNA)
2.11.2. Pre-Processing of Sequencing Data
2.11.3. Taxonomy Profiling
2.11.4. Statistical Analysis
2.12. Statistical Analysis
3. Results
3.1. Gardenia jasminoides Extract Improves AD Symptoms in the Dorsal Skin of DNCB-Applied Microbiome-Deficient Mice
3.2. Gardenia jasminoides Extract Improves AD Symptoms in the Dorsal Skin of DNCB-Applied Microbiome-Deficient Mice
3.3. Gardenia jasminoides Extract Regulates Immune Cell-Related Hematological Parameters and Cytokine Expression in the Serum of DNCB-Applied ABX-Induced Mice
3.4. Gardenia jasminoides Extract Reduces Epidermal Thickness, Mast Cell Infiltration and the Expression of Inflammation-Related Markers in the Dorsal Skin of DNCB-Applied ABX-Induced Mice
3.5. Gardenia jasminoides Extract Reduces Fecal Hemorrhage and Restores Elongated Intestines and Shortened Intestinal Villi to Normal in DNCB-Applied ABX-Induced Mice
3.6. Gardenia jasminoides Extract Restores Tight Junctions in the Intestine of DNCB-Applied ABX Mice
3.7. Gardenia jasminoides Extract Suppresses the Expression of Th17-Related Markers in the Intestine of DNCB-Applied ABX-Induced Mice
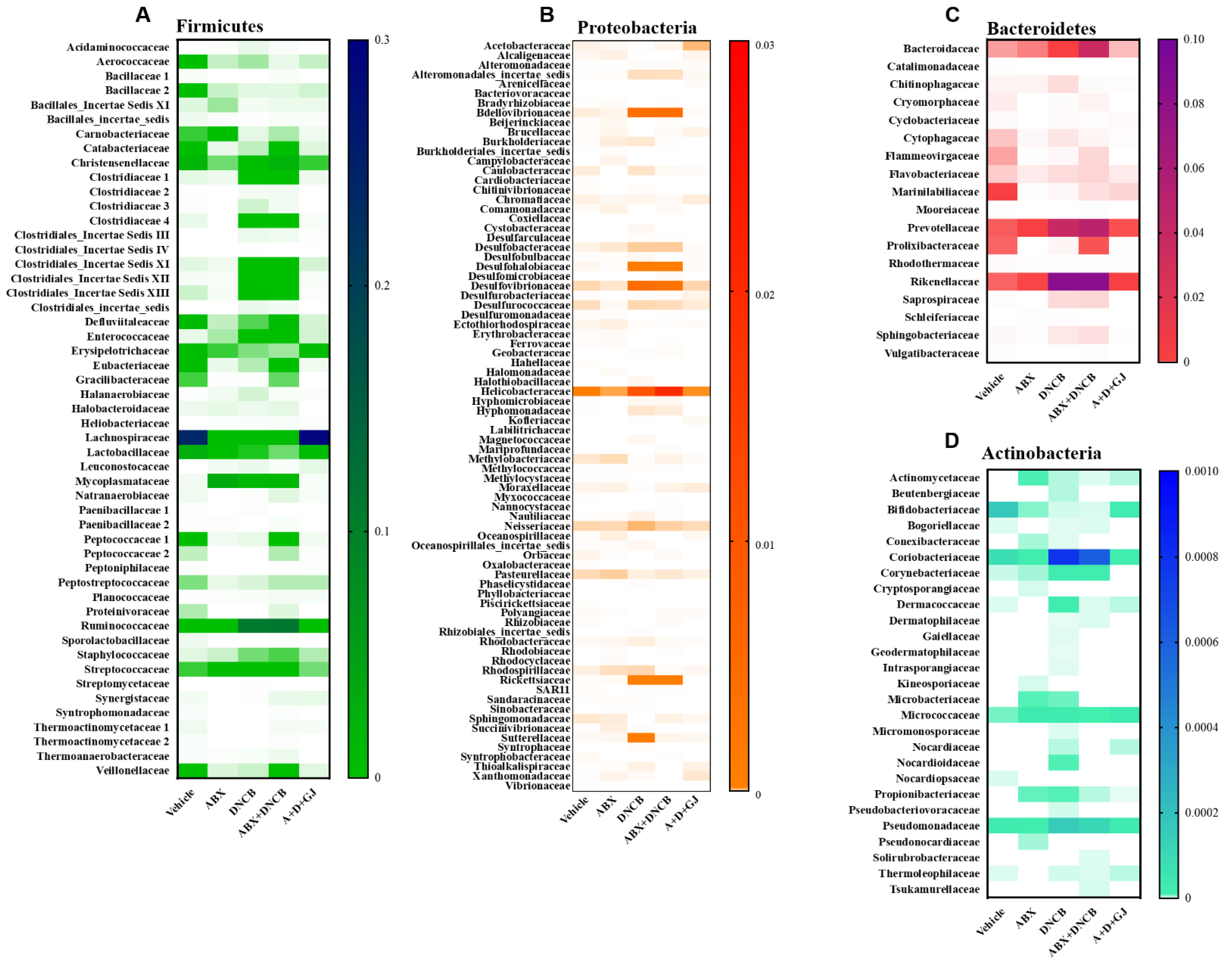
3.8. Gardenia jasminoides Extract Recovers Antibiotic Cocktail- and DNCB-Induced Changes in the Microbiome Composition of DNCB-Applied ABX-Induced Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, S.J.; Goethel, A.; Girardin, S.E.; Philpott, D.J. Innate Immune Influences on the Gut Microbiome: Lessons from Mouse Models. Trends Immunol. 2018, 39, 992–1004. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Lim, S.K.; Jang, J.Y.; Lee, J.; Park, H.K.; Kim, N.; Yun, M.; Shin, M.Y.; Jo, H.E.; Oh, Y.J.; et al. Lactobacillus sakei WIKIM30 Ameliorates Atopic Dermatitis-Like Skin Lesions by Inducing Regulatory T Cells and Altering Gut Microbiota Structure in Mice. Front. Immunol. 2018, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Im, D.S. FFA2 Activation Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice. Biomol. Ther. (Seoul) 2020, 28, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell Immunol. 2011, 2. [Google Scholar] [CrossRef]
- Yamanaka, K.I.; Mizutani, H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 80–92. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Sun, P.; Qian, J.; Shi, Y.; Wang, R. Preventive effect of Gardenia jasminoides on HCl/ethanol induced gastric injury in mice. J. Pharmacol. Sci. 2017, 133, 1–8. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Z.; Hu, P.; Tian, Q.; Xu, Z.; Li, Z.; Tian, X.; Chen, M.; Huang, C. Determining the protective effects of Yin-Chen-Hao Tang against acute liver injury induced by carbon tetrachloride using 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2019, 174, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, H.K. Crocin Ameliorates Atopic Dermatitis Symptoms by down Regulation of Th2 Response via Blocking of NF-κB/STAT6 Signaling Pathways in Mice. Nutrients 2018, 10, 1625. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ku, J.M.; Kim, H.I.; Kim, T.Y.; Seo, H.S.; Shin, Y.C.; Ko, S.G. Topical Application of KAJD Attenuates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis Symptoms Through Regulation of IgE and MAPK Pathways in BALB/C Mice and Several Immune Cell Types. Front. Pharmacol. 2019, 10, 1097. [Google Scholar] [CrossRef]
- Ku, J.M.; Hong, S.H.; Kim, S.R.; Choi, H.S.; Kim, H.I.; Kim, D.U.; Oh, S.M.; Seo, H.S.; Kim, T.Y.; Shin, Y.C.; et al. The prevention of 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in BALB/c mice by Jawoongo. BMC Complement. Altern. Med. 2018, 18, 215. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Uttarkar, S.; Brembilla, N.C.; Boehncke, W.H. Regulatory cells in the skin: Pathophysiologic role and potential targets for anti-inflammatory therapies. J. Allergy Clin. Immunol. 2019, 143, 1302–1310. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.J.; Guha, G.; Li, S.; Kyrylkova, K.; Kioussi, C.; Leid, M.; Ganguli-Indra, G.; Indra, A.K. Selective ablation of Ctip2/Bcl11b in epidermal keratinocytes triggers atopic dermatitis-like skin inflammatory responses in adult mice. PLoS ONE 2012, 7, e51262. [Google Scholar] [CrossRef]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Keane-Myers, A.; Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005, 202, 829–839. [Google Scholar] [CrossRef]
- Cianferoni, A.; Spergel, J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev. Clin. Immunol. 2014, 10, 1463–1474. [Google Scholar] [CrossRef]
- Yu, S.L.; Kuan, W.P.; Wong, C.K.; Li, E.K.; Tam, L.S. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 715190. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–s76. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Heitmiller, K.D.; Czarnowicki, T. An Update on the Pathophysiology of Atopic Dermatitis. Dermatol. Clin. 2017, 35, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Haug, U.; Kuhn, K.; Brenner, H. Comparison and combination of blood-based inflammatory markers with faecal occult blood tests for non-invasive colorectal cancer screening. Br. J. Cancer 2012, 106, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.S.W.; Clemency, N.; Klein, E.; Provenzano, M.; Iyer, R.; Niederhuber, J.E.; Hourigan, S.K. Collection of non-meconium stool on fecal occult blood cards is an effective method for fecal microbiota studies in infants. Microbiome 2017, 5, 114. [Google Scholar] [CrossRef]
- Baxter, N.T.; Ruffin, M.T.t.; Rogers, M.A.; Schloss, P.D. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016, 8, 37. [Google Scholar] [CrossRef]
- Chen, Y.; Si, J.M.; Liu, W.L.; Cai, J.T.; Du, Q.; Wang, L.J.; Gao, M. Induction of experimental acute ulcerative colitis in rats by administration of dextran sulfate sodium at low concentration followed by intracolonic administration of 30% ethanol. J. Zhejiang Univ. Sci. B 2007, 8, 632–637. [Google Scholar] [CrossRef]
- Sovran, B.; Planchais, J.; Jegou, S.; Straube, M.; Lamas, B.; Natividad, J.M.; Agus, A.; Dupraz, L.; Glodt, J.; Da Costa, G.; et al. Enterobacteriaceae are essential for the modulation of colitis severity by fungi. Microbiome 2018, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zhang, Q.; Zeng, W.; Liu, G.; Shao, H. The effect of antibiotic cocktails on host immune status is dynamic and does not always correspond to changes in gut microbiota. Appl. MicroBiol. Biotechnol. 2020, 104, 4995–5009. [Google Scholar] [CrossRef]
- Wang, F.; Sun, N.N.; Li, L.L.; Zhu, W.W.; Xiu, J.; Shen, Y.; Xu, Q. Hepatic progenitor cell activation is induced by the depletion of the gut microbiome in mice. Microbiologyopen 2019, 8, e873. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.H.; Weber, C.R. Molecular aspects of tight junction barrier function. Curr. Opin. Pharmacol. 2014, 19, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 103. [Google Scholar] [CrossRef] [PubMed]
- Tash, B.R.; Bewley, M.C.; Russo, M.; Keil, J.M.; Griffin, K.A.; Sundstrom, J.M.; Antonetti, D.A.; Tian, F.; Flanagan, J.M. The occludin and ZO-1 complex, defined by small angle X-ray scattering and NMR, has implications for modulating tight junction permeability. Proc. Natl. Acad. Sci. USA 2012, 109, 10855–10860. [Google Scholar] [CrossRef]
- Dubin, P.J.; Kolls, J.K. Interleukin-17A and interleukin-17F: A tale of two cytokines. Immunity 2009, 30, 9–11. [Google Scholar] [CrossRef]
- Mumcu, G.; Direskeneli, H. Triggering agents and microbiome as environmental factors on Behçet’s syndrome. Intern. Emerg. Med. 2019, 14, 653–660. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.C.; Yahav, D.; Paul, M. Gut bacterial microbiota and obesity. Clin. MicroBiol. Infect. 2013, 19, 305–313. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Huang, G.; Xu, J.; Guo, T.L. Chapter 15—Exposure to Polyphenolic Compounds Modulates Type 1 Diabetes: The Case of Genistein. In Polyphenols: Mechanisms of Action in Human Health and Disease (Second Edition); Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 193–203. [Google Scholar] [CrossRef]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. MicroBiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS MicroBiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.; Bulach, D.; Carter, G.; Albert, M.J. Gut microbiome of native Arab Kuwaitis. Gut Pathog. 2020, 12, 10. [Google Scholar] [CrossRef]
- Yang, J.; McDowell, A.; Seo, H.; Kim, S.; Min, T.K.; Jee, Y.-K.; Choi, Y.; Park, H.-S.; Pyun, B.Y.; Kim, Y.-K. Diagnostic Models for Atopic Dermatitis Based on Serum Microbial Extracellular Vesicle Metagenomic Analysis: A Pilot Study. Allergy Asthma Immunol. Res. 2020, 12. [Google Scholar] [CrossRef]
- Mitchell, H.M.; Rocha, G.A.; Kaakoush, N.O.; O’Rourke, J.L.; Queiroz, D.M.M. The Family Helicobacteraceae. In The Prokaryotes: Deltaproteobacteria and Epsilonproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 337–392. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal. Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; Nicholl, C.E.; Alhaidan, Y.A.; Thomson, J.M.; Berry, S.H.; Pattinson, C.; Stead, D.A.; Russell, R.K.; El-Omar, E.M.; et al. A comprehensive evaluation of colonic mucosal isolates of Sutterella wadsworthensis from inflammatory bowel disease. PLoS ONE 2011, 6, e27076. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Mattarelli, P.; Brandi, G.; Calabrese, C.; Fornari, F.; Prati, G.M.; Biavati, B.; Sgorbati, B. Occurrence of Bifidobacteriaceae in human hypochlorhydria stomach. Microb. Ecol. Health Dis. 2014, 25. [Google Scholar] [CrossRef]
- Booth, S.J. Diseases Caused by Actinomyces Species. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Powers, C.E.; McShane, D.B.; Gilligan, P.H.; Burkhart, C.N.; Morrell, D.S. Microbiome and pediatric atopic dermatitis. J. Dermatol. 2015, 42, 1137–1142. [Google Scholar] [CrossRef]
- Silbergeld, E.K. The Microbiome. Toxicol. Pathol. 2017, 45, 190–194. [Google Scholar] [CrossRef]
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The human microbiome in evolution. BMC Biol. 2017, 15, 127. [Google Scholar] [CrossRef]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host. Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Vashee, S.; Oldfield, L.M.; Suzuki, Y.; Venter, J.C.; Telenti, A.; Nelson, K.E. The Human Microbiome and Cancer. Cancer Prev Res. (Phila) 2017, 10, 226–234. [Google Scholar] [CrossRef]
- Maguire, M.; Maguire, G. The role of microbiota, and probiotics and prebiotics in skin health. Arch. Dermatol. Res. 2017, 309, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Thiele, I. Modeling metabolism of the human gut microbiome. Curr. Opin. Biotechnol. 2018, 51, 90–96. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Kernbauer, E.; Ding, Y.; Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 2014, 516, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Isaac, S.; Scher, J.U.; Djukovic, A.; Jiménez, N.; Littman, D.R.; Abramson, S.B.; Pamer, E.G.; Ubeda, C. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J. Antimicrob. Chemother. 2017, 72, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440, 440.e1–440.e2. [Google Scholar] [CrossRef]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Nylund, L.; Satokari, R.; Nikkilä, J.; Rajilić-Stojanović, M.; Kalliomäki, M.; Isolauri, E.; Salminen, S.; de Vos, W.M. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC MicroBiol. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Clausen, S.K.; Pang, W.; Nielsen, D.S.; Möller, K.; Josefsen, K.E.; Hansen, A.K. Gastrointestinal microbiota and local inflammation during oxazolone-induced dermatitis in BALB/cA mice. Comp. Med. 2012, 62, 371–380. [Google Scholar] [PubMed]
- Debes, K.P.; Evdina, N.A.; Laigaard, A.; Larsen, J.M.; Zachariassen, L.F.; Hansen, C.H.F.; Hansen, A.K. Betamethasone Treatment for Atopic Dermatitis in Gut Microbiota Transplanted Mice. Comp. Med. 2020, 70, 6–15. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.H.; Hong, S.J. Antibiotics-Induced Dysbiosis of Intestinal Microbiota Aggravates Atopic Dermatitis in Mice by Altered Short-Chain Fatty Acids. Allergy Asthma Immunol. Res. 2020, 12, 137–148. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Abramson, S.B.; Scher, J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016, 12, 446–455. [Google Scholar] [CrossRef]
- Damsker, J.M.; Hansen, A.M.; Caspi, R.R. Th1 and Th17 cells: Adversaries and collaborators. Ann. N Y Acad. Sci. 2010, 1183, 211–221. [Google Scholar] [CrossRef]
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J., 3rd. Th17 cells in immunity and autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789. [Google Scholar] [CrossRef]
- Yuki, T.; Tobiishi, M.; Kusaka-Kikushima, A.; Ota, Y.; Tokura, Y. Impaired Tight Junctions in Atopic Dermatitis Skin and in a Skin-Equivalent Model Treated with Interleukin-17. PLoS ONE 2016, 11, e0161759. [Google Scholar] [CrossRef]
- Yuki, T.; Komiya, A.; Kusaka, A.; Kuze, T.; Sugiyama, Y.; Inoue, S. Impaired tight junctions obstruct stratum corneum formation by altering polar lipid and profilaggrin processing. J. Dermatol. Sci. 2013, 69, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kalamaha, K.; Reis, E.; Newton, S.; Roche, C.; Julson, J.; Fernandes, H.; Rodrigues, J. Atopic dermatitis: A review of evolving targeted therapies. Expert Rev. Clin. Immunol. 2019, 15, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; An, J.E.; Jang, S.; Kim, J.Y.; Lee, J.W.; Kim, H.K. Gardenia jasminoides extract without crocin improved atopic dermatitis-like skin lesions via suppression of Th2-related cytokines in Dfe-induced NC/Nga mice. J. EthnoPharmacol. 2019, 241, 112015. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Wisniewski, J.A.; Woodfolk, J.A. The role of regulatory T cells in atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Caridade, M.; Graca, L.; Ribeiro, R.M. Mechanisms Underlying CD4+ Treg Immune Regulation in the Adult: From Experiments to Models. Front. Immunol. 2013, 4, 378. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Kwon, M.S.; Lee, J.; Oh, Y.J.; Jang, J.Y.; Lee, J.H.; Park, H.W.; Nam, Y.D.; Seo, M.J.; Roh, S.W.; et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci. Rep. 2017, 7, 40040. [Google Scholar] [CrossRef]
- Jung, K.H.; Baek, H.; Kang, M.; Kim, N.; Lee, S.Y.; Bae, H. Bee Venom Phospholipase A2 Ameliorates House Dust Mite Extract Induced Atopic Dermatitis Like Skin Lesions in Mice. Toxins 2017, 9, 68. [Google Scholar] [CrossRef]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. ImmunoPharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef]
- Zhang, X.; Borbet, T.C.; Fallegger, A.; Wipperman, M.F.; Blaser, M.J.; Müller, A. An Antibiotic-Impacted Microbiota Compromises the Development of Colonic Regulatory T Cells and Predisposes to Dysregulated Immune Responses. mBio 2021, 12. [Google Scholar] [CrossRef]
- Chaudhry, A.; Rudra, D.; Treuting, P.; Samstein, R.M.; Liang, Y.; Kas, A.; Rudensky, A.Y. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009, 326, 986–991. [Google Scholar] [CrossRef]
- Liao, P.; Liu, L.; Wang, B.; Li, W.; Fang, X.; Guan, S. Baicalin and geniposide attenuate atherosclerosis involving lipids regulation and immunoregulation in ApoE-/- mice. Eur J. Pharmacol. 2014, 740, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, Y.J.; Chung, Y. Targeting IL-17 in autoimmunity and inflammation. Arch. Pharm. Res. 2016, 39, 1537–1547. [Google Scholar] [CrossRef]
- O’Connor, W., Jr.; Kamanaka, M.; Booth, C.J.; Town, T.; Nakae, S.; Iwakura, Y.; Kolls, J.K.; Flavell, R.A. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009, 10, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Hoffmann, C.; Abt, M.C.; Du, Y.; Kobuley, D.; Kirn, T.J.; Bushman, F.D.; Artis, D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal. Immunol. 2010, 3, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Monin, L.; Castillo, P.; Elsegeiny, W.; Horne, W.; Eddens, T.; Vikram, A.; Good, M.; Schoenborn, A.A.; Bibby, K.; et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 2016, 44, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Filipe, P. Small Molecules in the Treatment of Psoriasis. Drug Dev. Res. 2015, 76, 215–227. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Flavell, R.A. Recent advances in IL-22 biology. Int Immunol. 2011, 23, 159–163. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008, 29, 947–957. [Google Scholar] [CrossRef]
- Brand, S.; Beigel, F.; Olszak, T.; Zitzmann, K.; Eichhorst, S.T.; Otte, J.M.; Diepolder, H.; Marquardt, A.; Jagla, W.; Popp, A.; et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G827–G838. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Majamaa, H.; Isolauri, E. Evaluation of the gut mucosal barrier: Evidence for increased antigen transfer in children with atopic eczema. J. Allergy Clin. Immunol. 1996, 97, 985–990. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, A.; Satsangi, G.P.; Bhatnager, A.K.; Srivastava, J.N. Inhibitory effects of antibiotic from Nitrobacter spp. against Tinea capitis. Allelopathy J. 2007, 19, 535–542. [Google Scholar]
- Peng, J.H.; Leng, J.; Tian, H.J.; Yang, T.; Fang, Y.; Feng, Q.; Zhao, Y.; Hu, Y.Y. Geniposide and Chlorogenic Acid Combination Ameliorates Non-alcoholic Steatohepatitis Involving the Protection on the Gut Barrier Function in Mouse Induced by High-Fat Diet. Front. Pharmacol. 2018, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Fu, Z. Crocin-I ameliorates the disruption of lipid metabolism and dysbiosis of the gut microbiota induced by chronic corticosterone in mice. Food Funct. 2019, 10, 6779–6791. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Shu, R.; Wu, C.; Tong, Y.; Xiong, Z.; Zhou, J.; Yu, C.; Xie, X.; Fu, Z. Crocin-I alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J. Affect. Disord. 2020, 276, 476–486. [Google Scholar] [CrossRef]
- Lin, S.; Li, Q.; Jiang, S.; Xu, Z.; Jiang, Y.; Liu, L.; Jiang, J.; Tong, Y.; Wang, P. Crocetin ameliorates chronic restraint stress-induced depression-like behaviors in mice by regulating MEK/ERK pathways and gut microbiota. J. EthnoPharmacol. 2021, 268, 113608. [Google Scholar] [CrossRef]
- Tamura, M.; Nakagawa, H.; Tsushida, T.; Hirayama, K.; Itoh, K. Effect of pectin enhancement on plasma quercetin and fecal flora in rutin-supplemented mice. J. Food Sci. 2007, 72, S648–S651. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Power, K.A.; Lu, J.T.; Monk, J.M.; Lepp, D.; Wu, W.; Zhang, C.; Liu, R.; Tsao, R.; Robinson, L.E.; Wood, G.A.; et al. Purified rutin and rutin-rich asparagus attenuates disease severity and tissue damage following dextran sodium sulfate-induced colitis. Mol. Nutr. Food Res. 2016, 60, 2396–2412. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Condition | ||
|---|---|---|---|
| Flow Rate | 1.0 mL/min | ||
| Injection Volume | 10 µL | ||
| Column | YMC Pack-Pro C18 | ||
| Column Temp. | 30 °C | ||
| Heater Temp. | 250 °C | ||
| Sheath Gas Flow Rate | 35 arb (N2) | ||
| Spray Voltage | 5 kV | ||
| Capillary Temp. | 275 °C | ||
| Gradient Conditions | Time (min) | Water (0.1% formic acid) | Acetonitrile (0.1% formic acid) |
| 0 | 95 | 5 | |
| 10 | 95 | 5 | |
| 50 | 30 | 70 | |
| 55 | 10 | 90 | |
| 65 | 95 | 5 | |
| Primer Name | Sequence (5′->3′) | |
|---|---|---|
| Il6 | Forward | GATGCTACCAAACTGGATATAATC |
| Reverse | GGTCCTTAGCCACTCCTTCTGTG | |
| Il12 | Forward | ATGGCCATGTGGGAGCTGGAG |
| Reverse | TTTGGTGCTTCACACTTCAGG | |
| Il13 | Forward | CGGCAGCATGGTATGGAGTG |
| Reverse | ATTGCAATTGGAGATGTTGGTCAG | |
| Il17a | Forward | ATCAGGACGCGCAAACATGA |
| Reverse | TCAAAGCTCAGCGTGTCCAA | |
| Il17f | Forward | TGCTACTGTTGATGTTGGGAC |
| Reverse | TTCAACCAAAACCAGGGCATT | |
| Il22 | Forward | TTGAGGTGTCCAACTTCCAGCA |
| Reverse | AGCCGGACATCTGTGTTGTTA | |
| Gapdh | Forward | GAGGGGCCATCCACAGTCTTC |
| Reverse | CATCACCATCTTCCAGGAGCG | |
| Grade | Severity of Inflammation | Extent of Inflammation | Crypt Damage |
|---|---|---|---|
| 4 | – | – | Crypt and surface epithelium lost |
| 3 | Severe | Transmural | Crypts lost, surface and epithelium present |
| 2 | Moderate | Mucosa and submucosa | 2/3 damages |
| 1 | Mild | Mucosa | 1/3 damages |
| 0 | None | None | None |
| No. | Retention Time (min) | Positive/Negative Mode | Molecular Weight | Proposed Structure |
|---|---|---|---|---|
| 1 | 2.88 | [M-H]+ | 387.18 | geniposide |
| 2 | 11.06 | [M-H]+ | 723.43 | jasmigeniposide A |
| 3 | 16.50 | [M-H]+ | 329.17 | crocetin |
| 4 | 18.44 | [M-H]+ | 347.09 | picrocrocinic acid |
| 5 | 18.47 | [M-H]+ | 391.26 | shanzhiside |
| 6 | 23.76 | [M-H]+ | 373.26 | gardoside |
| 7 | 24.47 | [M-H]+ | 609.26 | rutin |
| 8 | 25.58 | [M-H]+ | 561.35 | caffeoyl sinapoyl quinic acid |
| 9 | 27.40 | [M-H]+ | 725.35 | 6”-O-trans-feruloyl genipin gentiobioside |
| 10 | 28.33 | [M-H]+ | 659.35 | 3,4-dicaffeoyl-5-(3-hydroxy-3-methylglutaroyl) quinic acid |
| 11 | 31.18 | [M-H]+ | 725.35 | 6”-O-trans-cinnamoyl genipin gentiobioside |
| 12 | 32.70 | [M-H]+ | 975.52 | crocin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.I.; Hong, S.H.; Lee, S.Y.; Ku, J.M.; Kim, M.J.; Ko, S.-G. Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile. Nutrients 2021, 13, 1349. https://doi.org/10.3390/nu13041349
Kim HI, Hong SH, Lee SY, Ku JM, Kim MJ, Ko S-G. Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile. Nutrients. 2021; 13(4):1349. https://doi.org/10.3390/nu13041349
Chicago/Turabian StyleKim, Hyo In, Se Hyang Hong, Seo Yeon Lee, Jin Mo Ku, Min Jeong Kim, and Seong-Gyu Ko. 2021. "Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile" Nutrients 13, no. 4: 1349. https://doi.org/10.3390/nu13041349
APA StyleKim, H. I., Hong, S. H., Lee, S. Y., Ku, J. M., Kim, M. J., & Ko, S.-G. (2021). Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile. Nutrients, 13(4), 1349. https://doi.org/10.3390/nu13041349





