Bone Marrow Adipocytes—Role in Physiology and Various Nutritional Conditions in Human and Animal Models
Abstract
1. Introduction
2. Types of Adipose Tissue
2.1. White Adipose Tissue
2.2. Brown Adipose Tissue
2.3. Bone Marrow Adipose Tissue
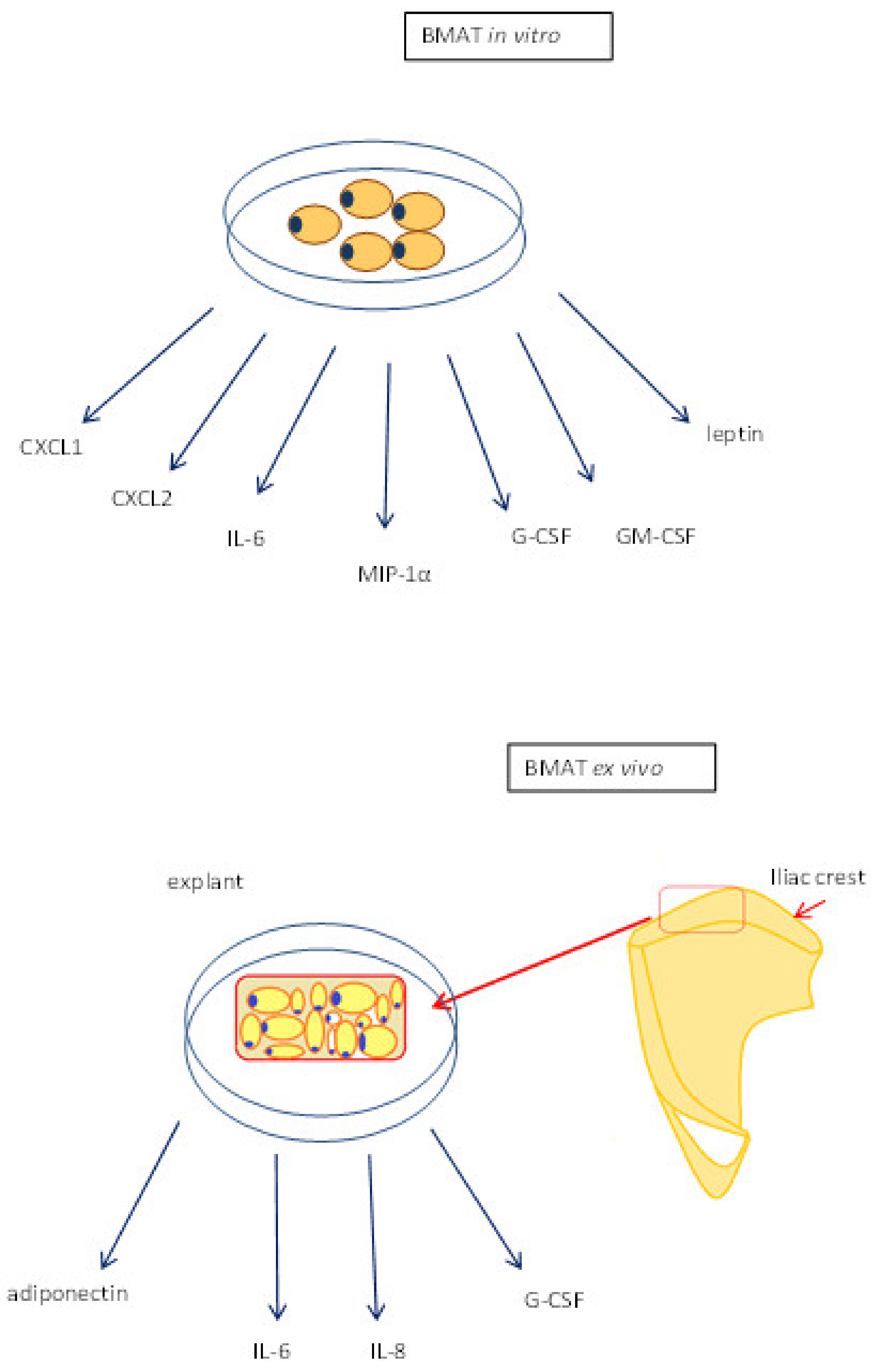
3. BMAT—Function
3.1. BMAT and Haematopoiesis
3.2. Cytokine Production
3.3. BMAT and the Endocrine System
3.4. BMAT in Metabolic Disorders
4. BMAT in Dietary Regimes
4.1. BMAT in Obesity and High Fat Diets
4.2. Dietary Restrictions and BMAT
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sulston, R.J.; Cawthorn, W.P. Bone Marrow Adipose Tissue as an Endocrine Organ: Close to The Bone? Horm. Mol. Biol. Clin. Investig. 2016, 28, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone Marrow Adipose Tissue Is an Endocrine Organ That Contributes to Increased Circulating Adiponectin during Caloric Restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Youmna, K.; Scadden, D.T. Mesenchymal Cell Contributions to the Stem Cell Niche. Cell. Stem. Cell. 2015, 16, 239–253. [Google Scholar]
- Nuttall, M.E.; Shah, F.; Singh, V.; Thomasporch, C.; Frazier, T.; Gimble, J.M. Adipocytes and the Regulation of Bone Remodeling: A Balancing Act. Calcif. Tissue Int. 2014, 94, 78–87. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Kang, K. Marrow Adipose Tissue: Its Origin, Function, and Regulation in Bone Remodeling and Regeneration. Stem. Cells Int. 2018, 7098456. [Google Scholar] [CrossRef]
- Hausman, D.B.; Digrolamo, M.; Bartness, T.J.; Hausman, G.J.; Matrin, R.J. The Biology of White Adipocyte Proliferation. Obes. Rev. 2001, 2, 239–254. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From Stem Cell to Adipocyte. Annu. Rev Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Ahmadian, M.; Wang, Y.; Sul, H.S. Lipolysis in Adipocytes. Int. J. Biochem. Cell. Biol. 2010, 42, 555–559. [Google Scholar] [CrossRef]
- Hawkes, C.P.; Mostoufi-Moab, S. Fat-Bone Interaction within the Bone Marrow Milieu: Impact on Hematopoiesis and Systemic Energy Metabolism. Bone 2019, 119, 57–64. [Google Scholar] [CrossRef]
- Rosen, E.D.; Macdougald, O.A. Adipocyte Differentiation from the Inside Out. Natrev. Mol. Cell. Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and Visceral Adipose Tissue: Structural and Functional Differences. Obesrev. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W.; Defuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte Death, Adipose Tissue Remodeling, And Obesity Complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity Increases the Production of Proinflammatory Mediators from Adipose Tissue T Cells and Compromises TCR Repertoire Diversity: Implications for Systemic Inflammation and Insulin Resistance. J. Immunol. 2010, 185, 1836–1845. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 Controls A Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nerdegaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification And Importance Of Brown Adipose Tissue In Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Galgani, J.E. The Implication of Brown Adipose Tissue for Humans. Annu. Rev. Nutr. 2011, 31, 33–47. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes Wn And Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Xu, M.; Gu, W.; Xi, Y.; Qi, L.; Li, B.; Wang, W. Brown Adipose Tissue Activation Is Inversely Related To Central Obesity And Metabolic Parameters In Adult Human. PLoS ONE 2015, 10, E0123795. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Fernández-Galilea, M.; Felix-Soriano, E.; Escoté, X.; González-Muniesa, P.; Moreno-Aliaga, M.J. Inflammation and Oxidative Stress. In Adipose Tissue Obesity Oxidative Stress and Dietary Antioxidants; Del Moral, A.M., García, C.M.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 63–92. [Google Scholar]
- Chechi, K.; Van Markenlichtenbelt, W.; Richard, D. Brown and Beige Adipose Tissues: Phenotype and Metabolic Potential in Mice and Men. J. Appl. Physiol. 2018, 124, 482–496. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Horowitz, M.C.; Macdougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.J.; Klibanski, A.J. Marrow Fat and Bone-New Perspectives. Clin. Endocrinol. Metab 2013, 98, 935–945. [Google Scholar] [CrossRef]
- Scheller, E.L.; Cawthorn, W.P.; Burr, A.A.; Horowitz, M.C.; Macdougald, O.A. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinolmetab 2016, 27, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramaniyan, K.; Lehnen, D.; Ghazanfari, R.; Sobiesiak, M.; Harichandan, A.; Mortha, E.; Petkova, N.; Grimm, S.; Cerabona, F.; De Zwart, P.; et al. Phenotypic and Functional Heterogeneity of Human Bone Marrow- And Amnion-Derived MSC Subsets. Ann. N.Y. Acad. Sci. 2012, 1266, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Kassem, M. New Factors Controlling the Balance between Osteoblastogenesis and Adipogenesis. Bone 2012, 50, 540–545. [Google Scholar] [CrossRef]
- Hardouin, P.; Rharass, T.; Lucas, S. Bone Marrow Adipose Tissue: To Be or Not to Be A Typical Adipose Tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Zhao, G.; Li, B.; Li, Y.; Cawthorn, W.P.; Macdougald, O.A.; Franceschi, R.T. Genetic Inhibition of Pparγ S112 Phosphorylation Reduces Bone Formation and Stimulates Marrow Adipogenesis. Bone 2018, 107, 1–9. [Google Scholar] [CrossRef]
- Moore, S.G.; Dawson, K.L. Red and Yellow Marrow in the Femur: Age-Related Changes in Appearance at MR Imaging. Radiology 1990, 175, 219–223. [Google Scholar] [CrossRef]
- Schellinger, D.; Lin, C.S.; Fertikh, D.; Lee, J.S.; Lauerman, W.C.; Henderson, F.; Davis, B. Normal Lumbar Vertebrae: Anatomic, Age, And Sex Variance in Subjects at Proton MR Spectroscopy-Initial Experience. Radiology 2000, 215, 910–916. [Google Scholar] [CrossRef]
- Meunier, P.; Aaron, J.; Edouard, C.; Vignon, G. Osteoporosis and the Replacement of Cell Populations of The Marrow by Adipose Tissue. A Quantitative Study of 84 Iliac Bone Biopsies. Clin. Orthop. 1971, 80, 147–154. [Google Scholar]
- Blebea, J.S.; Houseni, M.; Torigian, D.A.; Fan, C.; Mavi, A.; Zhuge, Y.; Iwanaga, T.; Mishra, S.; Udupa, J.; Zhuang, J.; et al. Structural and Functional Imaging of Normal Bone Marrow and Evaluation of Its Age-Related Changes. Seminnucl. Med. 2007, 37, 185–194. [Google Scholar] [CrossRef]
- Cornish, J.; Macgibbon, A.; Lin, J.M.; Watson, M.; Callon, K.E.; Tong, P.C.; Dunford, J.E.; Van Der Does, Y.; Williams, G.A.; Grey, A.B.; et al. Modulation of Osteoclastogenesis By Fatty Acids. Endocrinology 2008, 149, 5688–5695. [Google Scholar] [CrossRef]
- Krings, A.; Rahman, S.; Huang, S.; Lu, Y.; Czernik, P.J.; Lecka-Czernik, B. Bone Marrow Fat Has Brown Adipose Tissue Characteristics, Which Are Attenuated with Aging and Diabetes. Bone 2012, 50, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.C.; Mohan, S. Thyroid Hormone Acting Via Trβ Induces Expression of Browning Genes in Mouse Bone Marrow Adipose Tissue. Endocrine 2017, 56, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Suchacki, K.J.; Cawthorn, W.P. Molecular Interaction of Bone Marrow Adipose Tissue with Energy Metabolism. Cur. Rmolbiol. Rep. 2018, 4, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Khandaker, S.; Learman, B.S.; Cawthorn, W.P.; Anderson, L.M.; Pham, H.A.; Robles, H.; Wang, Z.; Li, Z.; Parlee, S.D.; et al. Bone Marrow Adipocytes Resist Lipolysis and Remodeling in Response Tobeta-Adrenergic Stimulation. Bone 2019, 118, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Styner, M.; Pagnotti, G.M.; Mcgrath, C.; Wu, X.; Sen, B.; Uzer, G.; Xie, Z.; Zong, X.; Styner, M.A.; Rubin, C.T.; et al. Exercise Decreases Marrow Adipose Tissue Through Ss-Oxidation in Obese Running Mice. J. Bone Min. Res. 2017, 32, 1692–1702. [Google Scholar] [CrossRef]
- Doucette, C.R.; Horowitz, M.C.; Berry, R.; Macdougald, O.A.; Anunciado-Koza, R.; Koza, R.A.; Rosen, C.J. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J. Cell. Physiol. 2015, 230, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Cuminetti, V.L. Bone Marrow Adipocytes: The Enigmatic Components of the Hematopoietic Stem Cell Niche. J. Clin. Med. 2019, 8, 707. [Google Scholar] [CrossRef]
- Lecka-Czernik, B. Marrow Fat Metabolism Is Linked to The Systemic Energy Metabolism. Bone 2012, 50, 534–539. [Google Scholar] [CrossRef]
- Devlin, M.J.; Cloutier, A.M.; Thomas, N.A.; Panus, D.A.; Lotinun, S.; Pinz, I.; Baron, R.; Rosen, C.J.; Bouxsein, M.L. Caloric Restriction Leads to High Marrow Adiposity and Low Bone Mass in Growing Mice. J. Bone Min. Res. 2010, 25, 2078–2088. [Google Scholar] [CrossRef]
- Martin, L.M.; Mccabe, L.R. Type I Diabetic Bone Phenotype Is Location but Not Gender Dependent. Histo. Chem. Cell Biol. 2007, 128, 125–133. [Google Scholar] [CrossRef]
- Poloni, A.; Maurizi, G.; Serrani, F.; Mancini, S.; Zingaretti, M.C.; Frontini, A.; Cinti, S.; Olivieri, A.; Leoni, P. Molecular and Functional Characterization of Human Bone Marrow Adipocytes. Exph. Ematol. 2013, 41, 558.E2–566.E2. [Google Scholar] [CrossRef]
- Ma, H.T.; Ren, R.; Chen, Y.; Griffith, J.F.; Leung, P.C.; Zhang, P. A Simulation Study of Marrow Fat Effect on Bone Biomechanics. Annu. IEEE. Eng. Med. Biolsoc. 2014, 2014, 4030–4033. [Google Scholar]
- Gurkan, U.A.; Akkus, O. The Mechanical Environment of Bone Marrow: A Review. Ann. Biomedeng. 2008, 36, 1978–1991. [Google Scholar] [CrossRef]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-Marrow Adipocytes as Negative Regulators of the Haematopoietic Microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef]
- Spindler, T.J.; Tseng, A.W.; Zhou, X.; Adams, G.B. Adipocytic Cells Augment the Support of Primitive Hematopoietic Cells In Vitro But Have No Effect in The Bone Marrow Niche under Homeostatic Conditions. Stem Cells Dev. 2014, 23, 434–441. [Google Scholar] [CrossRef]
- Mirantes, C.; Passegue, E.; Pietras, E.M. Pro-Inflammatory Cytokines: Emerging Players Regulating HSC Function in Normal and Diseased Hematopoiesis. Exp. Cell Res. 2014, 329, 248–254. [Google Scholar] [CrossRef]
- Gunaratnam, K.; Vidal, C.; Gimble, J.M.; Duque, G. Mechanisms of Palmitate-Induced Lipotoxicity in Human Osteoblasts. Endocrinology 2014, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Baxter, J.; Satapati, S.; Burgess, S.C. The Effect of Short-Term Fasting on Liver and Skeletal Muscle Lipid, Glucose, and Energy Metabolism in Healthy Women and Men. J. Lipidres 2012, 53, 577–586. [Google Scholar] [CrossRef]
- Scheller, E.L.; Doucette, C.R.; Learman, B.S.; Cawthorn, W.P.; Khandaker, S.; Schell, B.; Wu, B.; Ding, S.Y.; Bredella, M.A.; Fazeli, P.K.; et al. Region-Specific Variation in The Properties of Skeletal Adipocytes Reveals Regulated and Constitutive Marrow Adipose Tissues. Nat. Commun. 2015, 6, 7808. [Google Scholar] [CrossRef]
- Corre, J.; Barreau, C.; Cousin, B.; Chavoin, J.P.; Caton, D.; Fournial, G.; Penicaud, L.; Casteilla, L.; Laharrague, P. Human Subcutaneous Adipose Cells Support Complete Differentiation but Not Self-Renewal of Hematopoietic Progenitors. J. Cell Physiol. 2006, 208, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Laharrague, P.; Larrouy, D.; Fontanilles, A.M.; Truel, N.; Campfield, A.; Tenenbaum, R.; Galitzky, J.; Corberand, J.X.; Pénicaud, L.; Casteilla, L. High Expression of Leptin by Human Bone Marrow Adipocytes in Primary Culture. FASEB J. 1998, 12, 747–752. [Google Scholar] [CrossRef]
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Marrow Adipocyte-Derived CXCL1 and CXCL2 Contribute to Osteolysis In Metastatic Prostate Cancer. Clin. Exp. Metastasis. 2015, 32, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Shen, W.J.; Ueno, M.; Patel, S.; Kraemer, F.B. Characterization of Age-Related Gene Expression Profiling in Bone Marrow and Epididymal Adipocytes. BMC Genom. 2011, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Tencerova, M.; Figeac, F.; Ditzel, N.; Taipaleenmaki, H.; Nielsen, T.K.; Kassem, M. High-Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J. Bone Min. Res. 2018, 33, 1154–1165. [Google Scholar] [CrossRef]
- Trudel, G.; Payne, M.; Madler, B.; Ramachandran, N.; Lecompte, M.; Wade, C.; Biolo, G.; Blanc, S.; Hughson, R.; Bear, L.; et al. Bone Marrow Fat Accumulation After 60 Days of Bed Rest Persisted 1 Year After Activities Were Resumed Along with Hemopoietic Stimulation: The Women International Space Simulation for Exploration Study. J. Appl. Physiol. 2009, 107, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, C.; Chen, Y.; Ji, X.; Chen, X.; Tian, L.; Yu, X. Preservation of High-Fat Diet-Induced Femoral Trabecular Bone Loss Through Genetic Target of TNF-Alpha. Endocrine 2015, 50, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tian, L.; Zhang, K.; Chen, Y.; Chen, X.; Xie, Y.; Zhao, Q.; Yu, X. Interleukin-6 Gene Knockout Antagonizes High-Fat-Induced Trabecular Bone Loss. J. Mol. Endocrinol. 2016, 57, 161–170. [Google Scholar] [CrossRef]
- Tavassoli, M.; Marrow Adipose Cells. Histochemical Identification of Labile and Stable Components. Arch. Pathol. Lab. Med. 1976, 100, 16–18. [Google Scholar]
- Menagh, P.J.; Turner, R.T.; Jump, D.B.; Wong, C.P.; Lowry, M.B.; Yakar, S.; Rosen, C.J.; Iwaniec, U.T. Growth Hormone Regulates the Balance Between Bone Formation and Bone Marrow Adiposity. J. Bone Min. Res. 2010, 25, 757–768. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Failure to Generate Bone Marrow Adipocytes Does Not Protect Mice from Ovariectomy-Induced Osteopenia. Bone 2013, 53, 145–153. [Google Scholar] [CrossRef]
- Syed, F.A.; Oursler, M.J.; Hefferanm, T.E.; Peterson, J.M.; Riggs, B.L.; Khosla, S. Effects of Estrogen Therapy on Bone Marrow Adipocytes in Postmenopausal Osteoporotic Women. Osteoporosint 2008, 19, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Dutchak, P.A.; Wang, X.; Ding, X.; Wang, X.; Bookout, A.L.; Goetz, R.; Mohammadi, M.; Gerard, R.D.; Dechow, P.C.; et al. Fibroblast Growth Factor 21 Promotes Bone Loss by Potentiating the Effects of Peroxisome Proliferator-Activated Receptor Γ. Proc. Natl. Acad. Sci. USA 2012, 109, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Devogelaer, J.P.; Maldague, B.; Houssiau, F.A. Fat Conversion of Femoral Marrow in Glucocorticoid-Treated Patients: A Cross-Sectional and Longitudinal Study with Magnetic Resonance Imaging. Arthritis. Rheum. 1999, 42, 1405–1411. [Google Scholar] [CrossRef]
- Polineni, S.; Resulaj, M.; Faje, A.T.; Meenaghan, E.; Bredella, M.A.; Bouxsein, M.; Lee, H.; Macdougald, O.A.; Klibanski, A.; Fazeli, P.K. Red and White Blood Cell Counts Are Associated with Bone Marrow Adipose Tissue, Bone Mineral Density, and Bone Microarchitecture in Premenopausal Women. J. Bone Min. Res. 2020, 35, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Maridas, D.E.; Rendina-Ruedy, E.; Helderman, R.C.; Demambro, V.E.; Brooks, D.; Guntur, A.R.; Lanske, B.; Bouxsein, M.L.; Rosen, C.J. Progenitor Recruitment and Adipogenic Lipolysis Contribute to The Anabolic Actions of Parathyroid Hormone on The Skeleton. FASEB J. 2019, 33, 2885–2898. [Google Scholar] [CrossRef]
- Suresh, S.; Alvarez, J.C.; Dey, S.; Noguchi, C.T. Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity. Int. J. Mol. Sci. 2020, 21, 1657. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Parlee, S.D.; Pham, H.A.; Learman, B.S.; Redshaw, C.M.; Sulston, R.J.; Burr, A.A.; Das, A.K.; Simon, B.R.; et al. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated with Increased Circulating Glucocorticoids and Not with Hypoleptinemia. Endocrinology 2016, 157, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; Della Fera, M.A.; Choi, Y.H.; Hartzell, D.; Pennington, C.; Baile, C.A. Injections of Leptin into Rat Ventromedial Hypothalamus Increase Adipocyte Apoptosis in Peripheral Fat and in Bone Marrow. Cell Tissue Res. 2007, 327, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Li, Q.; Rayalam, S.; Hartzell, D.L.; Della-Fera, M.A.; Hamrick, M.W.; Baile, C.A. Central Leptin Versus Ghrelin: Effects on Bone Marrow Adiposity and Gene Expression. Endocrine 2010, 37, 115–123. [Google Scholar] [CrossRef]
- Bartell, S.M.; Rayalam, S.; Ambati, S.; Gaddam, D.R.; Hartzell, D.L.; Hamrick, M.; She, J.X.; Della-Fera, M.A.; Baile, C.A. Central (ICV) Leptin Injection Increases Bone Formation, Bone Mineral Density, Muscle Mass, Serum IGF-1, and the Expression of Osteogenic Genes in Leptin-Deficient Ob/Ob Mice. J. Bone Min. Res. 2011, 26, 1710–1720. [Google Scholar] [CrossRef]
- Ainslie, D.A.; Morris, M.J.; Wittert, G.; Turnbull, H.; Proietto, J.; Thorburn, A.W. Estrogen Deficiency Causes Central Leptin Insensitivity and Increased Hypothalamic Neuropeptide Y. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1680–1688. [Google Scholar] [CrossRef]
- Martin, R.B.; Chow, B.D.; Lucas, P.A. Bone Marrow Fat Content in Relation to Bone Remodeling and Serum Chemistry in Intact and Ovariectomized Dogs. Calcif. Tissue Int. 1990, 46, 189–194. [Google Scholar] [CrossRef]
- Scheller, E.L.; Song, J.; Dishowitz, M.I.; Soki, F.N.; Hankenson, K.D.; Krebsbach, P.H. Leptin Functions Peripherally to Regulate Differentiation of Mesenchymal Progenitor Cells. Stem Cells 2010, 28, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The Different Shades of Fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Della-Fera, M.A.; Choi, Y.H.; Pennington, C.; Hartzell, D.; Baile, C.A. Leptin Treatment Induces Loss of Bone Marrow Adipocytes and Increases Bone Formation in Leptin-Deficient Ob/Ob Mice. J. Bone Min. Res. 2005, 20, 994–1001. [Google Scholar] [CrossRef]
- Slade, J.M.; Coe, L.M.; Meyer, R.A.; Mccabe, L.R. Human Bone Marrow Adiposity Is Linked with Serum Lipid Levels Not T1-Diabetes. J. Diabetes Complicat. 2012, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V. Marrow Fat and Bone: Review of Clinical Findings. Front. Endocrinol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Baum, T.; Yap, S.P.; Karampinos, D.C.; Nardo, L.; Kuo, D.; Burghardt, A.J.; Masharani, U.B.; Schwartz, A.V.; Li, X.; Link, T.M. Does Vertebral Bone Marrow Fat Content Correlate with Abdominal Adipose Tissue, Lumbar Spine Bone Mineral Density, and Blood Biomarkers in Women with Type 2 Diabetes Mellitus? J. Magn. Reson. Imaging 2012, 35, 117–124. [Google Scholar] [CrossRef]
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Rosen, C.J.; Klibanski, A.; Miller, K.K. Vertebral Bone Marrow Fat Is Positively Associated with Visceral Fat and Inversely Associated With IGF-1 In Obese Women. Obesity 2011, 19, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Lin, E.; Gerweck, A.V.; Landa, M.G.; Thomas, B.J.; Torriani, M.; Bouxsein, M.L.; Miller, K.K. Determinants of Bone Microarchitecture and Mechanical Properties in Obese Men. J. Clin. Endocrinol. Metab. 2012, 97, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Casamassima, F.; Ruggiero, C.; Caramella, D.; Tinacci, E.; Villari, N.; Ruggiero, M. Hematopoietic Bone Marrow Recovery after Radiation Therapy: MRI Evaluation. Blood 1989, 73, 1677–1681. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of Fat Cell Turnover in Humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The Developmental Origins of Adipose Tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Burghardt, A.J.; Yap, S.P.; Baum, T.; Schwartz, A.V.; Joseph, G.B.; Link, T.M. Increased Cortical Porosity in Type 2 Diabetic Postmenopausal Women with Fragility Fractures. J. Bone Min. Res. 2013, 28, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Watanabe, K.; Maki, K. Serum Leptin Levels Negatively Correlate with Trabercular Bone Mineral Density in High- Fat Diet-Induced Obesity Mice. J. Musculoskelet. Neuronal Interact. 2012, 12, 84–94. [Google Scholar] [PubMed]
- Develin, M.J.; Robbins, A.; Cosman, M.N.; Moursi, C.A.; Cloutier, A.M.; Louis, L.; Van Vliet, M.; Conlon, C.; Bouxsein, M.L. Differential Effects of High Fat Diet and Diet-Induced Obesity on Skeletal Acquisition in Female C57BL/6 Vs.FBV/NJ Mice. Bone Rep. 2016, 88, 204–214. [Google Scholar]
- Bonnet, N.; Somm, E.; Rosen, C.J. Diet and Gene Interactions Influence the Skeletal Response to Polyunsaturated Fatty Acids. Bone 2014, 68, 100–107. [Google Scholar] [CrossRef]
- Halade, G.V.; Rahman, M.; Williams, P.J.; Fernandes, G. Conbination of Conjugated Linoleic Acid with Fish Oil Prevents Age-Associated Bone Marrow Adiposity in C57Bl/6J Mice. J. Nutr. Biochem. 2011, 22, 459–469. [Google Scholar] [CrossRef]
- Newton, A.L.; Hanks, L.J.; Davis, M.; Casazza, K. The Relationship among Total Body Fat, Bone Mineral Content and Bone Marrow Adipose Tissue in Early-Pubertal Girls. Bonekey. Rep. 2013, 315. [Google Scholar] [CrossRef][Green Version]
- De Araújo, I.M.; Salmon, C.E.; Nahas, A.K.; Nogueira-Barbosa, M.H.; Elias, J., Jr.; De Paula, F.J. Marrow Adipose Tissue Spectrum in Obesity and Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2017, 176, 21–30. [Google Scholar] [CrossRef]
- De Paula, F.J.; De Araújo, I.M.; Carvalho, A.L.; Elias, J., Jr.; Salmon, C.E.; Nogueira-Barbosa, M.H. The Relationship of Fat Distribution and Insulin Resistance with Lumbar Spine Bone Mass in Women. PLoS ONE 2015, 10, E0129764. [Google Scholar] [CrossRef]
- Yu, E.W.; Greenblatt, L.; Eajazi, A.; Torriani, M.; Bredella, M.A. Marrow Adipose Tissue Composition in Adults with Morbid Obesity. Bone 2017, 97, 38–42. [Google Scholar] [CrossRef]
- Blom-Høgestøl, I.K.; Mala, T.; Kristinsson, J.A.; Hauge, E.M.; Brunborg, C.; Gulseth, H.L.; Eriksen, E.F. Changes in Bone Marrow Adipose Tissue One Year After Roux-En-Y Gastric Bypass: A Prospective Cohort Study. J. Bone Min. Res. 2019, 34, 1815–1823. [Google Scholar] [CrossRef]
- Bredella, M.A.; Greenblatt, L.; Eajazi, A.; Torriani, M.; Yu, E.W. Effects of Roux-En-Y Gastric Bypass and Sleeve Gastrectomy on Bone Mineral Density and Marrow Adipose Tissue. Bone 2017, 95, 85–90. [Google Scholar] [CrossRef]
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Currdiab. Rep. 2017, 17, 123. [Google Scholar] [CrossRef]
- Bredella, M.A.; Fazeli, P.K.; Daley, S.M.; Miller, K.K.; Rosen, C.J.; Klibanski, A.; Torriani, M. Marrow Fat Composition in Anorexia Nervosa. Bone 2014, 66, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ecklund, K.; Vajepeyam, S.; Mulkern, R.V.; Feldman, H.A.; O’Donnell, J.M.; Divasta, A.D.; Gordon, C.M. Bone Marrow Fat Content In 70 Adolescent Girls with Anorexia Nervosa: Magnetic Resonance Imaging and Magnetic Resonance Spectroscopy Assessment. Pediatr. Radiol. 2017, 47, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Tulsiani, S.S.; Campoverde, K.J.; Mitchell, D.M.; Slattery, M.; Schorr, M.; Miller, K.K.; Bredella, M.A.; Misra, M.; Klibanski, A. Impaired Bone Strength Estimates at The Distal Tibia and Its Denerminants In Adolescence with Anorexia Nervosa. Bone 2018, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fazelli, P.K.; Faje, A.T.; Bredella, M.A.; Polineni, S.; Russel, S.; Resulaj, M.; Rosen, C.J.; Klibanski, A. Changes in Marrow Adipose Tissue with Short-Term Changes in Wiegth In Premenopausal Women With Anorexia Nervosa. EJ. Endocrinol. 2019, 180, 189–199. [Google Scholar]
- Abella, E.; Feliu, E.; Granada, I.; Millá, F.; Oriol, A.; Ribera, J.M.; Sánchez-Planell, L.; Berga, L.I.; Reverter, J.C.; Rozman, C. Bone Marrow Changes in Anorexia Nervosa Are Correlated with The Amount of Weight Loss and Not with Other Clinical Findings. Am. J. Clinpathol. 2002, 118, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Hariz, A.; Hamdi, M.S.; Boukhris, I.; Boujelbène, N.; Azzabi, S.; Khalfallah, N. Gelatinous Transformation of Bone Marrow in A Patient with Anorexia Nervosa: An Uncommon but Reversible Etiology. Am. J. Case Rep. 2018, 19, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, C.; Jia, J.; Zhang, C.; Yuan, W.; Leng, H.; Xu, Y.; Song, C. Short-Term Caloric Restriction Induced Bone Loss in Both Axial and Appendicular Bones by Increasing Adiponectin. Ann. N.Y. Acad. Sci. 2020, 1474, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Zgutka, K.; Kupnicka, P.; Chlubek, D.; Pawlik, A.; Baranowska-Bosiacka, I. Analysis of Bone Mineral Profile After Prolonged Every-Other-Day Feeding in C57BL/6J Male and Female Mice. Biol. Trace Elem. Res. 2020, 194, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mcgrath, C.; Sankaran, J.S.; Misaghian-Xanthos, N.; Sen, B.; Xie, Z.; Styner, M.A.; Zong, X.; Rubin, J.; Styner, M. Exercise Degrades Bone in Caloric Restriction, Despite Suppression of Marrow Adipose Tissue (MAT). J. Bone Min. Res. 2020, 35, 106–115. [Google Scholar] [CrossRef]
- Duque, G.; Saedi, A.; Rivas, D.; Miard, S.; Ferland, G.; Picard, F.; Gaudreau, P. Differential Effects of Long-Term Caloric Restriction and Dietary Protein Source on Bone and Marrow Fat of The Aging Rat. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Increased BMAT | Decreased BMAT |
|---|---|
| Ageing | Weight loss (gastric bypass) |
| Diet regimes (HFD, CR) | Oestrogen administration |
| Glucocorticoids | PTH administration (and Scl-Ab) (mice rats) |
| T1DM (mice), T2DM (mice) | EPO administration (mice) |
| Decreased oestrogen (ovariectomy—mice and rats) | Vitamin D3 administration |
| Anorexia nervosa | GH, IGF-1 |
| obesity |
| Species/ Strain | Age/Gender | Study Length | Type of Fat | Effects in Bones | Reference |
|---|---|---|---|---|---|
| Mice C57BL/6J | 12 months females | 6 months | linoleic acid + fish oil | ↑BMD, ↓BMAT, ↓total fat mass | [90] |
| corn oil | ↑BMAT | ||||
| Mice C57BL/6J | 6 weeks males | 12 weeks | 62% lard | ↓cortical bone cross-section area, ↑BMAT, ↓BMD | [87] |
| Mice C57BL/6J C3H-6T | 10 weeks females | sacrificed in 12th month of age | 22% fish oil | ↑BMAT in spine, ↓BMD more pronounced in 6T strain than in B6, | [89] |
| 22% safflower oil | prevented weight gain and bone loss in spine | ||||
| Mice C57BL/6J | 3 weeks males | 12 weeks | 60% lard | ↑BMAT volume, ↑total body weight, ≈ BMD | [38] |
| males and females | short term 2 weeks | 58% lard | ↑BMAT, ↑body weight | ||
| Mice C57BL/6J | 3 weeks females | 3,8,17 weeks | 39% lard+ 6% soybean oil | ↑BMAT, ↑body weight, ↓ trabecular bone architecture, ↓femoral cortical bone acquisition | [88] |
| Mice FVB | ≈ BMAT, body weight, | ||||
| Mice C57Bl/6J | 8 weeks males | 12 and 20 weeks | 6% fat from lard | ↑BMAT volume, ↓BMD, ↓ amount of stem cells in bone marrow | [56] |
| Species/ Strain | Age/ Gender | Study Length | Type of Restriction | Effects in Bones | Reference |
|---|---|---|---|---|---|
| Mice C57BL/6J Apo-/- | 11 weeks males | 4–12 weeks | 30% CR | ↓bone quality, ↓BM, ↑adiponectin in BMAT No influence of CR in apo-/- | [104] |
| Mice C57BL/6J | 3 weeks males | 3–9 weeks | 30% CR | ↓trabecular volume, leptin,↓ osteoblast number,↑trabecular separation, ↑ bone resorption, ↑BMAT | [41] |
| Mice C57BL/6J | 9 weeks males and females | 6 weeks | 30% CR | Males: ↓ BW,↓leptin,↑rBMAT (tibia), ↑adiponectin, ↑Glucocorticoids Females: ≈TBF, ≈leptin, ↑rBMAT (tibia), | [69] |
| Rabbits | 6 weeks males | 7 weeks | 30% CR | ≈adiponectin,↓BW,↓leptin, ↓bone quality, ≈BMAT | |
| Mice C57BL/6J | 4 weeks Males and females | 9 months | every-other day feeding | ↑BMAT in females, ≈mineral content in long bones | [105] |
| Mice C57BL/6J | 11 weeks females | 6 weeks + voluntary running | 30% CR | ↓bone quality, ↑BMAT (femurs), ↑CD36 | [106] |
| Sprague- Downey rats | 8 months of age | 12 months | 40% CR | ↓ BW, ↓bone quality, ↓leptin, ↑rBMAT(tibia), | [107] |
| Sprague- Downey rats | Males and females | 48h | fasting | ↓body mass, ↓BMAT in tibia | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, K.; Tarnowski, M. Bone Marrow Adipocytes—Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients 2021, 13, 1412. https://doi.org/10.3390/nu13051412
Piotrowska K, Tarnowski M. Bone Marrow Adipocytes—Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients. 2021; 13(5):1412. https://doi.org/10.3390/nu13051412
Chicago/Turabian StylePiotrowska, Katarzyna, and Maciej Tarnowski. 2021. "Bone Marrow Adipocytes—Role in Physiology and Various Nutritional Conditions in Human and Animal Models" Nutrients 13, no. 5: 1412. https://doi.org/10.3390/nu13051412
APA StylePiotrowska, K., & Tarnowski, M. (2021). Bone Marrow Adipocytes—Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients, 13(5), 1412. https://doi.org/10.3390/nu13051412






