Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety
Abstract
:1. Introduction
2. Methodology
3. Evolution of Trends in Research with Black Cumin
4. Ethnopharmacological Aspects
5. Phytochemical Profiles
5.1. Terpenes and Terpenoids
5.2. Phytosterols
5.3. Alkaloids
5.4. Tocols
5.5. Polyphenols
5.6. Miscellaneous Components
6. Benefits of Black Cumin on Human Health and Disease Conditions
6.1. Antioxidant Effects
6.2. Anti-Inflammatory Effects
6.3. Immunomodulatory Effects
6.4. Protection against Neurological Disorders
6.4.1. Protection against Neuroinflammation
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Neuroinflammation | |||
| TQ (12.5 μM for 24 h) | LPS/IFNγ or H2O2-activated BV-2 microglial cell | ↓H2O2; ↑GSH; ↑SOD and CAT | [32] |
| TQ (12.5 μM for 24 h) | LPS/IFNγ or H2O2-activated BV-2 microglial cell | ↑Glutaredoxin-3, biliverdin reductase A, 3-mercaptopyruvate sulfurtransferase, and mitochondrial Lon protease; ↓IL-2, IL-4, IL-6, IL-10, and IL-17a, CFB, CXCL3 and CCL5 | [49] |
| TQ (2.5–10 μM) | LPS-activated neuroinflammation in BV-2 microglial cell | ↓ROS; ↑LKB1 and AMPK; ↑nuclear accumulation of SIRT1 | [50] |
| Alzheimer’s disease | |||
| TQ (100 nM) | Aβ1–42-induced neurotoxicity in hiPSC-derived cholinergic neurons | ↑GSH; ↓ROS; ↓synaptic toxicity, attenuate cell death and apoptosis | [51] |
| TQ fraction rich nanoemulsion of seeds (TQRFNE) (250 and 500 mg/kg BW) | High fat/cholesterol diet-induced neurotoxicity in rats | ↓Aβ40 and Aβ42; ↑APP; ↓PSEN1 and PSEN2; ↓BACE1 and RAGE; ↑IDE and LRP1 | [52] |
| TQ fraction rich nanoemulsion of Nigella seeds (TQRFNE) (250 and 500 mg/kg BW) | High fat/cholesterol diet-induced neurotoxicity in rats | ↓Memory impairment; ↓lipid peroxidation and soluble Aβ levels; ↑total antioxidant status and antioxidants genes expression | [53] |
| TQ (10, 20, and 40 mg/kg/day p.o. for 14 days) | Combined AlCl3andD-Gal-induced AD in rats | Improved cognitive deficits; ↓Aβ formation and accumulation; ↓TNF-α and IL-1β; ↓TLRs pathway components; ↓NF-κB and IRF-3 mRNAs | [54] |
| TQ (intragastrically, 20 mg/kg/day once daily for 14 days) | Combined AlCl3 and D-Gal induced neurotoxicity in rats | ↑ Memory performance; ↑ SOD; ↓TAC; ↓MDA; ↓NO; ↓TNF-α; ↓AChE activity; ↑BDNF and Bcl-2 | [55] |
| TQ (intragastrically, 20 mg/kg/day for 15 days) | Aβ (1–42) infused rat model of AD | ↓Memory performance (Morris water maze test); ↓IFN-γ; ↑ DCX and MAP2 | [56] |
| Parkinson’s disease | |||
| TQ (100 nM) | α-Synuclein-induced rat hippocampal and hiPSC-derived neurons | ↑Synaptophysin; ↓synaptic vesicle recycling; ↑spontaneous firing activity | [57] |
| TQ (10 mg/kg BW, 1 week prior to MPTP at 25 mg/kg BW) | MPTP-induced mouse PD model | ↓MDA; ↑GSH; ↑SOD; ↑CAT; ↓IL-1β and IL-6; ↓TNF-α; ↓COX-2 and iNOS; ↓α-synuclein aggregation | [58] |
| TQ (7.5 and 15 mg/kg/day, p.o.) | Rotenone-induced rat PD model | ↓Oxidative stress; ↑Parkin; ↓ Drp1; ↑dopamine; ↑TH levels | [59] |
| Ischemic stroke | |||
| Hydroalcoholic seed extract (20 mg/kg BW) | Global ischemia in rats | ↓Brain edema and infarct volume; ↑VEGF, HIF and MMP9 | [60] |
| TQ | Stroke-prone spontaneously hypertensive rats | ↓Chemoattractant protein-1, Cox-2, IL-1β, and IL-6 | [61] |
| Traumatic brain injury | |||
| TQ (5 mg/kg/day for seven days) | Feeney’s falling weight-induced moderate head trauma | ↑Neuron densities; ↓MDA | [62] |
| Anxiety and Depression | |||
| Ethanolic seed extract | Chronic stress-induced depression model | ↓NO | [63] |
| TQ-loaded solid lipid nanoparticle (20 mg/kg, p.o.) and TQ (20 mg/kg, p.o.) | Chronic stress-induced depression model | ↓IL-6, TNFα; ↑BDNF; ↑5-HT; ↑IDO | [64] |
| NSO (0.2 mL/kg for 20 days) | Stress-induced depression model | ↑Memory performance (FST) | [65] |
| Hydroalcoholic seed extract (200 and 400 mg/kg) | Stress-induced depression and anxiety model | ↑Anxiolytic (Open field and elevated plus-maze test); ↓depression (FST) | [66] |
| Epilepsy | |||
| Ethanolic seed extract (400 mg/kg/day, p.o.) | PTZ-induced kindling mode | ↓Kindling development; ↑memory performance (Morris water maze test); ↓LTP | [67] |
| NSO (400 and 600 mg/kg BW) | Electroshock-induced seizures | ↑Anticonvulsant activity | [68] |
| TQ (10 mg/kg, i.p) | Lithium chloride and pilocarpine-induced seizure | ↑Memory performance; ↑SOD; ↑Nrf2, HO-1 | [69] |
| TQ (10 mg/kg, i.p) | Lithium chloride and pilocarpine-induced seizure | ↑Memory performance; ↓COX-2, TNF-α and NF-κB | [70] |
| Hydroalcoholic seed extract (200 and 400 mg/kg for 5 days) | PTZ-induced seizure model | ↑Memory performance (Morris water maze and passive avoidance test); ↑ total thiol; ↓MDA | [71] |
| Schizophrenia | |||
| TQ (20 mg/kg, daily for 28 days, i.p.) | Mice model of schizophrenia (haloperidol-induced catalepsy and apomorphine-induced climbing behavior) | Anti-amnesic effect; ↓AChE activity; ↓ TBARS; ↑GSH and catalase; ↑dopamine level | [72] |
| Miscellaneous effects | |||
| Chemical-induced toxicity | |||
| TQ (5 mg/kg, i.p. for 11days) | Acrylamide-induced neurotoxicity in rats | Improved gait abnormalities; ↑GSH; ↓MDA;↓caspases 3 and 9, and Bax/Bcl-2, pP38/P38 and pJNK/JNK; ↓pERK/ERK; restore BBB integrity | [73] |
| TQ (5 and 10 mg/kg, i.p., for 11 days) | Acrylamide-Induced Peripheral Nervous System Toxicity in rats | Improved gait abnormalities; ↑GSH and ↓MDA;↓caspases 3 and 9, and Bax/Bcl-2, pP38/P38 and pJNK/JNK; ↓pERK/ERK | [74] |
| TQ (10 µM and 20 µM) | Arsenic-induced cytotoxicity in SH-SY5Y cells | Promotes DNA repairing; ↓ROS, balanced transmembrane potential; ↓ Bax and PARP-1, and ↑Bcl-2 | [75] |
| TQ (5 mg/kg/day, for 3 days, p.o.) | Arsenic-induced hippocampal toxicity in rats | Improve anxiety behavior (Open field test and elevated plus maze); ↑GSH and SOD; ↓DNA damage; ↓TNF-α and INF-γ | [76] |
| TQ (2.5 and 5 mg/kg BW, for 8 days, p.o.) | Arsenic-induced hippocampal toxicity in Wistar rats | ↑Δψm | [77] |
| NSO (1 mL/kg BW for 7 days) | Dichlorvos-induced oxidative and neuronal damage in rats | ↓Vacuolation in the frontal and cerebellar cortices;↑TAC and GSH↓ROS | [78] |
| Radiotoxicity | |||
| TQ | Radiation-induced oxidative stress in brain tissue | ↑Antioxidant enzymes | [79] |
6.4.2. Protection against Alzheimer’s Disease

6.4.3. Protection Against Parkinson’s Disease
6.4.4. Protection Against Ischemic Stroke and Traumatic Brain Injury
6.4.5. Protection against Anxiety and Depression, Epilepsy, Schizophrenia, and Other Miscellaneous Neurological Problems
6.5. Anti-Cancer Effects
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Seeds incorporated silver nanoparticles (NS-AgNP) (25–200 µg/mL) | Human breast cancer cell line (HCC-712) | Dose-dependent cytotoxicity; ↓cell density | [95] |
| Aqueous seed extract (11.5 µg/mL) | Human breast cancer cell line (MCF-7) | Potent cytotoxic effect with IC50 11.5 µg/mL; ↑caspase-3,8 and 9, and Bax | [96] |
| NSO nanoemulsion (10–100 µL/mL) | Human breast cancer cell line (MCF-7) | ↓Cell proliferation; ↑apoptosis and necrosis | [97] |
| TQ (25 µmol/L) | Human breast cancer cell line (MCF-7) | Inhibit tumor cell growth; ↑p53; induce apoptosis | [98] |
| Seeds incorporated platinum nanoparticles (NS-PtNP) (25, 50, 100 and 150 µg/mL) | HeLa cervical cancer and MDA-MB-231 breast cancer cell lines | Dose-dependent cytotoxic effect with IC50 value 36.86 µg/mL (MDA-MB-231) and 19.83 µg/mL (HeLa), respectively | [99] |
| TQ (0.78 µM) | HeLa cervical cancer cell line | Dose-dependent antiproliferative effect | [100] |
| TQ (2, 4, 6 and 8 µM) | Human colon cancer cell line (LoVo) | Inhibit metastasis; ↑JNK, p38; ↓P13K, ERK1/2, IKKα/β and NF-κB | [101] |
| TQ (20 µmol/L) | Human colon cancer cell line (LoVo) | Reduce cell proliferation; ↓p-P13K, p-Akt, p-GSK3β, β-catenin and COX-2; ↓PGE2, LEF-1 and TCF-4 | [102] |
| TQ (10–120 µmol/L) | Human bladder cancer cell lines (253J and T24) | Inhibit proliferation and metastasis; ↓MYC, Axin-2, MMP-7, MET and cyclin-D1; ↓Wnt/β-catenin signaling cascade | [103] |
| TQ (40, 60 and 80 µM) | Human bladder cancer cell lines (253J and T24) | Significant cytotoxicity and reduction in cell proliferation; ↑caspase-3, cleaved PARP, Bax, cyt c and AIF; ↑ER-stress marker GRP78, IRE1, ATF6, ATF4 and CHOP; ↓Bcl-2 and Bcl-xl; induce apoptosis | [104] |
| TQ (10–50 µM) | Pancreatic ductal adenocarcinoma cell lines (AsPC1 and MiaPaCa-2) | Inhibit cell viability; reduce tumor size; ↑p53, p21; ↓Bcl-2 and HDAC; induce apoptosis and G2 cell cycle arrest | [105] |
| TQ (0.5–20 µM) | Human renal tubular epithelial cell line (HK2) and human renal cancer cell lines (769-P and 786-O) | Inhibit metastatic phenotype and epithelial-mesenchymal transition; ↑E-cadherin; ↓Snail, ZEB1 and vimentin; ↑LKB1/AMPK signaling | [106] |
| TQ (0–100 µmol/L) | Human renal cancer cell lines (ACHN and 786-O) | Inhibition of metastasis; ↑LC3; ↑AMPK/mTOR signaling; induce autophagy | [107] |
| TQ (40 and 50 µM) | Human kidney cancer cell lines (A498 and Caki-1) | Anti-proliferative effects with GI50 value 40.07 µM (A498) and 51.04 µM (Caki-1), respectively; ↑Bax; ↓Bcl-2 and p-Akt; induce apoptosis | [108] |
| Hexanic seed extract (0–150 µg/mL) | Human ovary cancer cell line (A2780) | Strong cytotoxic activity of SF2 with IC50 10.89 µg/mL; ↑caspase-3 and 9; ↓MMP; induce apoptosis | [109] |
| Seed extract and NSO with OM-90(0.5 and 2.4 mg/mL) | AGS human gastric adenocarcinoma cell line | Activates mitochondrial pathways; induce apoptosis | [110] |
| TQ (0.1–30 µM) | Human prostate cancer cell lines (PC3 and DU145) | Inhibit metastatic phenotype and epithelial-mesenchymal transition; ↓TGF-β, Smad2 and Smad3 | [111] |
| TQ (0–80 µM) | Head and neck squamous cells carcinoma cell lines (SCC25 and CAL27) | Dose-dependent cytotoxicity with IC50 value 12.12 µM (CAL27) and 24.62 µM (SCC25), respectively; induce apoptosis | [112] |
| TQ + Resveratrol (46 µM) | Hepatocellular carcinoma cell line (HepG2) | Significant cell inhibition; ↑caspase-3; ↓GSH and MDA; induce apoptosis | [113] |
| NSO (50–250 µg/mL) | Human liver cancer (HepG2), human breast cancer (MCF-7), human lung cancer (A-549) and normal human embryonic kidney (HEK293) cell lines | High cytotoxic effect in HepG2 cells with IC50 48µg/mL; ↑ROS and LPO; ↓GSH and MMP; ↑p53, caspase-3 and 9, Bax; ↓Bcl-2; induce apoptosis | [114] |
| TQ (In vitro: 1–50 µMIn vivo: 20 and 100 mg/kg for 3 days; i.v.) | TNBC cells and orthotopic TNBC xenograft mice model | Inhibit cell proliferation, migration and invasion; ↓tumor growth; ↓eEF-2K, Src/FAK and Akt | [115] |
| TQ + Paclitaxel (In vitro: 0–100 µM In vivo: 2.4 mg/kg/day for 12 days; i.p) | Mouse breast cancer cell line (4T1) and EAC cells-induced female Balb/c mice model | Dose-dependent cytotoxicity; ↑caspase-3,7 and 12, PARP; ↓p65, p53 and Akt1; ↓JAK-STAT signaling | [116] |
| Ethanolic seed extract (250 mg/kg/day for 5 days, p.o.) | Diethyl nitrosamine-induced hepatocarcinogenesis in Wistar rat model | Antiangiogenic effect; ↓serum VEGF and AFP levels, and liver HGFβ level | [94] |
| Ethanolic seed extract and TQ (150, 250 and 300 mg/kg (extract) 6 days/week and 20 mg/kg (TQ) for 3 days/week, p.o.) | Diethyl nitrosamine-induced hepatocellular carcinoma in albino-Wistar rat model | Reduction in cell proliferation; ↑Antioxidant activity; ↓PCNA, c-fos, Bcl-2; ↓EGFR/ERK1/2 signaling | [117] |
| TQ + 5-fluorouracil (35 mg/kg/day for 3 days/week for 9 weeks; p.o.) | Azoxymethane-induced colon cancer in Wistar rat model | Subdues tumor growth; ↑TGF-β1, TGF-β/RII, Smad4, DKK-1, CDNK-1A and GPx; ↓Wnt, β-catenin, NF-κB, VEGF, COX2, iNOS and TBRAS | [118] |
| TQ + Piperine (10 mg/kg/day for 14 days; i.p) | EMT6/P cells- inoculated Balb/c mice | Inhibit angiogenesis; ↓Tumor size; ↑serum INF-ᵧ level; ↓VEGF; induce apoptosis | [119] |
| TQ + Resveratrol (50 mg/kg/day for 14 days; i.p) | EMT6/P cells- inoculated Balb/c mice | Inhibit angiogenesis; ↓Tumor size; ↑serum INF-ᵧ level; ↓VEGF; induce apoptosis | [120] |
6.6. Anti-Obesity and Anti-Dyslipidemic Effects
6.7. Anti-Diabetic Effects
6.8. Cardioprotective and Antihypertensive Effects
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Hydroalcoholic extract of Seed (200 g of powder) | Rats with ischemia-reperfusion (I/R) injury (black cumin post-conditioning) | ↑LVDP, RPP, and maximum up/down rate of the left ventricular pressure; protection against oxidative stress (↑SOD and CAT activities; ↓MDA and 4-HNE levels during early reperfusion) | [143] |
| Ethanolic seed extract (800 mg/kg) | Isoproterenol-induced myocardial infarction in albino rats | ↑MI associated alteration and cardiac biomarkers, antioxidant markers, and biochemical activity of cardiac tissue | [144] |
| TQ (10 and 20 mg/kg, b/w, p.o. for 14 days) | Doxorubicin-induced cardiotoxicity in mice | ↓Serum marker and ↑antioxidant enzymes; ↑heart antioxidant defense mechanisms; ↓LPO levels; ↓IL2 level | [148] |
| TQ (2.5, 5 and 10 mg/ kg, for 28 days) | Diazinon-induced cardiotoxicity in Wistar rats | Act as a natural antioxidant, lessen DZN cardio-toxicity and ameliorated cholinesterase activity | [149] |
| Hydroalcoholic seed extract (600 mg/kg) and TQ (40 mg/kg) | Angiotensin II-induced hypertension in rats | ↓ SBP, MAP, and HR | [145] |
| Seed extract (300 mg twice daily for 28 days) | Randomized controlled clinical trial in elderly patients with hypertension | A slight but insignificant reduction of blood pressure | [146] |
| Black cumin virgin oil (twice a day in a dose of 0.5 mL p.o. for 45 days) | Clinical study on patients with mild-moderate hypertension | ↓Total cholesterol, LDL and TGs; ↑HDL; ↓systolic pressure and diastolic pressure | [147] |
6.9. Hepatoprotective Effects
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| NSO (2.5 mL/kg BW) | Ibuprofen-induced hepatotoxicity in Swiss albino mice | ↓ALT, AST, and ALP | [162] |
| NSO (2 mL/kg) | STZ-induced diabetic male Wistar rats | ↑CAT and GSH; histopathological picture and hepatic glycogen contents | [136] |
| NSO (300 mg oil/kg BW) | CCl4-induced liver injury in rats | ↓ MDA, NO and TNF-α, AST and ALT; ↑unsaturated fatty acids | [154] |
| TQ (100 mg/kg/day BW) | High-dose atorvastatin-induced hepatotoxicity in male SD rats | ↓Serum hepatic enzymes, MDA, protein carbonylation, and caspase 3 activity;↑GSH and CAT; histopathological and ultrastructural changes | [155] |
| TQ (20 or 40 mg/kg, p.o., daily) | Ethanol-induced injury in C57BL/6 mice; TGF-β-induced injury in hepatic stellate cells | ↑PPAR-γ; ↑LKB1 and AMPK phosphorylation; ↑SIRT1 | [177] |
| Hydroethanolic seed extract (100, 200, or 400 mg/kg) | LPS-treated rats | ↓MDA, NO and IL-6, AST, ALT and alkaline phosphatase; ↑ thiol content, SOD, CAT, serum protein, albumin | [166] |
| NSO (2 mg/kg/day) | Irradiation-induced liver damage in rats | ↓AST, ALT, MDA, SOD, IL-6, TNF-α, TGF-β; ↑IL-10 | [158] |
| NSO (100 mg/kg) | Gibberellic acid-treated pregnant albino rats | ↓ALT, AST, MDA, Bax, Hydropic degeneration, Cellular infiltration, periportalFibrosis; ↑SOD, CAT, GPx and Bcl-2 | [165] |
| TQ (400 mg/kg) | Rat model of NAFLD associated with 6-propyl-2-thiouracil (PTU)-induced hypothyroidism | ↑CAT, NO, GSH, SOD; ↓MDA, steatosis score, lobular inflammation, NAFLD Activity Score, alpha-smooth muscle actin, intralobular and portal tract and CD68+ | [156] |
| TQ (5 or 20 mg/kg) | Rat model of acetaminophen overdose-induced acute liver injury | ↓ALT, AST, MAPK Phosphorylation (JNK, ERK and P38 phosphorylation); PI3K/mTOR signaling pathway (PI3K, AKT, mTOR, IL-1B, P70S6K); DNA fragmentation and cellular damage; STAT3 phosphorylation, JNK phosphorylation; ↑GSH, GPx, AMPK and LKB1 | [159] |
| NSO (4 mL/kg 48 h) | Carboplatin-induced liver damage in female Wistar-albino rats | ↓Apoptotic index, collagen fiber distribution around the central vein, hepatocyte cords preserved | [160] |
| Seed extract (100, 200, or 400 mg/kg) | PTU-induced hypothyroid rats | ↓MDA, ALK-P, AST and ALT; ↑thiol concentration, CAT and SOD, and body weight gain | [157] |
| TQ (4.5, 9, and 18 mg/kg) | Morphine-induced liver damage in male mice | ↑Liver weight; ↓mean diameter of hepatocyte, central hepatic vein, AST, ALT, and NO | [164] |
| Seed (1 g twice a day for 3 months) | Randomized, double-blind, placebo-controlled clinical trial with NAFLD patients | ↓Bodyweight, normal fatty liver grading | [178] |
| Seed (5 g, drink as tea for 3 months) | Randomized, double-blind, placebo-controlled clinical trial with NAFLD patients | ↓AST, ALT, body mass index, and grade of fatty liver | [161] |
| NSO (2.5 mL/person, every 12 h for 3 months) | Randomized, double-blind, placebo-controlled clinical trial with NAFLD patients | ↓Grade of hepatic steatosis, ALT, AST, TG, LDL-C, and HDL-C | [167] |
| Seed (2 g/day/person, for 12 weeks) | Randomized, double-blind, placebo-controlled clinical trial with NAFLD patients | ↑ Insulin sensitivity check index; ↓serum glucose, serum insulin, insulin resistance, hepatic steatosis percentage | [179] |
| Seed (2 g/day/person, for 12 weeks) | Randomized, double-blind, placebo-controlled clinical trial with NAFLD patients | ↓ hs-CRP and NF-κB, TNF-α, hepatic steatosis and its percentage | [168] |
| NSO (1 g/person, for 8 weeks) | Randomized, double-blind, placebo-controlled clinical trial with NAFLD patients | ↑HDL-C; ↓ FBS, lipid profiles (total cholesterol, triglyceride, VLDL, LDL), Liver enzyme (ALT and AST), inflammatory markers (Hs-CRP and IL-6) | [163] |
6.10. Pulmonary Protective Effects
6.11. Gastroprotective Effects
6.12. Effects on Fertility and Reproduction
6.13. Protection against Skin Diseases
6.13.1. Wound Healing
6.13.2. Acne Vulgaris
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Hydroethanolic seed extracts (20% or 40%) | Diabetic skin wounded male Wistar rats | ↑ Anti-inflammatory and antimicrobial effect | [131] |
| NSO-containing cold cream | Wounded (dermis + epidermis) male albino Wistar rats | ↑Epithelialization rate | [227] |
| A mixture of 1:1 ratio of honey and cold-pressed NSO | A circular excision wound in the back region of male albino Wistar rats | ↓ Wound surface area | [220] |
| Cold pressed NSO (3 mL) | Double-blind randomized study with Albino rabbits | ↑ Wound contraction; ↓inflammation | [228] |
| NSO cream (50%) | Female Wistar-albino rats with 84 excisional skin wounds on the backs | ↑ Epithelialization and granulation | [221] |
| 70% hot methanolic seed extract | Male adult rabbits with skin incision | ↑Wound healing without any infection | [222] |
| Ethanolic seed extract (IC50 values of 71.54 ± 3.22 μg/mL) | Murine macrophage leukemia cell line (RAW 264.7), human promyelocytic leukemia cell line (HL-60), Murine embryonic fibroblast cell line (3T3-CCL92) | ↑Wound closure | [229] |
| Hydrodistillation of seed [0.6% (w/w) of essential oil] (10 μL) | Diabetic Sprague–Dawley male rats with 2 excision wounds on the upper back of each animal with a dermal punch | ↓ Oxidative stress and lipid peroxidation | [219] |
| NSO (2 g/kg b.w.) | Albino Wister male rats | ↑Formation of wound collagen | [230] |
| Ethyl acetate seed extract (15%) | Gel prepared with the seed extract | ↓Growth of S. aureus and P. acnes | [224] |
| TQ | Ethosomes gel with TQ | ↓Number and size of sebaceous glands | [225] |
| Pure NSO (5 mg/kg b.w.) | Male Albino Rats having psoriasis-like skin inflammation | ↓ IMQ-induced psoriasis-like inflammation | [231] |
| Hydrogel of hydro-ethanol seed extract | Randomized double-blind controlled clinical trial with mild-to-moderate acne vulgaris patients | ↓Number of comedones, papules, and pustules;no side effect | [226] |
| NSO-containing cream | Vitiligo patients, 47 body surface areas were affected | ↑Repigmentation | [232] |
| NSO | Three patients with contact dermatitis | Controlled contact dermatitis | [233] |
6.13.3. Vitiligo
6.14. Bone Regenerative Effects
6.15. Nephroprotective Effects
6.16. Anti-Arthritis Effects
6.17. Protection against Emerging Diseases
6.18. Black Cumin and TQ as a Promising Antidote
6.19. Black Cumin as a Galactagogue
7. Molecular Mechanisms Underlying the Pharmacological Effects across Health and Disease Conditions
8. Drug Interaction and Nanoparticle-Mediated Drug Delivery
| Active Compound/ Extract | Interacting Drug | Experimental Model | Route of Drug Administration | Dose of Drug | Effects on Pharmacological Parameters | Reference |
|---|---|---|---|---|---|---|
| TQ | Cyclosporine A (CsA) | Rodents | PO * IP | TQ (PO, 10 mg/kg) CsA: 10 mg/KG (PO and IP) | Bioavailability: oral CsA reduced by 32% but IP CsA was not affected Chronic CsA effect (Increase fasting glucose, cysteine C and marked kidney alteration) was reversed by TQ | [303] |
| TQ | Glibenclamide (GBC) | Rat | PO | 10 mg/kg | Plasma concentration of GBC increased by 13.4% (Single dose) and 21.8% (multiple doses) with TQ Synergistic effect on glucose level | [306] |
| TQ | Quercetin (QR) | Fluorescence- assays | TQ (purity ≥95%, HPLC | Assay | An insignificant inhibitory effect on the activity of CYP1A2 or CYP2E1. Moderate to a strong inhibitory effect on CYP3A4 activity. Moderate inhibitors of the CYP2C9. QR has a moderate inhibitory effect on CYP2C19 and a strong inhibitory effect on CYP2D6. | [312] |
| Black cumin | Amoxicillin | Rat Model | PO | 25 mg/kg BW | Methanol and hexane extracts increased the permeation of amoxicillin significantly; Enhanced amoxicillin availability in both in vivo and in vitro | [307] |
| Black cumin | Amoxicillin | Rat sac model | Rat sac | Seed extract | The methanolic extract improved intestinal permeability of amoxicillin in the in vitro experiments in a dose-dependent manner | [313] |
| Black cumin | Sildenafil | Beagle dogs | PO | Sildenafil 100 mg | Reduced AUC0-∞, C max and t 1/2 of Sildenafil | [304] |
| Black cumin | Cyclosporine (CCS) | Rabbit | PO | 200 mg/kgCCS (30 mg/kg) | Co-administration significantly decreased the C(max)-35.5% and AUC (0-∞)-55.9% | [314] |
| Black cumin | Phenytoin | Beagle dogs | PO | Phenytoin 50 mg | Drastic reduction of elimination and to a lesser extent on VoD at steady state (Vss) with a consequent reduction of area under the curve (AUC0-∞) by about 87% | [305] |
| Black cumin | Theophylline | Beagle dogs | PO | 200 mg | No significant effect on theophylline disposition as measured by Cmax, Tmax, AUC0–∞, and CL/F | [308] |
| Black cumin | Carbamazepine (CBZ) | Rabbit | PO | Black cumin (200 mg/kg) or Lepidium sativum (150 mg/kg) | Concurrent use of Lepidium sativum but not black cumin alters the pharmacokinetics of CBZ | [309] |
| Black cumin | CYP2C11 | Wistar rats | PO | 300 mg/kg | Significantly inhibited the mRNA and protein expression levels of CYP2C11 in a dose-dependent manner. | [315] |
| Nanoparticles | Method of Preparation | Size | Zeta Potential | Dose (EC) | Experimental Model | Indication | Benefits/ Advantages | References |
|---|---|---|---|---|---|---|---|---|
| TQ-loaded nanocapsule | Nanoprecipitation | 70.21 nm | –45.3 mV | 10 mg/kg TQ-loaded NCs | Streptozotocin plus nicotinamide–induced diabetic Wistar female albino rats | Anti-diabetes |
| [316] |
| TQ-loaded phospholipid nanoconstructs | Micro-emulsification technique | 83.44 nm | −0.65 mV | 20 mg/kg | Wistar rats | Hepatoprotection |
| [317] |
| TQ-capped iron oxide nanoparticles | Co-precipitation method | 10 nm | −33.4 ± 1.5 mV | 0.05, 0.1, 0.15, 0.2, and 0.25 mg/mL | MDA-MB-231 (epithelial, human breast cancer cell) | Chemo-Photothermal Therapy of Cancer |
| [318] |
| TQ-loaded chitosan-lecithin micelles | 50 nm to 100 nm | - | 200 µL of 20 mg/mL of TQ-PMs | Balb/c mice | Wound healing efficacy |
| [319] | |
| TQ-loaded nanoformulation | Emulsion solvent evaporation method | 97.36 ± 2.01 nm | −17.98 ± 1.09 | 10 mg kg−1 | Albino rats | Epilepsy |
| [320] |
| TQ-loaded Chitosan nanoparticles (TQ-TPP-Cs NPs) | Bio-fabrication and statistical optimization | 391.4 ± 78.35 | 30.9 ± 3.02 mV | (141.91 mg/kg | Wistar rodents | Depression |
| [321] |
| TQ-loaded, hyaluronic acid (HA)-conjugated Pluronic® P123 and F127 copolymer nanoparticles (HA-TQ-Nps) | 15–20 nm | -- | 1.5, 2, 3 μg/mL | Two human TNBC cell lines such as MDA-MB-231 and MDA-MB-468 | Triple-negative breast cancer |
| [322] | |
| TQ-loaded polymeric nanocapsules | nanoprecipitation technique | 217 to 231.5 nm | −36 to −39 mV | 100, 200, 300, 400 μM | Colon cancer cell lines (HT-29, HCT-116, Caco-2) | Colon cancer |
| [323] |
| TQ solid lipid nanoparticles | solvent injection methods | 20 mg/kg | Male Albino Wistar rats | Depression |
| [64] | ||
| Ethosomic TQ | Conventional method | 20 ± 1 nm | −63 ± 2 mv | Human epithelial breast cancer cell lines MCF-7 | Breast cancer |
| [324] |
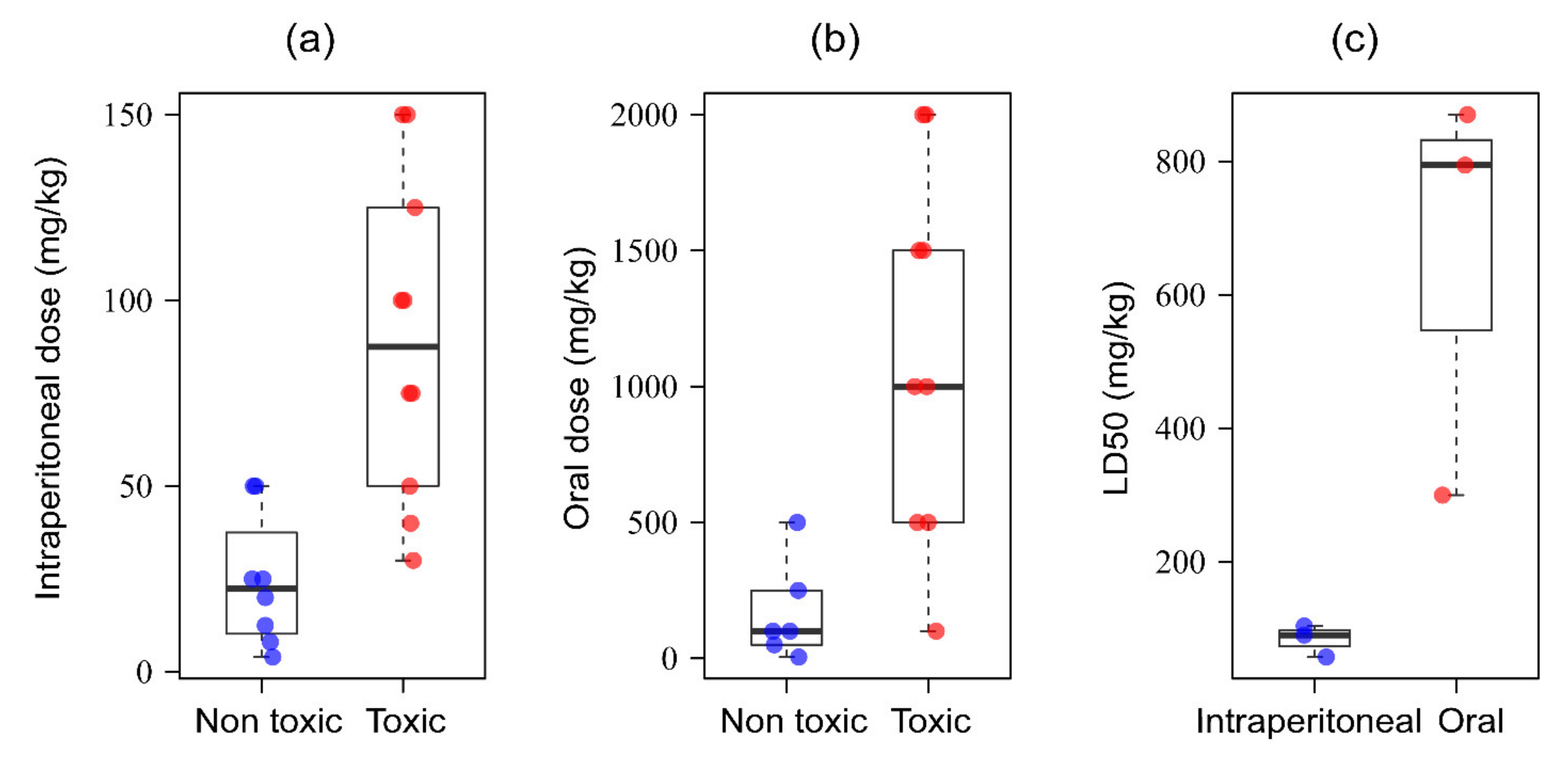
9. Safety Evaluation of Black Cumin-Based Therapeutics
10. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gupta, C.; Prakash, D. Nutraceuticals for geriatrics. J. Tradit. Complement. Med. 2015, 5, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Chapter 13—Cumin. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–178. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Kabir, Y.; Shirakawa, H.; Komai, M. Nutritional composition of the indigenous cultivar of black cumin seeds from Bangladesh. Prog. Nutr. 2019, 21, 428–434. [Google Scholar] [CrossRef]
- Greenish, H.G. Beiträge zur Chemie von Nigella sativa. Fresenius Z. F. Anal. Chem. 1882, 21, 462. [Google Scholar] [CrossRef]
- Mustafa, Z.; Soliman, G. Melanthigenin and its identity with hederagenin. J. Chem. Soc. 1943, 70–71. [Google Scholar] [CrossRef]
- Toppozada, H.H.; Mazloum, H.A.; el-Dakhakhny, M. The antibacterial properties of the Nigella sativa L. seeds. Active principle with some clinical applications. J. Egypt Med. Assoc. 1965, 48, 187–202. [Google Scholar]
- Kapoor, B.M. Contributions to the Cytology of Endosperm in some Angiosperms. X. Nigella Damascena and N. Sativa. Caryologia 1966, 19, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Tripathi, J.; Manik, S.; Umar, L.; Rabia, J. Preliminary Phytochemical Studies of the Miracle Herb of the Century, Nigella sativa L. (Black Seed). Indo Am. J. Pharm. Res. 2013, 3, 3000–3007. [Google Scholar]
- al-Bukhari, S. Sahih Al-Bukhari 5687 Book 76 Hadith 10. Available online: https://sunnah.com/bukhari/76 (accessed on 30 June 2020).
- Tariq, M. Nigella sativa seeds: Folklore treatment in modern day medicine. Saudi J. Gastroenterol. 2008, 14, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Parveen, N.; Ali, A.S. Links between the Prophet Muhammad (PBUH) recommended foods and disease management: A review in the light of modern superfoods. Int. J. Health Sci. 2018, 12, 61–69. [Google Scholar]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: Traditional uses, chemical constituents, and nutraceutical effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Carroll, J.F.; Babish, J.G.; Pacioretty, L.M.; Kramer, M. Repellency to ticks (Acari: Ixodidae) of extracts of Nigella sativa (Ranunculaceae) and the anti-inflammatory DogsBestFriend™. Exp. Appl. Acarol. 2016, 70, 89–97. [Google Scholar] [CrossRef]
- Sitara, U.; Niaz, I.; Naseem, J.; Sultana, N. Antifungal effect of essential oils on in vitro growth of pathogenic fungi. Pak. J. Bot. 2008, 40, 409–414. [Google Scholar]
- Cheikh-Rouhou, S.; Besbes, S.; Lognay, G.; Blecker, C.; Deroanne, C.; Attia, H. Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J. Food Compos. Anal. 2008, 21, 162–168. [Google Scholar] [CrossRef]
- Akram Khan, M.; Afzal, M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology 2016, 24, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Kiralan, M.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind. Crop. Prod. 2014, 57, 52–58. [Google Scholar] [CrossRef]
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogoşan, C.; Mocan, A. Comparative studies on polyphenolic composition, antioxidant and diuretic effects of nigella sativa L. (black cumin) and nigella damascena L. (Lady-in-a-Mist) seeds. Molecules 2015, 20, 9560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharm. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M. Phytochemical composition, antioxidant, anti-inflammatory and antimicrobial activity of Nigella sativa L. essential oil. J. Essent. Oil Bear. Plants 2014, 17, 1002–1011. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Schuff, C.; De Lampasona, M.P.; Catalán, C.A.N. Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gindy, Y.; Zeweil, H.; Zahran, S.; El-Rahman, M.A.; Eisa, F. Hematologic, lipid profile, immunity, and antioxidant status of growing rabbits fed black seed as natural antioxidants. Trop. Anim. Health Prod. 2020, 52, 999–1004. [Google Scholar] [CrossRef]
- Imam, A.; Sulaiman, N.A.; Oyewole, A.L.; Amin, A.; Shittu, S.T.T.; Ajao, M.S. Pro-neurogenic and antioxidant efficacy of Nigella sativa oil reduced vulnerability cholinesterase dysfunction and disruption in amygdala-dependent behaviours in chlorpyrifos exposure. J. Krishna Inst. Med. Sci. Univ. 2018, 7, 1–12. [Google Scholar]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Abu Bakar, M.F.; Basri, H.; Abdullah, M.A. Modulation of hydrogen peroxide-induced oxidative stress in human neuronal cells by thymoquinone-rich fraction and thymoquinone via transcriptomic regulation of antioxidant and apoptotic signaling genes. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, A. Protective effect of thymoquinone against lead-induced antioxidant defense system alteration in rat liver. Acta Biol. Hung. 2017, 68, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Bauer, D.; Soliman, K.F.A. The Antioxidant Effects of Thymoquinone in Activated BV-2 Murine Microglial Cells. Neurochem. Res. 2016, 41, 3227–3238. [Google Scholar] [CrossRef] [Green Version]
- Ardiana, M.; Pikir, B.S.; Santoso, A.; Hermawan, H.O.; Al-Farabi, M.J. Effect of Nigella sativa Supplementation on Oxidative Stress and Antioxidant Parameters: A Meta-Analysis of Randomized Controlled Trials. Sci. World J. 2020, 2020. [Google Scholar] [CrossRef]
- Dwita, L.P.; Yati, K.; Gantini, S.N. The anti-inflammatory activity of nigella sativa balm sticks. Sci. Pharm. 2019, 87, 3. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and anti-inflammatory properties of nigella sativa oil in human pre-adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, H.N.; Ibrahim, F.M.; Maklad, Y.A.; Ahmed, K.A.; Ramadan, M.F. Characterization of antiradical and anti-inflammatory activities of some cold pressed oils in carrageenan-induced rat model of acute inflammation. Der Pharma Chem. 2016, 8, 148–158. [Google Scholar]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.; Son, Y.J.; Cho, J.Y. Thymoquinone suppresses irf-3-mediated expression of type i interferons via suppression of tbk1. Int. J. Mol. Sci. 2018, 19, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheik, N.M.M.; Jaikumar, K.; Marimuthu, S.; Wyson, W.J.; Anand, D.; Saravanan, P. In vitro immunostimulation activity of Nigella sativa Linn. And psoralea Corylifolia Linn. seeds using a murine macrophage cell line. Asian J. Pharm. Clin. Res. 2017, 10, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Hakim, A.S.; Abouelhag, H.A.; Abdou, A.M.; Fouad, E.A.; Khalaf, D.D. Assessment of immunomodulatory effects of black cumin seed (Nigella Sativa) extract on macrophage activity in vitro. Int. J. Vet. Sci. 2019, 8, 385–389. [Google Scholar]
- Koshak, A.E.; Yousif, N.M.; Fiebich, B.L.; Koshak, E.A.; Heinrich, M. Comparative immunomodulatory activity of Nigella sativa L. preparations on proinflammatory mediators: A focus on asthma. Front. Pharm. 2018, 9, 1075. [Google Scholar] [CrossRef] [Green Version]
- Meles, D.K.; Safitri, E.; Mustofa, I.; Susilowati, S.; Putri, D.K.S.C. Immunomodulatory Activity of Black Jinten Oil (Nigella sativa) as Macrophage Activator for Salmonella typimurium Infected Rat. Indian Vet. J. 2020, 97, 12–14. [Google Scholar]
- Eladl, A.H.; Arafat, N.; El-Shafei, R.A.; Farag, V.M.; Saleh, R.M.; Awadin, W.F. Comparative immune response and pathogenicity of the H9N2 avian influenza virus after administration of Immulant®, based on Echinacea and Nigella sativa, in stressed chickens. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 165–175. [Google Scholar] [CrossRef]
- Karmous, A.M.; Ben Naser, K.M.; Abuzaid, L.; Asheg, A. Duration of feeding black seed (Nigella sativa) to broiler chicks and its effect on immune response, cholesterol and gut microflora. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 183–187. [Google Scholar]
- El-Shanshory, M.; Hablas, N.M.; Aboonq, M.S.; Fakhreldin, A.R.; Attia, M.; Arafa, W.; Mariah, R.A.; Baghdadi, H.; Ayat, M.; Zolaly, M.; et al. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J. Herb. Med. 2019, 16, 100245. [Google Scholar] [CrossRef]
- Kheirouri, S.; Hadi, V.; Alizadeh, M. Immunomodulatory Effect of Nigella sativa Oil on T Lymphocytes in Patients with Rheumatoid Arthritis. Immunol. Investig. 2016, 45, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. A review on possible therapeutic effect of nigella sativa and thymoquinone in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Mitra, S.; Ali, M.C.; Oktaviani, D.F.; Hannan, M.A.; Choi, S.M.; Moon, I.S. Phytosterols: Targeting Neuroinflammation in Neurodegeneration. Curr. Pharm. Des. 2021, 27, 383–401. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Velagapudi, R.; El-Bakoush, A.; Lepiarz, I.; Ogunrinade, F.; Olajide, O.A. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol. Cell Biochem. 2017, 435, 149–162. [Google Scholar] [CrossRef]
- Alhibshi, A.H.; Odawara, A.; Suzuki, I. Neuroprotective efficacy of thymoquinone against amyloid beta-induced neurotoxicity in human induced pluripotent stem cell-derived cholinergic neurons. Biochem. Biophys. Rep. 2019, 17, 122–126. [Google Scholar] [CrossRef]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Abdullah, M.A.; Basri, H. Thymoquinone-rich fraction nanoemulsion (TQRFNE) decreases Aβ40 and Aβ42 levels by modulating APP processing, up-regulating IDE and LRP1, and down-regulating BACE1 and RAGE in response to high fat/cholesterol diet-induced rats. Biomed. Pharmacother. 2017, 95, 780–788. [Google Scholar] [CrossRef]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Stanslas, J.; Sani, D.; Basri, H.; Abdullah, M.A. Beneficial effects of TQRF and TQ nano- and conventional emulsions on memory deficit, lipid peroxidation, total antioxidant status, antioxidants genes expression and soluble Aβ levels in high fat-cholesterol diet-induced rats. Chem. Biol. Interact. 2017, 275, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Badary, O.A. Protective effects of thymoquinone on D-galactose and aluminum chloride induced neurotoxicity in rats: Biochemical, histological and behavioral changes. Neurol. Res. 2018, 40, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Terzioglu-Usak, S.; Beker, M.; Sahbaz, C. Thymoquinone (TQ) demonstrates its neuroprotective effect via an anti-inflammatory action on the Aβ(1–42)-infused rat model of Alzheimer’s disease. Psychiatry Clin. Psychopharmacol. 2019, 29, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Alhebshi, A.H.; Odawara, A.; Gotoh, M.; Suzuki, I. Thymoquinone protects cultured hippocampal and human induced pluripotent stem cells-derived neurons against α-synuclein-induced synapse damage. Neurosci. Lett. 2014, 570, 126–131. [Google Scholar] [CrossRef]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates α-synuclein aggregation in vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef]
- Soleimannejad, K.; Rahmani, A.; Hatefi, M.; Khataminia, M.; Hafezi Ahmadi, M.R.; Asadollahi, K. Effects of Nigella sativa Extract on Markers of Cerebral Angiogenesis after Global Ischemia of Brain in Rats. J. Stroke Cerebrovasc. Dis. 2017, 26, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Li, Y.; Peng, X.; Zhao, H.; Mao, Y.; Cui, Y. Thymoquinone protects against cerebral small vessel disease: Role of antioxidant and anti-inflammatory activities. J. Biol. Regul. Homeost. Agents 2018, 32, 225–231. [Google Scholar]
- Gülşen, İ.; Ak, H.; Çölçimen, N.; Alp, H.H.; Akyol, M.E.; Demir, İ.; Atalay, T.; Balahroğlu, R.; Rağbetli, M.Ç. Neuroprotective Effects of Thymoquinone on the Hippocampus in a Rat Model of Traumatic Brain Injury. World Neurosurg. 2016, 86, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, D.; Ahirwar, B. Antidepressant effect of nigella sativa in stress-induced depression. Res. J. Pharm. Technol. 2020, 13, 1611–1614. [Google Scholar] [CrossRef]
- Alam, M.; Zameer, S.; Najmi, A.K.; Ahmad, F.J.; Imam, S.S.; Akhtar, M. Thymoquinone Loaded Solid Lipid Nanoparticles Demonstrated Antidepressant-Like Activity in Rats via Indoleamine 2,3- Dioxygenase Pathway. Drug Res. 2020, 70, 206–213. [Google Scholar] [CrossRef]
- Farh, M.; Kadil, Y.; Tahri, E.H.; Abounasr, M.; Riad, F.; El Khasmi, M.; Tazi, A. Evaluation of anxiolytic, antidepressant, and memory effects of Nigella sativa seeds oil in rat. Phytotherapie 2017, 1–9. [Google Scholar] [CrossRef]
- Beheshti, F.; Norouzi, F.; Abareshi, A.; Anaeigoudari, A.; Hosseini, M. Acute administration of Nigella sativa showed anxiolytic and anti-depression effects in rats. Curr. Nutr. Food Sci. 2018, 14, 422–431. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Oryan, S.; Mohajerani, H.R.; Akbari, N.; Palizvan, M.R. Probiotics and Nigella sativa extract supplementation improved behavioral and electrophysiological effects of PTZ-induced chemical kindling in rats. Epilepsy Behav. 2020, 104. [Google Scholar] [CrossRef]
- Bepari, A.; Parashivamurthy, B.M.; Niazi, S.K. Evaluation of the effect of volatile oil extract of Nigella sativa seeds on maximal electroshock-induced seizures in albino rats. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 273–284. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Li, B.; Huang, Y.M.; Luo, Q.; Xie, Y.M.; Chen, Y.H. Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model. Transl. Neurosci. 2017, 8, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Feng, Y.; Xie, Y.; Luo, Q.; Chen, L.; Li, B.; Chen, Y. Protective Effects of Thymoquinone Against Convulsant Activity Induced by Lithium-Pilocarpine in a model of Status Epilepticus. Neurochem. Res. 2016, 41, 3399–3406. [Google Scholar] [CrossRef]
- Vafaee, F.; Hosseini, M.; Hassanzadeh, Z.; Edalatmanesh, M.A.; Sadeghnia, H.R.; Seghatoleslam, M.; Mousavi, S.M.; Amani, A.; Shafei, M.N. The effects of Nigella sativa hydro-alcoholic extract on memory and brain tissues oxidative damage after repeated seizures in rats. Iran. J. Pharm. Res. 2015, 14, 547–557. [Google Scholar]
- Khan, R.A.; Najmi, A.K.; Khuroo, A.H.; Goswami, D.; Akhtar, M. Ameliorating effects of thymoquinone in rodent models of schizophrenia. Afr. J. Pharm. Pharmacol. 2014, 8, 413–421. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Role of Oxidative Stress, MAPKinase and Apoptosis Pathways in the Protective Effects of Thymoquinone Against Acrylamide-Induced Central Nervous System Toxicity in Rat. Neurochem. Res. 2020, 45, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Neuroprotective Effects of Thymoquinone in Acrylamide-Induced Peripheral Nervous System Toxicity Through MAPKinase and Apoptosis Pathways in Rat. Neurochem. Res. 2019, 44, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer, M.F.; Anis, E.; Ahmad, F.; Hossain, M.M.; Ali, A.; Afzal, M. Evaluation of phyto-medicinal efficacy of thymoquinone against Arsenic induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. Phytomedicine 2019, 54, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer, M.F.; Ahmad, M.; Afzal, M. Anxiolytic and anti-inflammatory role of thymoquinone in arsenic-induced hippocampal toxicity in Wistar rats. Heliyon 2018, 4, e00650. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer, M.F.; Waseem, M.; Ullah, R.; Ahmad, M.; Afzal, M. Thymoquinone alleviates arsenic induced hippocampal toxicity and mitochondrial dysfunction by modulating mPTP in Wistar rats. Biomed. Pharmacother. 2018, 102, 1152–1160. [Google Scholar] [CrossRef]
- Imam, A.; Ogunniyi, A.; Ibrahim, A.; Abdulmajeed, W.I.; Oyewole, L.A.; Lawan, A.H.; Sulaimon, F.A.; Adana, M.Y.; Ajao, M.S. Dichlorvos induced oxidative and neuronal responses in rats: Mitigative efficacy of Nigella sativa (Black Cumin). Niger. J. Physiol. Sci. 2018, 33, 83–88. [Google Scholar]
- Demir, E.; Taysi, S.; Ulusal, H.; Kaplan, D.S.; Cinar, K.; Tarakcioglu, M. Nigella sativa oil and thymoquinone reduce oxidative stress in the brain tissue of rats exposed to total head irradiation. Int. J. Radiat. Biol. 2020, 96, 228–235. [Google Scholar] [CrossRef]
- Dash, R.; Ali, M.C.; Jahan, I.; Munni, Y.A.; Mitra, S.; Hannan, M.A.; Timalsina, B.; Oktaviani, D.F.; Choi, H.J.; Moon, I.S. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res. Rev. 2021, 65, 101209. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Biswas, P.; Hossain, M.S.; Islam, R.; Hannan, M.A.; Uddin, M.J.; Rhim, H. Potential Therapeutic Role of Phytochemicals to Mitigate Mitochondrial Dysfunctions in Alzheimer’s Disease. Antioxidants 2020, 10, 23. [Google Scholar] [CrossRef]
- Dash, R.; Jahan, I.; Ali, M.C.; Mitra, S.; Munni, Y.A.; Timalsina, B.; Hannan, M.A.; Moon, I.S. Potential roles of natural products in the targeting of proteinopathic neurodegenerative diseases. Neurochem. Int. 2021, 145, 105011. [Google Scholar] [CrossRef] [PubMed]
- Azzubaidi, M.S.; Al-Ani, I.M.; Saxena, A.K.; Faisal, G.G. Prevention of brain hypoperfusion-induced neurodegeneration in rat’s hippocampus by black cumin fixed oil treatment. Int. Med. J. Malays. 2018, 17, 103–112. [Google Scholar] [CrossRef]
- Cascella, M.; Bimonte, S.; Barbieri, A.; Del Vecchio, V.; Muzio, M.R.; Vitale, A.; Benincasa, G.; Ferriello, A.B.; Azzariti, A.; Arra, C.; et al. Dissecting the potential roles of Nigella sativa and its constituent thymoquinone on the prevention and on the progression of Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Cho, D.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neuropharmacological potential and delivery prospects of thymoquinone for neurological disorders. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Isaev, N.K.; Chetverikov, N.S.; Stelmashook, E.V.; Genrikhs, E.E.; Khaspekov, L.G.; Illarioshkin, S.N. Thymoquinone as a Potential Neuroprotector in Acute and Chronic Forms of Cerebral Pathology. Biochemistry 2020, 85, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Li, P.; Stetler, R.A.; Leak, R.K.; Shi, Y.; Li, Y.; Yu, W.; Bennett, M.V.L.; Chen, J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 2018, 134, 208–217. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Wilson, J.R.; Fehlings, M.G. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019, 18, 24–25. [Google Scholar] [CrossRef] [Green Version]
- Imam, A.; Lawal, A.; Oyewole, L.A.; Ajibola, M.I.; Williams, V.; Chengetanai, S.; Shittu, T.S.T.; Ajao, M.S. Nigella sativa conserved hippocampal oxidative and neurogenic activities to salvage neuro-cognitive integrities in chlorpyrifos insult. Sci. Afr. 2018, 1, e00008. [Google Scholar] [CrossRef]
- Çelik, F.; Göçmez, C.; Karaman, H.; Kamaşak, K.; Kaplan, İ.; Akıl, E.; Tufek, A.; Guzel, A.; Uzar, E. Therapeutic Effects of Thymoquinone in a Model of Neuropathic Pain. Curr. Ther. Res. 2014, 76, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Amin, B.; Taheri, M.M.; Hosseinzadeh, H. Effects of intraperitoneal thymoquinone on chronic neuropathic pain in rats. Planta Med. 2014, 80, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk. J. Med. Sci. 2018, 48, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Khadri, H.; Azam, M.; Rahmani, A.H.; Khaleefah, A.; Khaleefah, F.; Khateef, R.; Ansari, M.A.; Allemailem, K.S. Antibacterial, Antibiofilm and Anticancer Activity of Biologically Synthesized Silver Nanoparticles Using Seed Extract of Nigella sativa. Processes 2020, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Bumidin, M.S.; Johari, F.A.; Risan, N.F.; Nasir, M.H.M. The effect of aqueous extracts of nigella sativa on breast cancer cell line Mcf-7: An in vitro study. Sci. Herit. J. 2018, 2, 13–17. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016, 31, 449–455. [Google Scholar] [CrossRef]
- Dastjerdi, M.N.; Mehdiabady, E.M.; Iranpour, F.G.; Bahramian, H. Effect of thymoquinone on P53 gene expression and consequence apoptosis in breast cancer cell line. Int. J. Prev. Med. 2016, 7, 66. [Google Scholar] [CrossRef]
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 112961. [Google Scholar] [CrossRef]
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella sativa L. and Thymus vulgaris L., and its anti-proliferative effect on HeLa cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017, 32, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-H.; Chen, M.-C.; Day, C.H.; Lin, Y.-M.; Li, S.-Y.; Tu, C.-C.; Padma, V.V.; Shih, H.-N.; Kuo, W.-W.; Huang, C.-Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J. Gastroenterol. 2017, 23, 1171. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Wang, L.; Yue, Y.; Zhang, P.; Huang, Z.; Lv, W.; Ma, J.; Shao, Q.; Ma, M.; et al. Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2020, 320, 109022. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Huang, Z.; Zhang, P.; Yue, Y.; Wang, W.; Liu, W.; Zeng, J.; Ma, J.; Chen, G.; et al. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact. 2018, 292, 65–75. [Google Scholar] [CrossRef]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016, 2016, 1407840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, B.; Kou, Q.; Ma, B.; Zhang, J.; Sun, B.; Yang, Y.; Li, J.; Zhou, J.; Liu, W. Thymoquinone inhibits metastatic phenotype and epithelial-mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol. Rep. 2018, 40, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Fan, Y.; Huang, S.; Wang, G.; Han, R.; Lei, F.; Luo, A.; Jing, X.; Zhao, L.; Gu, S. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018, 109, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Dera, A.; Rajagopalan, P. Thymoquinone attenuates phosphorylation of AKT to inhibit kidney cancer cell proliferation. J. Food Biochem. 2019, 43, e12793. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Bahrami, G.; Taherabadi, F.; Jaffari, F.; Hosseinzadeh, L. Apoptosis cell death effect of linoleic acid from nigella sativa on human ovary cancer cells through mitochondrial intrinsic pathway. J. Rep. Pharm. Sci. 2018, 7, 20–26. [Google Scholar]
- Czajkowska, A.; Gornowicz, A.; Pawłowska, N.; Czarnomysy, R.; Nazaruk, J.; Szymanowski, W.; Bielawska, A.; Bielawski, K. Anticancer Effect of a Novel Octahydropyrazino[2,1-a:5,4-a’]diisoquinoline Derivative and Its Synergistic Action with Nigella sativa in Human Gastric Cancer Cells. Biomed. Res. Int. 2017, 2017, 9153403. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.; Liu, W.; Zhao, W.; Duan, P.; Yang, Y.; Yi, Q.; Guo, F.; Li, J.; Zhou, J.; Kou, Q. Thymoquinone inhibits epithelial-mesenchymal transition in prostate cancer cells by negatively regulating the TGF-β/Smad2/3 signaling pathway. Oncol. Rep. 2017, 38, 3592–3598. [Google Scholar] [CrossRef] [Green Version]
- Kotowski, U.; Heiduschka, G.; Kadletz, L.; Fahim, T.; Seemann, R.; Schmid, R.; Schneider, S.; Mitterbauer, A.; Thurnher, D. Effect of thymoquinone on head and neck squamous cell carcinoma cells in vitro: Synergism with radiation. Oncol. Lett. 2017, 14, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Abdel–Mottaleb, Y.; Ahmed, A.A.E.; El-Maraghy, N.N. Novel combination of thymoquinone and resveratrol enhances anticancer effect on hepatocellular carcinoma cell line. Future J. Pharm. Sci. 2018, 4, 41–46. [Google Scholar] [CrossRef]
- Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Farshori, N.N. Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. S. Afr. J. Bot. 2017, 112, 70–78. [Google Scholar] [CrossRef]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef]
- Şakalar, Ç.; İzgi, K.; İskender, B.; Sezen, S.; Aksu, H.; Çakır, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Shahin, Y.R.; Elguindy, N.M.; Abdel Bary, A.; Balbaa, M. The protective mechanism of Nigella sativa against diethylnitrosamine-induced hepatocellular carcinoma through its antioxidant effect and EGFR/ERK1/2 signaling. Environ. Toxicol. 2018, 33, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Kensara, O.A.; El-Shemi, A.G.; Mohamed, A.M.; Refaat, B.; Idris, S.; Ahmad, J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des. Dev. Ther. 2016, 10, 2239–2253. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.H. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef] [Green Version]
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- Ahmad, S.; Chughtai, A.; Hussain, R.; Iqbal, S. Physiological and biochemical role of nigella sativa in hyperlipidemic albino rats a comparative study. Pak. J. Med. Health Sci. 2017, 11, 195–196. [Google Scholar]
- Bhatti, I.; Inayat, S.; Uzair, B.; Menaa, F.; Bakhsh, S.; Khan, H.; Naz, F.; Khan, B.A. Effects of Nigella sativa (Kalonji) and honey on lipid profile of hyper lipidemic smokers. Indian J. Pharm. Educ. Res. 2016, 50, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, M.; Khodakarami, F.; Feizabad, E.; Ghaemi, M. The effects of nigella sativa on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome. Endocrine 2020, 69, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Rachman, P.; Darmawan, E. The efficacy of black cumin seed (Nigella sativa) oil and hypoglycemic drug combination to reduce HbA1c level in patients with metabolic syndrome risk. IOP Conf. Ser. Mater. Sci. Eng. 2017, 259, 012018. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Tajmiri, S. The effects of powdered black cumin seeds on markers of oxidative stress, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in patients with Hashimoto’s thyroiditis. Clin. Nutr. Espen. 2020, 37, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, A.; Niazmand, S.; Mahmoudabady, M.; Soukhtanloo, M.; Rezaee, S.A.; Mousavi, S.M. Nigella sativa improve redox homeostasis in heart and aorta of diabetic rat. Curr. Nutr. Food Sci. 2016, 12, 35–41. [Google Scholar] [CrossRef]
- Widodo, G.P.; Herowati, R.; Perangin-Angin, J.M.; Kamlasi, J.E.Y. Antihyperglycemic, antioxidant, and pancreas regeneration activities of black cumin (Nigella sativa L.) seeds ethanol extract in alloxan-induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Bensiameur-Touati, K.; Kacimi, G.; Haffaf, E.M.; Berdja, S.; Aouichat-Bouguerra, S. In vivo Subacute Toxicity and Antidiabetic Effect of Aqueous Extract of Nigella sativa. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Abbasnezhad, A.; Niazmand, S.; Mahmoudabady, M.; Rezaee, S.A.; Soukhtanloo, M.; Mosallanejad, R.; Hayatdavoudi, P. Nigella sativa L. seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J. Ethnopharmacol. 2019, 228, 142–147. [Google Scholar] [CrossRef]
- Nourbar, E.; Mirazi, N.; Yari, S.; Rafieian-Kopaei, M.; Nasri, H. Effect of hydroethanolic extract of Nigella sativa L. on skin wound healing process in diabetic male rats. Int. J. Prev. Med. 2019, 10. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Hussein, R.H.; Hamza, A. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J. Biol. Sci. 2020, 27, 2410–2419. [Google Scholar] [CrossRef]
- Cüce, G.; Sözen, M.E.; Çetinkaya, S.; Canbaz, H.T.; Seflek, H.; Kalkan, S. Effects of Nigella sativa L. seed oil on intima-media thickness and Bax and Caspase 3 expression in diabetic rat aorta. Anatol. J. Cardiol. 2016, 16, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altun, E.; Avci, E.; Yildirim, T.; Yildirim, S. Protective effect of Nigella sativa oil on myocardium in streptozotocin-induced diabetic rats. Acta Endocrinol. 2019, 15, 289–294. [Google Scholar] [CrossRef]
- Balbaa, M.; El-Zeftawy, M.; Ghareeb, D.; Taha, N.; Mandour, A.W. Nigella sativa Relieves the Altered Insulin Receptor Signaling in Streptozotocin-Induced Diabetic Rats Fed with a High-Fat Diet. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrazek, H.M.A.; Kilany, O.E.; Muhammad, M.A.A.; Tag, H.M.; Abdelazim, A.M. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male Wistar rats. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Al-Trad, B.; Al-Batayneh, K.; El-Metwally, S.; Alhazimi, A.; Ginawi, I.; Alaraj, M.; Alkofahi, E.; Aljumaili, O.; Kosba, A. Nigella sativa oil and thymoquinone ameliorate albuminuria and renal extracellular matrix accumulation in the experimental diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2680–2688. [Google Scholar]
- Akhtar, M.T.; Qadir, R.; Bukhari, I.; Ashraf, R.A.; Malik, Z.; Zahoor, S.; Murtaza, M.A.; Siddique, F.; Shah, S.N.H.; Saadia, M. Antidiabetic potential of Nigella sativa L seed oil in alloxan-induced diabetic rabbits. Trop. J. Pharm. Res. 2020, 19, 283–289. [Google Scholar] [CrossRef]
- Preety, R.; Anitha, R.; Rajeshkumar, S.; Lakshmi, T. Anti-diabetic activity of silver nanoparticles prepared from cumin oil using alpha amylase inhibitory assay. Int. J. Res. Pharm. Sci. 2020, 11, 1267–1269. [Google Scholar] [CrossRef]
- Ansari, Z.M.; Nasiruddin, M.; Khan, R.A.; Haque, S.F. Protective role of Nigella sativa in diabetic nephropathy: A randomized clinical trial. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ. Transplant. Saudi Arab. 2017, 28, 9–14. [Google Scholar] [CrossRef]
- Kooshki, A.; Tofighiyan, T.; Rastgoo, N.; Rakhshani, M.H.; Miri, M. Effect of Nigella sativa oil supplement on risk factors for cardiovascular diseases in patients with type 2 diabetes mellitus. Phytother. Res. 2020, 34, 2706–2711. [Google Scholar] [CrossRef]
- Badar, A.; Kaatabi, H.; Bamosa, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Alkhadra, A.; Al-Almaie, S. Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: Nonrandomized clinical trial. Ann. Saudi Med. 2017, 37, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Ghoreyshi, M.; Mahmoudabady, M.; Bafadam, S.; Niazmand, S. The Protective Effects of Pharmacologic Postconditioning of Hydroalcoholic Extract of Nigella sativa on Functional Activities and Oxidative Stress Injury During Ischemia–Reperfusion in Isolated Rat Heart. Cardiovasc. Toxicol. 2020, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Akhtar, M.; Ahmed, S.; Ahmad, A.; Najmi, A.K. Nigella sativa protects against isoproterenol-induced myocardial infarction by alleviating oxidative stress, biochemical alterations and histological damage. Asian Pac. J. Trop. Biomed. 2017, 7, 294–299. [Google Scholar] [CrossRef]
- Enayatfard, L.; Mohebbati, R.; Niazmand, S.; Hosseini, M.; Shafei, M.N. The standardized extract of Nigella sativa and its major ingredient, thymoquinone, ameliorates angiotensin II-induced hypertension in rats. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rizka, A.; Setiati, S.; Lydia, A.; Dewiasty, E. Effect of Nigella sativa Seed Extract for Hypertension in Elderly: A Double-blind, Randomized Controlled Trial. Acta Med. Indones. 2017, 49, 307–313. [Google Scholar]
- Hussain, N.; Majid, S.A.; Abbasi, M.S.; Hussain, M.A.; Rehman, K.; Khan, M.Q.; Dar, M.E.U.I.; Shaheen, H.; Habib, T. Use of black seed (Nigella Sativa L.) oil in the management of hypertensive and hyperlipidemic individuals of district Muzaffarabad, Azad Kashmir, Pakistan. Appl. Ecol. Environ. Res. 2017, 15, 31–48. [Google Scholar] [CrossRef]
- Alam, M.F.; Khan, G.; Safhi, M.M.; Alshahrani, S.; Siddiqui, R.; Sivagurunathan Moni, S.; Anwer, T. Thymoquinone ameliorates doxorubicin-induced cardiotoxicity in swiss albino mice by modulating oxidative damage and cellular inflammation. Cardiol. Res. Pract. 2018, 2018, 1483041. [Google Scholar] [CrossRef] [Green Version]
- Danaei, G.H.; Memar, B.; Ataee, R.; Karami, M. Protective effect of thymoquinone, the main component of Nigella Sativa, against diazinon cardio-toxicity in rats. Drug Chem. Toxicol. 2019, 42, 585–591. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Vishnubhatla, S.; Amarapurkar, D.N.; Das, K.; Sood, A.; Chawla, Y.K.; Eapen, C.E.; Boddu, P.; Thomas, V.; Varshney, S.; et al. Etiology and mode of presentation of chronic liver diseases in India: A multi centric study. PLoS ONE 2017, 12, e0187033. [Google Scholar] [CrossRef] [Green Version]
- Noorbakhsh, M.F.; Hayati, F.; Samarghandian, S.; Shaterzadeh-Yazdi, H.; Farkhondeh, T. An Overview of Hepatoprotective Effects of Thymoquinone. Recent Pat. Food Nutr. Agric. 2018, 9, 14–22. [Google Scholar] [CrossRef]
- Tabassum, H.; Ahmad, A.; Ahmad, I.Z. Nigella sativa L. And its bioactive constituents as hepatoprotectant: A review. Curr. Pharm. Biotechnol. 2018, 19, 43–67. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Edris, A.E.; Fouda, K. Hepatic regeneration and reno-protection by fish oil, nigella sativa oil and combined fish oil/Nigella sativa volatiles in CCL4 treated rats. J. Oleo Sci. 2018, 67, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.S.; Razzaque, A.; Ahmad, Z.; Pazdernik, V.; Amin, S.N. Does posttreatment thymoquinone reverse high-dose atorvastatin-induced hepatic oxidative injury in rats? Can. J. Physiol. Pharmacol. 2018, 96, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ayuob, N.N.; Abdel-Hamid, A.A.H.M.; Helal, G.M.M.; Mubarak, W.A. Thymoquinone reverses nonalcoholic fatty liver disease (NAFLD) associated with experimental hypothyroidism. Rom. J. Morphol. Embryol. 2019, 60, 479–486. [Google Scholar]
- Hosseini, M.; Ghasemi, S.; Hadjzadeh, M.A.R.; Ghorbani, A.; Aghili, S.; Aghaei, A.; Soukhtanloo, M.; Beheshti, F. Administration of Nigella sativa during neonatal and juvenile growth period improved liver function of propylthiouracil-induced hypothyroid rats. J. Matern. Fetal Neonatal Med. 2020, 33, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Radwan, R.R.; Mohamed, H.A. Nigella sativa oil modulates the therapeutic efficacy of mesenchymal stem cells against liver injury in irradiated rats. J. Photochem. Photobiol. B Biol. 2018, 178, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.W.; Bai, T.; Yang, Y.; Zhang, Y.; Jiang, M.; Yang, H.X.; Wu, M.; Liu, J.; Qiao, C.Y.; Zhan, Z.Y.; et al. Thymoquinone attenuates acetaminophen overdose-induced acute liver injury and inflammation via regulation of JNK and AMPK signaling pathway. Am. J. Chin. Med. 2019, 47, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Erisgin, Z.; Atasever, M.; Cetinkaya, K.; Akarca Dizakar, S.Ö.; Omeroglu, S.; Sahin, H. Protective effects of Nigella sativa oil against carboplatin-induced liver damage in rats. Biomed. Pharmacother. 2019, 110, 742–747. [Google Scholar] [CrossRef]
- Hosseini, S.M.R.; Razmgah, G.R.G.; Nematy, M.; Esmaily, H.; Yousefi, M.; Kamalinejad, M.; Mosavat, S.H. Efficacy of black seed (Nigella sativa) and lemon balm (melissa officinalis) on non-alcoholic fatty liver disease: A randomized controlled clinical trial. Iran. Red Crescent Med. J. 2018, 20. [Google Scholar] [CrossRef]
- Husna, M.; Sumera, S.; Laiba, S.; Anam, A. The effect of crude nigella sativa oil against the acute toxicity of diclofenac sodium and ibuprofen on the liver of albino mice. Slov. Vet. Res. 2017, 54, 21–27. [Google Scholar]
- Rashidmayvan, M.; Mohammadshahi, M.; Seyedian, S.S.; Haghighizadeh, M.H. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J. Diabetes Metab. Disord. 2019, 18, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.R.; Vahabi, A.; Roshankhah, S.; Darehdori, A.S.; Jalili, C. The effects of thymoquinone against morphine-induced damages on male mice liver. Int. J. Prev. Med. 2018, 9. [Google Scholar] [CrossRef]
- Alsemeh, A.E.; Moawad, R.S.; Abdelfattah, E.R. Histological and biochemical changes induced by gibberellic acid in the livers of pregnant albino rats and their offspring: Ameliorative effect of Nigella sativa. Anat. Sci. Int. 2019, 94, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, F.; Norouzi, F.; Abareshi, A.; Khazaei, M.; Alikhani, V.; Moussavi, S.; Biglari, G.; Soukhtanloo, M.; Hosseini, M. Nigella sativa prevented liver and renal tissue damage in lipopolysaccharide-treated rats. Saudi J. Kidney Dis. Transplant. 2018, 29, 554–566. [Google Scholar] [CrossRef]
- Khonche, A.; Huseini, H.F.; Gholamian, M.; Mohtashami, R.; Nabati, F.; Kianbakht, S. Standardized Nigella sativa seed oil ameliorates hepatic steatosis, aminotransferase and lipid levels in non-alcoholic fatty liver disease: A randomized, double-blind and placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 234, 106–111. [Google Scholar] [CrossRef]
- Darand, M.; Darabi, Z.; Yari, Z.; Saadati, S.; Hedayati, M.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A. Nigella sativa and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Results from a randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 44, 204–209. [Google Scholar] [CrossRef]
- Clemens, M.G. Nitric oxide in liver injury. Hepatology 1999, 30, 1–5. [Google Scholar] [CrossRef]
- Chen, T.; Zamora, R.; Zuckerbraun, B.; Billiar, T.R. Role of nitric oxide in liver injury. Curr. Mol. Med. 2003, 3, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharm. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Farghali, H.; Hynie, S.; Vohnikova, Z.; Masek, K. Possible dual role of nitric oxide in oxidative stress injury: A study in perfused hepatocytes. Int. J. Immunopharmacol. 1997, 19, 599–605. [Google Scholar] [CrossRef]
- Eissa, L.A.; Eisa, N.H.; Ebrahim, M.A.; Ragab, M.; El-Gayar, A.M. Nitric Oxide is a Potential Diagnostic Marker for Hepatocellular Carcinoma. Sci. Pharm. 2013, 81, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hon, W.M.; Lee, K.H.; Khoo, H.E. Nitric oxide in liver diseases: Friend, foe, or just passerby? Ann. N. Y. Acad. Sci. 2002, 962, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.D.; Behary, J.; Zekry, A. Non-alcoholic fatty liver disease (NAFLD): A review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 2019, 50, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, T.; Yao, Y.L.; Zhang, D.Q.; Wu, Y.L.; Lian, L.H.; Nan, J.X. Upregulation of SIRT1-AMPK by thymoquinone in hepatic stellate cells ameliorates liver injury. Toxicol. Lett. 2016, 262, 80–91. [Google Scholar] [CrossRef]
- Hussain, M.; Tunio, A.G.; Akhtar, L.; Shaikh, G.S. Effects of nigella sativa on various parameters in Patients of non-alcoholic fatty liver disease. J. Ayub Med. Coll. Abbottabad JAMC 2017, 29, 403–407. [Google Scholar] [PubMed]
- Darand, M.; Darabi, Z.; Yari, Z.; Hedayati, M.; Shahrbaf, M.A.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A. The effects of black seed supplementation on cardiovascular risk factors in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2369–2377. [Google Scholar] [CrossRef]
- Aggarwal, T.; Wadhwa, R.; Thapliyal, N.; Sharma, K.; Rani, V.; Maurya, P.K. Oxidative, inflammatory, genetic, and epigenetic biomarkers associated with chronic obstructive pulmonary disorder. J. Cell. Physiol. 2019, 234, 2067–2082. [Google Scholar] [CrossRef]
- Sethi, G.S.; Dharwal, V.; Naura, A.S. Immunological basis of Oxidative stress-induced lung inflammation in asthma and COPD. In Oxidative Stress in Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 195–223. [Google Scholar]
- Noorbakhsh, M.-F.; Shaterzadeh-Yazdi, H.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Protective effects of thymoquinon on pulmonary disorders in experimental studies. Tanaffos 2018, 17, 211. [Google Scholar]
- Koshak, A.; Koshak, E.; Heinrich, M. Medicinal benefits of Nigella sativa in bronchial asthma: A literature review. Saudi Pharm. J. 2017, 25, 1130–1136. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Shakeri, F.; Saadat, S.; Ghorani, V.; Boskabady, M.H. Clinical and experimental effects of Nigella sativa and its constituents on respiratory and allergic disorders. Avicenna J. Phytomed. 2019, 9, 195–212. [Google Scholar]
- Mokhtari-Zaer, A.; Norouzi, F.; Askari, V.R.; Khazdair, M.R.; Roshan, N.M.; Boskabady, M.; Hosseini, M.; Boskabady, M.H. The protective effect of Nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J. Ethnopharmacol. 2020, 253, 112653. [Google Scholar] [CrossRef]
- Poursalehi, H.R.; Fekri, M.S.; Far, F.S.; Mandegari, A.; Izadi, A.; Mahmoodi, R.; Nematollahi, H.; Porgholamhosein, F.; Ghorani, V.; Fekri, M.S. Early and late preventive effect of Nigella sativa on the bleomycin-induced pulmonary fibrosis in rats: An experimental study. Avicenna J. Phytomed. 2018, 8, 263. [Google Scholar] [PubMed]
- Ahmad, A.; Alkharfy, K.M.; Jan, B.L.; Ahad, A.; Ansari, M.A.; Al-Jenoobi, F.I.; Raish, M. Thymoquinone treatment modulates the Nrf2/HO-1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Exp. Lung Res. 2020, 46, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.; Robbe, A.; Kourda, N.; Ben Khamsa, S.; Legrand, A. Nigella sativa, a traditional Tunisian herbal medicine, attenuates bleomycin-induced pulmonary fibrosis in a rat model. Biomed. Pharmacother. 2017, 90, 626–637. [Google Scholar] [CrossRef]
- Yetkin, N.A.; Büyükoğlan, H.; Sönmez, M.F.; Tutar, N.; Gülmez, I.; Yilmaz, I. The protective effects of thymoquinone on lung damage caused by cigarette smoke. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2020, 95, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.M. Evaluation of antioxidant and anti-lipid peroxidation potentials of Nigella sativa and onion extract on nicotine-induced lung damage. Folia Morphol. 2019, 78, 554–563. [Google Scholar] [CrossRef] [PubMed]
- El-Ebiary, A.A.; El-Ghaiesh, S.; Hantash, E.; Alomar, S. Mitigation of cadmium-induced lung injury by Nigella sativa oil. Environ. Sci. Pollut. Res. 2016, 23, 25356–25363. [Google Scholar] [CrossRef]
- Ikhsan, M.; Hiedayati, N.; Maeyama, K.; Nurwidya, F. Nigella sativa as an anti-inflammatory agent in asthma. BMC Res. Notes 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Salem, A.M.; Bamosa, A.O.; Qutub, H.O.; Gupta, R.K.; Badar, A.; Elnour, A.; Afzal, M.N. Effect of Nigella sativa supplementation on lung function and inflammatory mediators in partly controlled asthma: A randomized controlled trial. Ann. Saudi Med. 2017, 37, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella sativa supplementation improves asthma control and biomarkers: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2017, 31, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, F.; Farooqui, Z.; Khan, F. Cisplatin-induced gastrointestinal toxicity: An update on possible mechanisms and on available gastroprotective strategies. Eur. J. Pharmacol. 2018, 827, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Farooqui, Z.; Rizwan, S.; Abidi, S.; Parwez, I.; Khan, F. Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on brush border membrane enzymes, carbohydrate metabolism and antioxidant system in rat intestine. Exp. Toxicol. Pathol. 2017, 69, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Farooqui, Z.; Abidi, S.; Parwez, I.; Khan, F. Oral administration of thymoquinone mitigates the effect of cisplatin on brush border membrane enzymes, energy metabolism and antioxidant system in rat intestine. Biomed. Pharmacother. 2017, 94, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Farooqui, Z.; Khan, A.A.; Khan, F. Oral Nigella sativa oil and thymoquinone administration ameliorates the effect of long-term cisplatin treatment on the enzymes of carbohydrate metabolism, brush border membrane, and antioxidant defense in rat intestine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Manjegowda, S.B.; Rajagopal, H.M.; Dharmesh, S.M. Polysaccharide of Black cumin (Nigella sativa) modulates molecular signaling cascade of gastric ulcer pathogenesis. Int. J. Biol. Macromol. 2017, 101, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Khan, A.M.; Karim, S.; Kamal, M.A.; Damanhouri, G.A.; Mirza, Z. Panacea seed “Nigella”: A review focusing on regenerative effects for gastric ailments. Saudi J. Biol. Sci. 2016, 23, 542–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleem, U.; Rehman, A.; Shah, R.; Jan, T.U.; Jan, M.; Jan, R. Effects of thymoquinone obtained from seeds of Nigella sativa on volume & Acidity of stimulated gastric secretion. Pak. J. Med. Health Sci. 2020, 14, 49–50. [Google Scholar]
- Bali, I.; Polat, F.R.; Aziret, M.; Sözen, S.; Oruç, C.; Coskunkan, U.; Emir, S.; Bilir, B.; Koç, A. Protective effect of Nigella sativa in an animal model of colon anastomosis with ischemia/ reperfusion injury. Int. Surg. 2019, 103, 139–148. [Google Scholar] [CrossRef]
- Hashem-Dabaghian, F.; Agah, S.; Taghavi-Shirazi, M.; Ghobadi, A. Combination of Nigella sativa and honey in eradication of gastric helicobacter pylori infection. Iran. Red Crescent Med. J. 2016, 18. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah-Bodaghi, M.; Darabi, Z.; Agah, S.; Hekmatdoost, A. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytother. Res. 2019, 33, 1027–1032. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on Clinical Trials of Black Seed (Nigella sativa) and Its Active Constituent, Thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar] [CrossRef]
- Mosbah, R.; Djerrou, Z.; Mantovani, A. Protective effect of Nigella sativa oil against acetamiprid induced reproductive toxicity in male rats. Drug Chem. Toxicol. 2018, 41, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Pang, W.-K.; Ryu, D.-Y.; Park, Y.-J.; Pang, M.-G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020, 35, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kwon, W.-S.; Karmakar, P.C.; Yoon, S.-J.; Ryu, B.-Y.; Pang, M.-G. Gestational exposure to bisphenol A affects the function and proteome profile of F1 spermatozoa in adult mice. Environ. Health Perspect. 2017, 125, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, R.; Yousef, M.I.; Maranghi, F.; Mantovani, A. Protective role of Nigella sativa oil against reproductive toxicity, hormonal alterations, and oxidative damage induced by chlorpyrifos in male rats. Toxicol. Ind. Health 2016, 32, 1266–1277. [Google Scholar] [CrossRef]
- Abouzaripour, M.; Rezaie, M.J.; Nikkho, B.; Allahveisi, A.; Roshani, D.; Khalesro, S. Protective effect of Nigella sativa on sperm parameters in mice exposed to titanium dioxide during embryonic development. Sci. J. Kurd. Univ. Med. Sci. 2019, 24, 66–73. [Google Scholar] [CrossRef]
- Alyoussef, A.; Al-Gayyar, M.M.H. Thymoquinone ameliorated elevated inflammatory cytokines in testicular tissue and sex hormones imbalance induced by oral chronic toxicity with sodium nitrite. Cytokine 2016, 83, 64–74. [Google Scholar] [CrossRef]
- Assi, M.A.; Hezmee, M.N.M.; Abba, Y.; Md Yusof, M.S.; Haron, A.W.; Rajion, M.A.; Al-Zuhairy, M.A. Prophylactic effect of nigella sativa against lead acetate induced changes in spermiogram, reproductive hormones and gonadal histology of rats. Vet. World 2016, 9, 1305–1311. [Google Scholar] [CrossRef]
- Kolahdooz, M.; Nasri, S.; Modarres, S.Z.; Kianbakht, S.; Huseini, H.F. Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2014, 21, 901–905. [Google Scholar] [CrossRef]
- Latiff, L.A.; Parhizkar, S.; Dollah, M.A.; Hassan, S.T.S. Alternative supplement for enhancement of reproductive health and metabolic profile among perimenopausal women: A novel role of Nigella sativa. Iran. J. Basic Med. Sci. 2014, 17, 980. [Google Scholar]
- Chananeh, M.; Janati Ataei, P.; Dolatian, M.; Mojab, F.; Nasiri, M. Effects of the combination of nigella sativa and mefenamic acid and mefenamic acid alone on the severity of postpartum pain in multiparous women: A double-blind clinical trial. Iran. J. Obstet. Gynecol. Infertil. 2018, 21, 62–71. [Google Scholar]
- Arif, M.; Thakur, S.C.; Datta, K. Implication of thymoquinone as a remedy for polycystic ovary in rat. Pharm. Biol. 2016, 54, 674–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, W.K.; Hassan, M.S.; Keam, G.G. Study the effect of Nigella sativa on thyroid function and reproductive hormone of female rat. J. Contemp. Med. Sci. 2016, 2, 67–69. [Google Scholar]
- Mohammed, A.E.N.A. Nigella sativa oil improves physiological parameters, oocyte quality after ovarian transplantation, and reproductive performance of female mice. Pak. J. Zool. 2019, 51, 2225–2231. [Google Scholar] [CrossRef]
- Betul, A.Y.; Semin, G. Wound healing effects of Nigella sativa L. essential oil in streptozotocin induced in diabetic rats. GSC Biol. Pharm. Sci. 2019, 7, 30–40. [Google Scholar]
- Javadi, S.M.R.; Hashemi, M.; Mohammadi, Y.; MamMohammadi, A.; Sharifi, A.; Makarchian, H.R. Synergistic effect of honey and nigella sativa on wound healing in rats. Acta Cir. Bras. 2018, 33, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmet Cengiz, H.; Durmuş, A.S.; Sağliyan, A.; Günay, C.; Özkaraca, M.; Kandemir, F.M.; Comakli, S.; Öztopalan, D.F. Effects of Nigella sativa and Hypericum perforatum on wound healing. Turk. J. Vet. Anim. Sci. 2017, 41, 99–105. [Google Scholar]
- Bannai, J.A.A.; Alrashid, I.M.; Jabbar, L.A.A.J.P.A. Effect of hot methanolic extract of Nigella sativa on the healing of infected cutaneous wounds in rabbits. Plant Arch. 2020, 20, 756–760. [Google Scholar]
- Monton, C.; Settharaksa, S.; Suksaeree, J.; Chankana, N.; Charoenchai, L. Optimization of plant compositions of Trisattakula to maximize antibacterial activity and formulation development of film-forming polymeric solution containing Nigella sativa ethanolic extract. Adv. Tradit. Med. 2021, 1–12. [Google Scholar] [CrossRef]
- Nawarathne, N.W.; Wijesekera, K.; Wijayaratne, W.M.D.G.B.; Napagoda, M. Development of Novel Topical Cosmeceutical Formulations from Nigella sativa L. with Antimicrobial Activity against Acne-Causing Microorganisms. Sci. World J. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, M.H. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Soleymani, S.; Zargaran, A.; Farzaei, M.H.; Iranpanah, A.; Heydarpour, F.; Najafi, F.; Rahimi, R. The effect of a hydrogel made by Nigella sativa L. on acne vulgaris: A randomized double-blind clinical trial. Phytother. Res. 2020, 34, 3052–3062. [Google Scholar] [CrossRef]
- Kumandaş, A.; Karslı, B.; Kürüm, A.; Çınar, M.; Elma, E. Comparison of the effects of zinc-silver cream and Nigella sativa oil on wound healing and oxidative stress in the wound model in rats. Ank. Üniversitesi Vet. Fakültesi Derg. 2020, 67, 33–40. [Google Scholar] [CrossRef]
- Elgohary, H.M.; Al Jaouni, S.K.; Selim, S.A. Effect of ultrasound-enhanced Nigella sativa seeds oil on wound healing: An animal model. J. Taibah Univ. Med. Sci. 2018, 13, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Siriwattanasatorn, M.; Itharat, A.; Thongdeeying, P.; Ooraikul, B. In vitro Wound Healing Activities of Three Most Commonly Used Thai Medicinal Plants and Their Three Markers. Evid. Based Complement. Altern. Med. 2020, 2020, 6795383. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.; Samanta, A.; Roy, A. Study of wound healing activity of different formulations of Nigella sativa seed extract. J. Pharm. Technol. 2016, 9, 2097–2105. [Google Scholar] [CrossRef]
- Okasha, E.F.; Bayomy, N.A.; Abdelaziz, E.Z. Effect of Topical Application of Black Seed Oil on Imiquimod-Induced Psoriasis-like Lesions in the Thin Skin of Adult Male Albino Rats. Anat. Rec. 2018, 301, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarac, G.; Kapicioglu, Y.; Sener, S.; Mantar, I.; Yologlu, S.; Dundar, C.; Turkoglu, M.; Pekmezci, E. Effectiveness of topical Nigella sativa for vitiligo treatment. Dermatol. Ther. 2019, 32, e12949. [Google Scholar] [CrossRef]
- Gaudin, O.; Toukal, F.; Hua, C.; Ortonne, N.; Assier, H.; Jannic, A.; Giménez-Arnau, E.; Wolkenstein, P.; Chosidow, O.; Ingen-Housz-Oro, S. Association between severe acute contact dermatitis due to Nigella sativa oil and epidermal apoptosis. JAMA Dermatol. 2018, 154, 1062–1065. [Google Scholar] [CrossRef]
- Ezirganli, S.; Kazancioglu, H.O.; Ozdemir, H.; Inan, D.S.; Tek, M. The effects of Nigella sativa seed extract on bone healing in an experimental model. J. Craniofac. Surg. 2016, 27, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Abd Elrahman, S.M.; Younes, S.A.; Kawana, K.Y. Evaluation of Nigella sativa on socket healing in rabbits. Alex. Dent. J. 2019, 44, 60–64. [Google Scholar] [CrossRef]
- Arslan, A.H.; Tomruk, C.Ö.; Meydanlı, E.G.; Özdemir, İ.; Duygu Çapar, G.; Kütan, E.; Yılmaz, A.; Yalçın Ülker, G.M. Histopathological evaluation of the effect of systemic thymoquinone administration on healing of bone defects in rat tibia. Biotechnol. Biotechnol. Equip. 2017, 31, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Mendi, A. Nigella sativa oil could induce osteogenic differentiation of dental pulp mesenchymal stem cells: Clinical nutrition for dentistry. Food Health 2018, 4, 19–24. [Google Scholar] [CrossRef]
- Ahmed, Z.M.; Attia, M.S.; Mahmoud, A.M.; Shoreibah, E.A. Evaluation of Topical Application of Nigella Sativa (Black Seeds) on Delayed Dental Implant. Al-Azhar Dent. J. Girls 2020, 7, 255–261. [Google Scholar] [CrossRef]
- Hsu, R.K.; Hsu, C.Y. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Kim, E.H.; Hannan, M.A.; Ha, H. Pharmacotherapy against Oxidative Stress in Chronic Kidney Disease: Promising Small Molecule Natural Products Targeting Nrf2-HO-1 Signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef]
- Ahmed, J.H.; Abdulmajeed, I.M. Effect of Nigella sativa (black seeds) against methotrexate-induced nephrotoxicity in mice. J. Intercult. Ethnopharmacol. 2017, 6, 9–13. [Google Scholar] [CrossRef]
- Ahmed, E.; Abd-ellatief, R.; Ali, M.; Saleh, T.; Ahmed, E. Optimization of the effectiveness and cytocompatibility of Nigella sativa as a co-treatment for reducing methotrexate-related adverse effects. Comp. Clin. Pathol. 2020, 29, 287–296. [Google Scholar] [CrossRef]
- Alsuhaibani, A.M.A. Effect of nigella sativa against cisplatin induced nephrotoxicity in rats. Ital. J. Food Saf. 2018, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, Z.; Ahmed, F.; Rizwan, S.; Shahid, F.; Khan, A.A.; Khan, F. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed. Pharmacother. 2017, 85, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, Z.; Shahid, F.; Abidi, S.; Parwez, I.; Khan, F. Oral thymoquinone administration ameliorates: The effect of cisplatin on brush border membrane enzymes, energy metabolism, and redox status in rat kidney. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Palma, G.; Barbieri, A.; Bimonte, S.; Amruthraj, N.J.; Muzio, M.R.; del Vecchio, V.; Rea, D.; Falco, M.; Luciano, A.; et al. Role of nigella sativa and its constituent thymoquinone on chemotherapy-induced nephrotoxicity: Evidences from experimental animal studies. Nutrients 2017, 9, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asif, S.; Malik, L. Protective effects of Nigella sativa on acetylsalicylic acid-induced nephrotoxicity in albino rats. J. Coll. Physicians Surg. Pak. 2017, 27, 536–539. [Google Scholar] [PubMed]
- Asif, S.; Mudassir, S.; Toor, R.S. Histological Effects of Nigella Sativa on Aspirin-Induced Nephrotoxicity in Albino Rats. J. Coll. Physicians Surg. Pak. 2018, 28, 735–738. [Google Scholar] [PubMed]
- Canayakin, D.; Bayir, Y.; Kilic Baygutalp, N.; Sezen Karaoglan, E.; Atmaca, H.T.; Kocak Ozgeris, F.B.; Keles, M.S.; Halici, Z. Paracetamol-induced nephrotoxicity and oxidative stress in rats: The protective role of Nigella sativa. Pharm. Biol. 2016, 54, 2082–2091. [Google Scholar] [CrossRef] [Green Version]
- Akintunde, J.K.; Abubakar, O.K. Novel therapeutic approaches of natural oil from black seeds and its underlying mechanisms against kidney dysfunctions in haloperidol-induced male rats. Drug Metab. Personal. Ther. 2017, 32, 97–107. [Google Scholar] [CrossRef]
- Khair, N.S.; Nooreldien, N.M. The protective effect of Nigella Sativa oil on Penconazole induced -renal toxicity in adult albino rats: Histological, Immunohistochemical and Biochemical study. Egypt. J. Histol. 2019, 42, 99–120. [Google Scholar] [CrossRef]
- Al-Brakati, A.Y.; Kassab, R.B.; Lokman, M.S.; Elmahallawy, E.K.; Amin, H.K.; Abdel Moneim, A.E. Role of thymoquinone and ebselen in the prevention of sodium arsenite–induced nephrotoxicity in female rats. Hum. Exp. Toxicol. 2019, 38, 482–493. [Google Scholar] [CrossRef]
- Erboga, M.; Kanter, M.; Aktas, C.; Sener, U.; Fidanol Erboga, Z.; Bozdemir Donmez, Y.; Gurel, A. Thymoquinone Ameliorates Cadmium-Induced Nephrotoxicity, Apoptosis, and Oxidative Stress in Rats is Based on its Anti-Apoptotic and Anti-Oxidant Properties. Biol. Trace Elem. Res. 2016, 170, 165–172. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Maysarah, N.M.; El-Sherbiny, M.; Al-Gayyar, M.M. Renal protective effects of thymoquinone against sodium nitrite-induced chronic toxicity in rats: Impact on inflammation and apoptosis. Life Sci. 2017, 180, 1–8. [Google Scholar] [CrossRef]
- Al-Gayyar, M.M.H.; Hassan, H.M.; Alyoussef, A.; Abbas, A.; Darweish, M.M.; El-Hawwary, A.A. Nigella sativa oil attenuates chronic nephrotoxicity induced by oral sodium nitrite: Effects on tissue fibrosis and apoptosis. Redox Rep. 2016, 21, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebuehi, O.A.T.; Olowojaiye, A.A.; Erukainure, O.L.; Ajagun-Ogunleye, O.M. Nigella sativa (black seed) oil ameliorates CCl4-induced hepatotoxicity and mediates neurotransmitter levels in male Sprague Dawley albino rats. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef]
- Caskurlu, T.; Kanter, M.; Erboga, M.; Erboga, Z.F.; Ozgul, M.; Atis, G. Protective effect of Nigella Sativa on renal reperfusion injury in rat. Iran. J. Kidney Dis. 2016, 10, 135–143. [Google Scholar] [PubMed]
- Benhelima, A.; Kaid-Omar, Z.; Hemida, H.; Benmahdi, T.; Addou, A. Nephroprotective and diuretic effect of Nigella sativa L seeds oil on lithiasic wistar rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, S.; Bideskan, A.E.; Shafei, M.N.; Sadeghnia, H.R.; Soukhtanloo, M.; Shahraki, S.; Noshahr, Z.S.; Rad, A.K. Nigella sativa extract is a potent therapeutic agent for renal inflammation, apoptosis, and oxidative stress in a rat model of unilateral ureteral obstruction. Phytother. Res. 2018, 32, 2290–2298. [Google Scholar] [CrossRef]
- Alanizy, L.; Almatham, K.; Al Basheer, A.; Al Fayyad, I. Complementary and alternative medicine practice among saudi patients with chronic kidney disease: A cross-sectional study. Int. J. Nephrol. Renov. Dis. 2020, 13, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Nasiruddin, M.; Haque, S.F.; Khan, R.A. Evaluation of safety and efficacy profile of Nigella sativa oil as an add-on therapy, in addition to alpha-keto analogue of essential amino acids in patients with chronic kidney disease. Saudi J. Kidney Dis. Transpl. 2020, 31, 21–31. [Google Scholar] [CrossRef]
- Khabbazi, A.; Javadivala, Z.; Seyedsadjadi, N.; Malek Mahdavi, A. A Systematic Review of the Potential Effects of Nigella sativa on Rheumatoid Arthritis. Planta Med. 2020, 86, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Mahboubi, M.; Mohammad Taghizadeh Kashani, L.; Mahboubi, M. Nigella sativa fixed oil as alternative treatment in management of pain in arthritis rheumatoid. Phytomedicine 2018, 46, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Fedeli, D.; Bordoni, L.; Piangerelli, M.; Servili, M.; Selvaggini, R.; Gabbianelli, R. Anti-inflammatory, anti-arthritic and anti-nociceptive activities of Nigella sativa oil in a rat model of arthritis. Antioxidants 2019, 8, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidin, K.S.; Jais, M.R.; Ismail, E.N.; Ishak, R. The effect of nigella sativa and eucheuma cottonii in collagen-induced arthritis mice. Res. J. Pharm. Technol. 2020, 13, 1319–1323. [Google Scholar] [CrossRef]
- Arjumand, S.; Shahzad, M.; Shabbir, A.; Yousaf, M.Z. Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-α IL-1, and NFκB expression levels. Biomed. Pharmacother. 2019, 111, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Turhan, Y.; Arıcan, M.; Karaduman, Z.O.; Turhal, O.; Gamsızkan, M.; Aydın, D.; Kılıç, B.; Özkan, K. Chondroprotective effect of Nigella sativa oil in the early stages of osteoarthritis: An experimental study in rabbits. J. Musculoskelet. Neuronal Interact. 2019, 19, 362–369. [Google Scholar]
- Azizi, F.; Ghorat, F.; Hassan Rakhshani, M.; Rad, M. Comparison of the effect of topical use of Nigella Sativa oil and diclofenac gel on osteoarthritis pain in older people: A randomized, double-blind, clinical trial. J. Herb. Med. 2019, 16, 100259. [Google Scholar] [CrossRef]
- Salimzadeh, A.; Ghourchian, A.; Choopani, R.; Hajimehdipoor, H.; Kamalinejad, M.; Abolhasani, M. Effect of an orally formulated processed black cumin, from Iranian traditional medicine pharmacopoeia, in relieving symptoms of knee osteoarthritis: A prospective, randomized, double-blind and placebo-controlled clinical trial. Int. J. Rheum. Dis. 2017, 20, 691–701. [Google Scholar] [CrossRef]
- Tuna, H.I.; Babadag, B.; Ozkaraman, A.; Balci Alparslan, G. Investigation of the effect of black cumin oil on pain in osteoarthritis geriatric individuals. Complement. Ther. Clin. Pract. 2018, 31, 290–294. [Google Scholar] [CrossRef]
- Koshak, D.A.E.; Koshak, P.E.A. Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: A mini review of in silico studies. Curr. Ther. Res. 2020, 93, 100602. [Google Scholar] [CrossRef]
- Siddiqui, S.; Upadhyay, S.; Ahmad, R.; Gupta, A.; Srivastava, A.; Trivedi, A.; Husain, I.; Ahmad, B.; Ahamed, M.; Khan, M.A. Virtual screening of phytoconstituents from miracle herb nigella sativa targeting nucleocapsid protein and papain-like protease of SARS-CoV-2 for COVID-19 treatment. J. Biomol. Struct. Dyn. 2020, 1–21. [Google Scholar] [CrossRef]
- Jakhmola Mani, R.; Sehgal, N.; Dogra, N.; Saxena, S.; Pande Katare, D. Deciphering underlying mechanism of Sars-CoV-2 infection in humans and revealing the therapeutic potential of bioactive constituents from Nigella sativa to combat COVID19: In-silico study. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef]
- Kulyar, M.F.-E.A.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential influence of Nigella sativa (Black cumin) in reinforcing immune system: A hope to decelerate the COVID-19 pandemic. Phytomed. Int. J. Phytother. Phytopharm. 2020, 85, 153277. [Google Scholar] [CrossRef]
- Maideen, N.M.P. Prophetic Medicine-Nigella Sativa (Black cumin seeds)—Potential herb for COVID-19? J. Pharmacopunct. 2020, 23, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Rehman, M.U.; Ahmad, P.; Alkharfy, K.M. Covid-19 and thymoquinone: Connecting the dots. Phytother. Res. 2020, 34, 2786–2789. [Google Scholar] [CrossRef]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.T.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 2021, 35, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Onifade, A.A.; Jewell, A.P.; Adedeji, W.A. Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr. J. Tradit. Complement. Altern. Med. Afr. Netw. Ethnomed. 2013, 10, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Onifade, A.A.; Jewell, A.P.; Okesina, A.B. Seronegative conversion of an HIV positive subject treated with Nigella sativa and honey. Afr. J. Infect. Dis. 2015, 9, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Amadi, C.N.; Offor, S.J.; Frazzoli, C.; Orisakwe, O.E. Natural antidotes and management of metal toxicity. Environ. Sci. Pollut. Res. Int. 2019, 26, 18032–18052. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Khalil, S.U.; Hussain, A.; Rehman, S.; Sajjad, S.; Rahman, A.U.; Da Rocha, J.B.T. Ethanolic extract of Nigella sativa protects Fe(II) induced lipid peroxidation in rat’s brain, kidney and liver homogenates. Pak. J. Pharm. Sci. 2016, 29, 231–237. [Google Scholar]
- Kishwar, F.; Mahmood, T.; Mahmood, I. Complexation of active ingredient thymoquinone of nigella sativa (black seed) with chromium(vi). Fuuast J. Biol. 2016, 6, 65–72. [Google Scholar]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bungău, S.G.; Bin-Jumah, M.; El-kott, A.F.; Shati, A.A.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone and diallyl sulphide against malathion-induced toxicity in rats. Environ. Sci. Pollut. Res. 2020, 27, 10228–10235. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Shaheen, H.M.; Abushouk, A.I.; Toraih, E.A.; Fawzy, M.S.; Alansari, W.S.; Aleya, L.; Bungau, S. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ. Sci. Pollut. Res. 2018, 25, 23909–23916. [Google Scholar] [CrossRef]
- Danaei, G.H.; Karami, M. Protective effect of thymoquinone against diazinon-induced hematotoxicity, genotoxicity and immunotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 55, 217–222. [Google Scholar] [CrossRef]
- Alhazza, I.M.; Ebaid, H.; Abdel-Salam, B.; Al-Tamimi, J.H.; Hassan, I.; Rady, A.M.; Mashaly, A.M.A. Thymoquinone ameliorates Pachycondyla sennaarensis venom-induced acute toxic shock in male rats. BMC Pharmacol. Toxicol. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Karimi, Z.; Ghaffari, M.; Ezzati Nazhad Dolatabadi, J.; Dehghan, P. The protective effect of thymoquinone on tert-butylhydroquinone induced cytotoxicity in human umbilical vein endothelial cells. Toxicol. Res. 2019, 8, 1050. [Google Scholar] [CrossRef] [PubMed]
- Alinia-Ahandani, E. Milk-increasing medicinal plants. J. Pharm. Sci. Res. 2018, 10, 1. [Google Scholar]
- Javan, R.; Javadi, B.; Feyzabadi, Z. Breastfeeding: A review of its physiology and galactogogue plants in view of traditional Persian medicine. Breastfeed. Med. 2017, 12, 401–409. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Tafaghodi, M.; Mosavi, M.J.; Taghiabadi, E. Effect of Aqueous and Ethanolic Extracts of Nigella sativa Seeds on Milk Production in Rats. JAMS J. Acupunct. Meridian Stud. 2013, 6, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020, 57, 4671–4687. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Butt, M.S.; Sohail, M.; Suleria, H.A.R. The antioxidant potential of black cumin (Nigella sativa l.) extracts through different extraction methods. Curr. Bioact. Compd. 2019, 15, 623–630. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Hussin, A.S.M. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa L.) Oil. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staniek, K.; Gille, L. Is thymoquinone an antioxidant? BMC Pharm. 2010, 10, A9. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Rahman, M.S.; Sohag, A.A.M.; Dash, R.; Hossain, K.S.; Farjana, M.; Uddin, M.J. Intermittent fasting, a possible priming tool for host defense against SARS-CoV-2 infection: Crosstalk among calorie restriction, autophagy and immune response. Immunol. Lett. 2020, 226, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Jrah-Harzallah, H.; Ben-Hadj-Khalifa, S.; Almawi, W.Y.; Maaloul, A.; Houas, Z.; Mahjoub, T. Effect of thymoquinone on 1,2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur. J. Cancer 2013, 49, 1127–1135. [Google Scholar] [CrossRef]
- Linjawi, S.A.; Khalil, W.K.; Hassanane, M.M.; Ahmed, E.S. Evaluation of the protective effect of Nigella sativa extract and its primary active component thymoquinone against DMBA-induced breast cancer in female rats. Arch. Med. Sci. 2015, 11, 220–229. [Google Scholar] [CrossRef]
- Mu, G.G.; Zhang, L.L.; Li, H.Y.; Liao, Y.; Yu, H.G. Thymoquinone Pretreatment Overcomes the Insensitivity and Potentiates the Antitumor Effect of Gemcitabine Through Abrogation of Notch1, PI3K/Akt/mTOR Regulated Signaling Pathways in Pancreatic Cancer. Dig. Dis. Sci. 2015, 60, 1067–1080. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Alrashedi, M.G.; Ali, A.S.; Ali, S.S.; Khan, L.M. Impact of thymoquinone on cyclosporine A pharmacokinetics and toxicity in rodents. J. Pharm. Pharmacol. 2018, 70, 1332–1339. [Google Scholar] [CrossRef]
- Al-Mohizea, A.M.; Ahad, A.; El-Maghraby, G.M.; Al-Jenoobi, F.I.; AlKharfy, K.M.; Al-Suwayeh, S.A. Effects of Nigella sativa, Lepidium sativum and Trigonella foenum-graecum on sildenafil disposition in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Al-Suwayeh, S.A.; Khan, R.M.; Ahmad, A. Effects of Lepidium sativum, Nigella sativa and Trigonella foenum-graceum on phenytoin pharmacokinetics in beagle dogs. Phytother. Res. 2013, 27, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, R.M.; Alkharfy, K.M.; Raish, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Effects of Thymoquinone on the Pharmacokinetics and Pharmacodynamics of Glibenclamide in a Rat Model. Nat. Prod. Commun. 2015, 10, 1395–1398. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Amin, S.; Ahmad, J.; Ali, A.; Mir, S.R. Bioavailability enhancement studies of amoxicillin with Nigella. Indian J. Med. Res. 2012, 135, 555–559. [Google Scholar]
- Al-Jenoobi, F.I.; Ahad, A.; Mahrous, G.M.; Al-Mohizea, A.M.; AlKharfy, K.M.; Al-Suwayeh, S.A. Effects of fenugreek, garden cress, and black seed on theophylline pharmacokinetics in beagle dogs. Pharm. Biol. 2015, 53, 296–300. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Jenoobi, F.I.; Alam, M.A.; Al-Mohizea, A.M.; Al-Suwayeh, S.A.; Korashy, H.M.; Khan, R.M.; Muzaffar, I. Lepidium sativum but not Nigella sativa affects carbamazepine disposition in an animal model. Drug Metab. Lett. 2013, 7, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Rathore, C.; Rathbone, M.J.; Chellappan, D.K.; Tambuwala, M.M.; Pinto, T.D.J.A.; Dureja, H.; Hemrajani, C.; Gupta, G.; Dua, K.; Negi, P. Nanocarriers: More than tour de force for thymoquinone. Expert Opin. Drug Deliv. 2020, 17, 479–494. [Google Scholar] [CrossRef]
- El-Far, A.H.; Al Jaouni, S.K.; Li, W.; Mousa, S.A. Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases. Nutrients 2018, 10, 1369. [Google Scholar] [CrossRef] [Green Version]
- Elbarbry, F.; Ung, A.; Abdelkawy, K. Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities. Pharm. Mag. 2018, 13, S895–S899. [Google Scholar] [CrossRef]
- Ali, B.; Ali, M.; Amin, S.; Mir, S.R. Enhancement of gut permeation of amoxicillin with Nigella sativa seed extract and its phytochemical screening. Chin. J. Nat. Med. 2018, 16, 125–130. [Google Scholar] [CrossRef]
- Al-Jenoobi, F.I.; Al-Suwayeh, S.A.; Muzaffar, I.; Alam, M.A.; Al-Kharfy, K.M.; Korashy, H.M.; Al-Mohizea, A.M.; Ahad, A.; Raish, M. Effects of Nigella sativa and Lepidium sativum on cyclosporine pharmacokinetics. Biomed. Res. Int. 2013, 2013, 953520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korashy, H.M.; Al-Jenoobi, F.I.; Raish, M.; Ahad, A.; Al-Mohizea, A.M.; Alam, M.A.; Alkharfy, K.M.; Al-Suwayeh, S.A. Impact of Herbal Medicines like Nigella sativa, Trigonella foenum-graecum, and Ferula asafoetida, on Cytochrome P450 2C11 Gene Expression in Rat Liver. Drug Res. 2015, 65, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kaushik, A.; Kim, K.-H.; Kumar, S. Antidiabetic activity enhancement in streptozotocin + nicotinamide-induced diabetic rats through combinational polymeric nanoformulation. Int. J. Nanomed. 2019, 14, 4383–4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, C.; Upadhyay, N.; Kaundal, R.; Dwivedi, R.P.; Rahatekar, S.; John, A.; Dua, K.; Tambuwala, M.M.; Jain, S.; Chaudari, D.; et al. Enhanced oral bioavailability and hepatoprotective activity of thymoquinone in the form of phospholipidic nano-constructs. Expert Opin. Drug Deliv. 2020, 17, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.M. Multifunctional Thymoquinone-Capped Iron Oxide Nanoparticles for Combined Chemo-Photothermal Therapy of Cancer. J. Supercond. Nov. Magn. 2020, 33, 2125–2131. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, G.; Verma, C.; Garg, P.; Rathore, C.; Kulshrestha, S.; Lal, U.R.; Gupta, B.; Pathania, D. Novel thymoquinone loaded chitosan-lecithin micelles for effective wound healing: Development, characterization, and preclinical evaluation. Carbohydr. Polym. 2020, 230, 115659. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al Qatifi, S.; Alessa, M.; Al Hajji, H.; Sarafroz, M. A bioanalytical UHPLC based method used for the quantification of Thymoquinone-loaded-PLGA-nanoparticles in the treatment of epilepsy. BMC Chem. 2020, 14, 10. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Khadrawy, Y.A.; Abd-El Daim, T.M.; Elfeky, A.S.; Abd Rabo, A.A.; Mustafa, A.B.; Mostafa, I.T. Thymoquinone-encapsulated chitosan nanoparticles coated with polysorbate 80 as a novel treatment agent in a reserpine-induced depression animal model. Physiol. Behav. 2020, 222, 112934. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control Release 2020, 322, 357–374. [Google Scholar] [CrossRef]
- Ramzy, L.; Metwally, A.A.; Nasr, M.; Awad, G.A.S. Novel thymoquinone lipidic core nanocapsules with anisamide-polymethacrylate shell for colon cancer cells overexpressing sigma receptors. Sci. Rep. 2020, 10, 10987. [Google Scholar] [CrossRef] [PubMed]
- Nasri, S.; Ebrahimi-Hosseinzadeh, B.; Rahaie, M.; Hatamian-Zarmi, A.; Sahraeian, R. Thymoquinone-loaded ethosome with breast cancer potential: Optimization, in vitro and biological assessment. J. Nanostruct. Chem. 2020, 10, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Yazan, L.S.; Mohd Azlan, S.N.; Zakarial Ansar, F.H.; Gopalsamy, B. Acute toxicity study of intravenous administration of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) in Sprague Dawley rats. Malays. J. Med. Health Sci. 2019, 15, 51–57. [Google Scholar]
- Lou, W.P.; Assaw, S.; Lokman, M.A.; Suhaimin, N.; Yusof, H.M. Sub-acute toxicity of black seed (Nigella sativa) and honey mixture. Malays. Appl. Biol. 2018, 47, 11–18. [Google Scholar]
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badary, O.A.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Bekairi, A.M.; Elmazar, M.M.A. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev. Res. 1998, 44, 56–61. [Google Scholar] [CrossRef]
- Bamosa, A.O.; Ali, B.A.; Al-Hawsawi, Z.A. The effect of thymoquinone on blood lipids in rats. Indian J. Physiol. Pharm. 2002, 46, 195–201. [Google Scholar]
- Mashayekhi-Sardoo, H.; Rezaee, R.; Karimi, G. An overview of in vivo toxicological profile of thymoquinone. Toxin Rev. 2020, 39, 115–122. [Google Scholar] [CrossRef]
- Jrah Harzallah, H.; Grayaa, R.; Kharoubi, W.; Maaloul, A.; Hammami, M.; Mahjoub, T. Thymoquinone, the Nigella sativa bioactive compound, prevents circulatory oxidative stress caused by 1,2-dimethylhydrazine in erythrocyte during colon postinitiation carcinogenesis. Oxid. Med. Cell. Longev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Abukhader, M. Thymoquinone in The clinical Treatment of cancer: Fact or fiction. Pharmacogn. Rev. 2013, 7, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Al-Ali, A.; Alkhawajah, A.A.; Randhawa, M.A.; Shaikh, N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J. Ayub Med. Coll. Abbottabad 2008, 20, 25–27. [Google Scholar]
- Mansour, M.A.; Ginawi, O.T.; El-Hadiyah, T.; El-Khatib, A.S.; Al-Shabanah, O.A.; Al-Sawaf, H.A. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: Evidence for antioxidant effects of thymoquinone. Res. Commun. Mol. Pathol. Pharm. 2001, 110, 239–251. [Google Scholar]
- Abukhader, M.M. The effect of route of administration in thymoquinone toxicity in male and female rats. Indian J. Pharm. Sci. 2012, 74, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, Y.S.; Saiful Yazan, L.; Ng, W.K.; Noordin, M.M.; Sapuan, S.; Foo, J.B.; Tor, Y.S. Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int. J. Nanomed. 2016, 11, 5905–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salarinia, R.; Rakhshandeh, H.; Oliaee, D.; Gul Ghasemi, S.; Ghorbani, A. Safety evaluation of Phytovagex, a pessary formulation of Nigella sativa, on pregnant rats. Avicenna J. Phytomed. 2016, 6, 117–123. [Google Scholar] [PubMed]
- Wadaan, M.A.M. Long-term effects of black seed and garlic oil on the offspring of two consecutive pregnancies in rats. J. King Saud. Univ. Sci. 2009, 21, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Mouhajir, F.; Pedersen, J.A.; Rejdali, M.; Towers, G.H.N. Antimicrobial thymohydroquinones of Moroccan Nigella sativa seeds detected by electron spin resonance. Pharm. Biol. 1999, 37, 391–395. [Google Scholar] [CrossRef]
- Agarwal, S.; Tripathi, R.; Mohammed, A.; Rizvi, S.I.; Mishra, N. Effects of thymol supplementation against type 2 diabetes in streptozotocin-induced rat model. Plant Arch. 2020, 20, 863–869. [Google Scholar]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef]
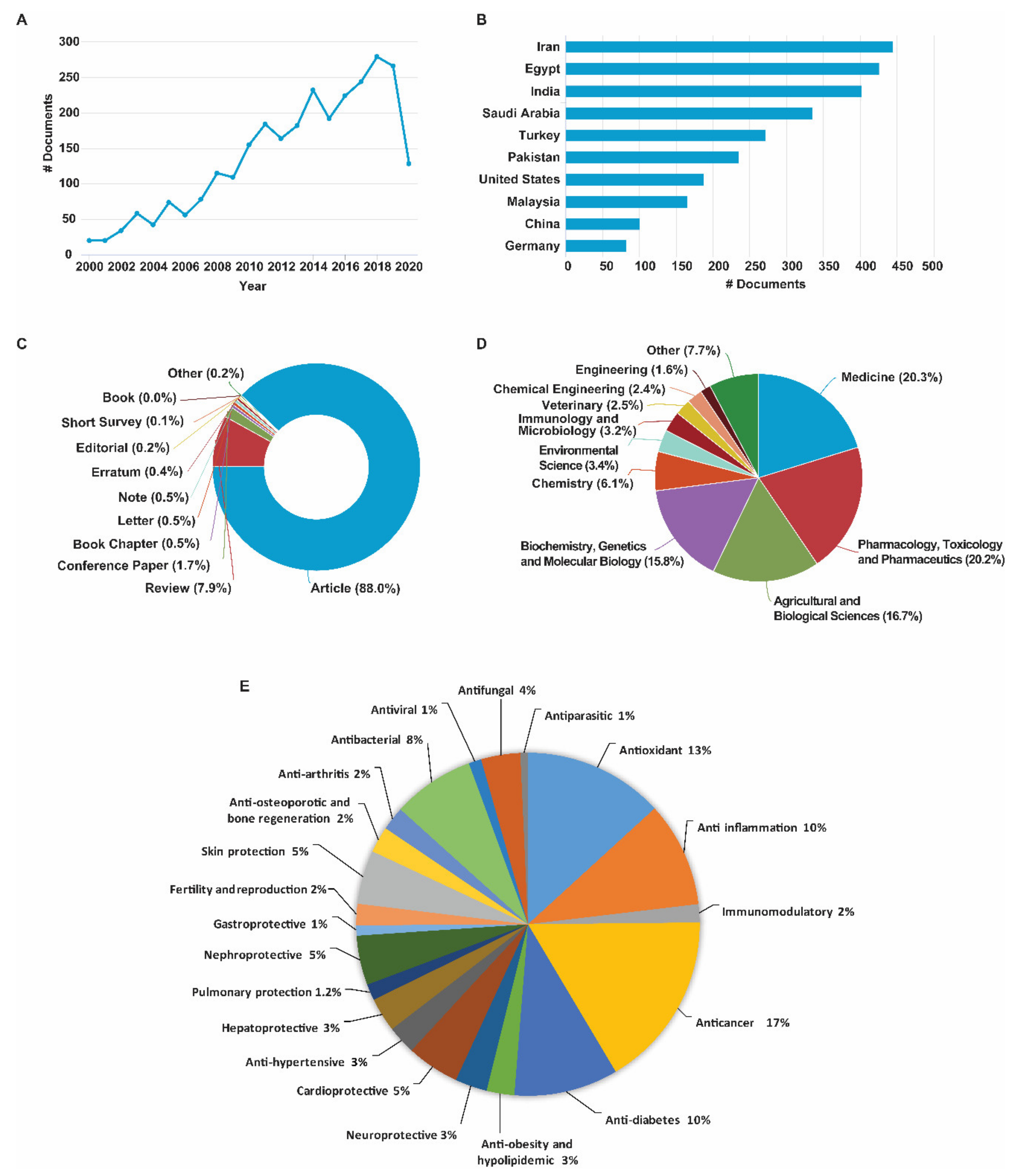



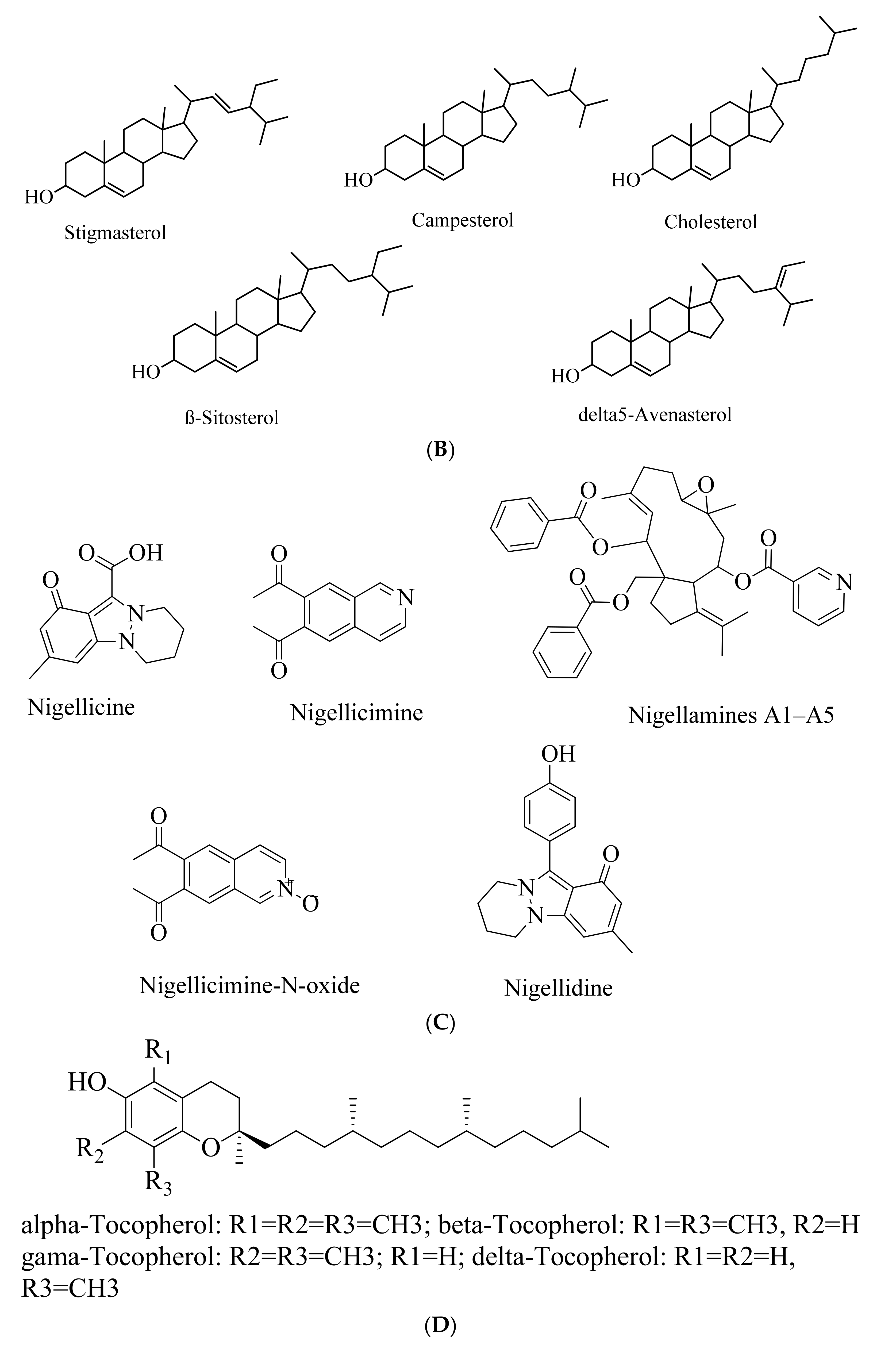

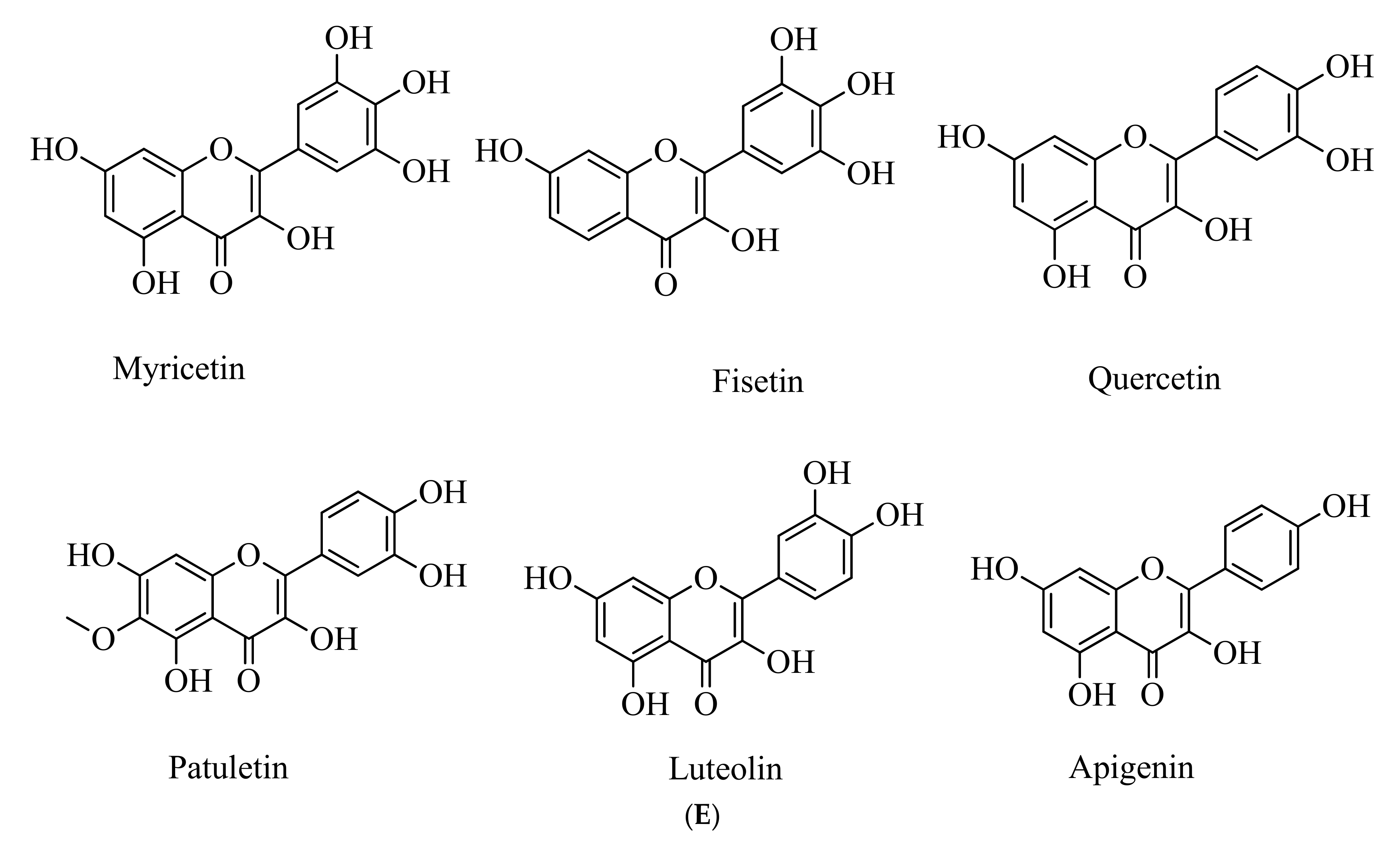



| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Seed powder (500 mg/kg) | Hyperlipidemic albino rats | ↓Serum cholesterol, Triglycerides, LDL levels; ↑HDL levels | [122] |
| Seed (1 g) (+ 1 tablespoon of honey + 10 mg atorvastatin) | Hyperlipidemic patients with a history of smoking habit | ↓TC, LDL, TG and ↑HDL | [123] |
| Seed extract (treated with vinegar for a week to make 500 mg capsule) | Double-blind, randomized placebo-controlled trial among menopausal women with metabolic syndrome | ↓LDL, TG, TC, and fasting blood sugar | [124] |
| NSO and hypoglycemic drug (1.5 mL and 3 mL) | Single-blind, randomized controlled trial on outpatients with metabolic syndrome | ↓HbA1c levels | [125] |
| Seed powder (2 g) | Patients with Hashimoto’s thyroiditis | ↑Serum TAC, SOD, HDL and ↓MDA, VCAM-1, BMI, LDL, and TG; no significant change in GPX | [126] |
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Silver nanoparticles prepared from NSO | In vitro biochemical assay | ↓α-amylase activity | [139] |
| Seed extract (125 and 250 mg/kg) | Alloxan-induced diabetic rats | ↓Glucose and MDA levels; ↑SOD and GPx; ↑diameter and amount of Langerhans islet cells | [128] |
| Seed extract (2 g/kg) | Alloxan-induced diabetic mice | ↓Blood glucose, TG, T-cholesterol, LDL-c, and TBARS; ↑HDL-c | [129] |
| Seed extract (20% or 40%) | STZ-induced diabetic rats | Shortens duration of wound healing (15 days in 40% seed extract-treated vs 27 days in untreated) | [131] |
| Seed extract (100, 200, and 400 mg/kg) | STZ-induced diabetic rat | ↓Serum glucose and lipids; ↑AIP; ↑eNOS expression; ↓VCAM-1 and LOX-1 expressions | [130] |
| Green synthesis of silver nanoparticles using seed extract (200 mg/kg) | STZ-induced model of diabetic neuropathy in rats | ↓Glucose and AGE and aldose reductase level; ↓TNF-α, NF-κB and S100B; ↓MDA and NO; ↑SOD and GSH; ↑TKr A; ↑nitrotyrosine | [132] |
| NSO (400 mg/kg) | STZ -induced diabetic rats | ↓Myositis, hyaline degeneration, and Zenker’s necrosis; ↑Bcl-2 expression | [134] |
| NSO (0.2 mg/kg) | STZ-induced diabetic rats | ↓Blood glucose; ↓Bax and Caspase 3 expression in aortic medial layer | [133] |
| NSO (100 mg/ Kg) | STZ-induced diabetic rats | ↑Insulin-like growth factor-1 and phosphoinositide-3 kinase; ↓ADAM-17; ↓blood glucose level, lipid profile, TBARS, NO, serum insulin/insulin receptor ratio, and the tumor necrosis factor | [135] |
| NSO (2 mL/kg) | STZ-induced diabetic rats | ↓FBG; ↑insulin levels; ↑pancreatic and hepatic CAT and GSH; ↑insulin immunoreactive parts % and mean pancreatic islet diameter | [136] |
| NSO (2 mL/kg) and TQ (50 mg/kg) | STZ-induced model of diabetic nephropathy | ↓Albuminuria and the kidney weight/body weight ratio; ↑podocyte-specific marker; ↓collagen IV, TGF-β1 and VEGF-A | [137] |
| NSO (2.5 mL/kg) | Alloxan-induced diabetic rabbits | ↓CAT activity, TC, TGs, LDL- cholesterol and VLDL- cholesterol levels, serum blood glucose levels and lipid contents; ↑mean body weight, HDL- cholesterol, vitamin C levels and normalized bilirubin levels | [138] |
| NSO (2.5 mL/kg) | Randomized clinical trial on patients with diabetic nephropathy accompanying chronic kidney disease | ↓Blood glucose, serum creatinine, blood urea, and 24 h total urinary protein levels; ↑glomerular filtration rate, 24 h total urinary volume, and hemoglobin level | [140] |
| NSO (1 g as two capsules) | Double-blind randomized clinical trial on T2DM patients | ↓Lipid profile and glucose level, C-reactive protein level, and lipid peroxidation | [141] |
| Seed capsules (2 g daily) | Single-blind, nonrandomized controlled clinical trials on T2DM patients | ↓TC, LDL-C, TC/HDL-C and LDL-C/HDL-C ratios; ↑serum HDL-C; ↓DBP, MAP and HR | [142] |
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Seed extract (100, 200, 400 mg/kg, i.p. for 16 days started 2 days prior to LPS injection) | LPS-induced lung damage in rats | ↓Inflammatory leukocytes and oxidative stress markers in the blood and bronchoalveolar fluid; ↓inflammatory cytokines (IFN-γ, TGF-β1, PGE2); ↑IL-4 in bronchoalveolar fluid | [185] |
| Seed extract (500 mg/kg per day) | Bleomycin-induced lung fibrosis (BMILF) in rats | ↓Pulmonary inflammation and fibrosis; ↓hydroxyproline concentration in pulmonary tissue ↓lipid peroxidation; ↑CAT | [186] |
| NSO (1 mL/kg per day, p.o. for 50 days) | BMILF in rats | ↓Inflammatory index and fibrosis score; ↑urinary secretion of histidine fumarate, allantoin and malate | [188] |
| TQ (10 and 20 mg/kg, p.o. for 28 days) | BMILF in rats | Recovers weight loss; ↓MMP-7 expression; ↓apoptotic markers (caspase 3, Bax, and Bcl-2); ↓fibrotic changes (TGF-β and hydroxyproline in lung tissues); ↑Nrf2, HO-1 and ↓TGF-β expression | [187] |
| TQ (i.p. daily for the last 21 days of a total of 90 days exposure to cigarette smoke) | Cigarette smoke-exposed chronic obstructive pulmonary disease (COPD) rats | ↓IL-8 levels; ↓↑apoptosis (hence, it requires appropriate dosing of TQ while long term TQ or DMSO exposure might have toxicity) | [189] |
| NS seed extract (+ onion extracts) for 18 weeks | Nicotine-induced lung damage in SD rats | ↓MDA level; ↑SOD, CAT, and GSH to a normal level | [190] |
| NSO (1 mL/kg of NSO through gastric gavage for 28 days one hour before CdCl2 administration) | Cadmium-induced lung damage in adult male rats | ↓Cd-induced lung damage with minimal histopathological changes in lung architecture | [191] |
| Seed extract (0.1–0.5 mg/mL) | Wistar rat peritoneal mast cells | ↓Histamine level, no toxicity on mast cells | [192] |
| TQ-rich oily preparations | Human T lymphocyte, monocyte and A549 human lung epithelial cells (in vitro models of asthma-related mediators of inflammation) | ↓IL-2, IL-6, and PGE2 in T-lymphocytes; ↓IL-6 and PGE2 in monocytes; ↑ PGE2 in A549 cells | [41] |
| Black cumin supplement (NS-1, NS-2 =1, 2 g/day respectively, for 3 months) | Randomized single-blind, placebo-controlled clinical trial with asthma patients | ↑Peak expiratory flow by 25–75%; ↓fractional exhaled nitric oxide and serum IgE; ↑serum IFN-γ,Improvement in asthma control test score | [193] |
| NSO capsules (500 mg, twice daily for 4 weeks) | Randomized single-blind, placebo-controlled clinical trial with asthma patients | ↓Blood eosinophils; ↑asthma control with an overall improvement in pulmonary function | [194] |
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| NSO (2 mL/kg BW, p.o.) | Cisplatin (CP)-induced gastrointestinal dysfunction in rats | ↓BBM enzyme activities and BBMV; ↓carbohydrate metabolism enzyme activities and enzymatic as well as non-enzymatic antioxidant parameters in the intestine; ↑intestinal redox and metabolic status; restored BBM integrity | [196] |
| TQ (1.5 mg/kg BW) | CP-induced gastrointestinal damage in rats | ↓CP induced specific activities of BBM marker enzymes; restoring the redox and metabolic status of intestinal mucosal tissue and preserving intestinal histoarchitecture | [197] |
| NSO (2 mL/kg BW, p.o.) and TQ (1.5 mg/kg BW, p.o.), for 14 days | CP-induced intestinal toxicity in rats | ↑BBM enzyme activities; ↑carbohydrate metabolism enzymes and the enzymatic as well as non-enzymatic parameters of antioxidant defense system in the intestinal mucosa | [198] |
| Pectic polysaccharide of seed (200 mg/kg BW for 10 days) | Acetic acid-induced gastric ulcers in rats. | ↑Gastric mucin content, Cox-2, PGE2, ERK-2, MMP-2; ↓MMP-9 levels; ↓H+/K+-ATPase and free radical-mediated oxidation and cellular damages that improved speed and quality of ulcer healing | [199] |
| TQ (5 mg/kg) | Fasting-induced rabbit gastric ulcer model | ↓ Volume and the total acidity of gastric secretion | [201] |
| NSO (0.2 mL/kg/day for 5 days) | Male Wistar albino rats with ischemia/ reperfusion injury (colonic anastomosis model) | ↑Serum hydroxyproline levels, IL-6 level; ↓tissue levels and serum levels of TNF-α; ↓ edema and inflammatory cell infiltration; significant difference in Chiu classification | [202] |
| Dosin (6 g/day of black cumin seed and 12 g/day of honey) | Patients with H. pylori infection | Negative UBT after intervention; ↓median and interquartile range of total dyspepsia symptoms | [203] |
| Seed powder (2 g for 6 weeks) | Patients with moderate ulcerative colitis | ↓ Stool frequency | [204] |
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) | References |
|---|---|---|---|
| Seed extract | Socket healing in rabbits | ↑Active bone formation, thick trabeculae with highly vascular bone marrow and large numerous osteocytes | [235] |
| Seed extract | Calvarial defected ovariectomy-induced osteoporosis in rat models | ↑Bone formation; ↑bone healing | [234] |
| TQ (10 mg/kg/day, p.o.) | Tibias defected male rats | ↑Ratio of new bone per total defect area and new bone trabeculae lined by active osteoblasts; ↑capillary intensity in the defect area;↑osteogenesis | [236] |
| NSO | Dental pulp MSCs isolated from 15–20 years old human patients from third molars | ↑Calcium concentrations | [237] |
| Seeds | Clinical trial with healthy patients (for evaluation of Topical NS application on Delayed Dental Implant | ↑Bone density after six months | [238] |
| Treatment with Doses | Model of Toxicity | Mechanism of Antidote Action | References |
|---|---|---|---|
| TQ (2.5, 5, 10, 15, and 20 μM) | 2-tert-Butyl-4-hydroquinone-induced cytotoxicity in human umbilical vein endothelial cells | Anti-apoptotic and anti-DNA and chromatin fragmentation effects | [288] |
| TQ (500 μM) | Chromium-induced in vitro toxicity | Act as a chelating agent | [283] |
| Ethanolic seed extract (0–25 µL) | FeSO4-induced toxicity in rat | Act as a chelating agent; Antioxidant function | [282] |
| TQ (10 mg/kg daily for one month) | Malathion-induced toxicity in rats | Antioxidant function | [284] |
| TQ (10 mg/kg BW, p.o., once daily for 28 days) | Fipronil (phenylpyrazole insecticide)-induced oxidative injury in rats | Antioxidant function | [285] |
| TQ (2.5, 5, 10 mg/kg/day) | Diazinon-induced toxicity in rats | Antioxidant function | [286] |
| TQ (20 mg/kg for three weeks) | Samsun ant (Pachycondyla sennaarensis) venom-induced acute toxic shock in male rats | Antioxidant and antiallergic functions | [287] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. https://doi.org/10.3390/nu13061784
Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, Rahman MS, Timalsina B, Munni YA, Sarker PP, et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients. 2021; 13(6):1784. https://doi.org/10.3390/nu13061784
Chicago/Turabian StyleHannan, Md. Abdul, Md. Ataur Rahman, Abdullah Al Mamun Sohag, Md. Jamal Uddin, Raju Dash, Mahmudul Hasan Sikder, Md. Saidur Rahman, Binod Timalsina, Yeasmin Akter Munni, Partha Protim Sarker, and et al. 2021. "Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety" Nutrients 13, no. 6: 1784. https://doi.org/10.3390/nu13061784
APA StyleHannan, M. A., Rahman, M. A., Sohag, A. A. M., Uddin, M. J., Dash, R., Sikder, M. H., Rahman, M. S., Timalsina, B., Munni, Y. A., Sarker, P. P., Alam, M., Mohibbullah, M., Haque, M. N., Jahan, I., Hossain, M. T., Afrin, T., Rahman, M. M., Tahjib-Ul-Arif, M., Mitra, S., ... Kim, B. (2021). Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients, 13(6), 1784. https://doi.org/10.3390/nu13061784





















