Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies
Abstract
1. Introduction
2. Pathophysiology of OA
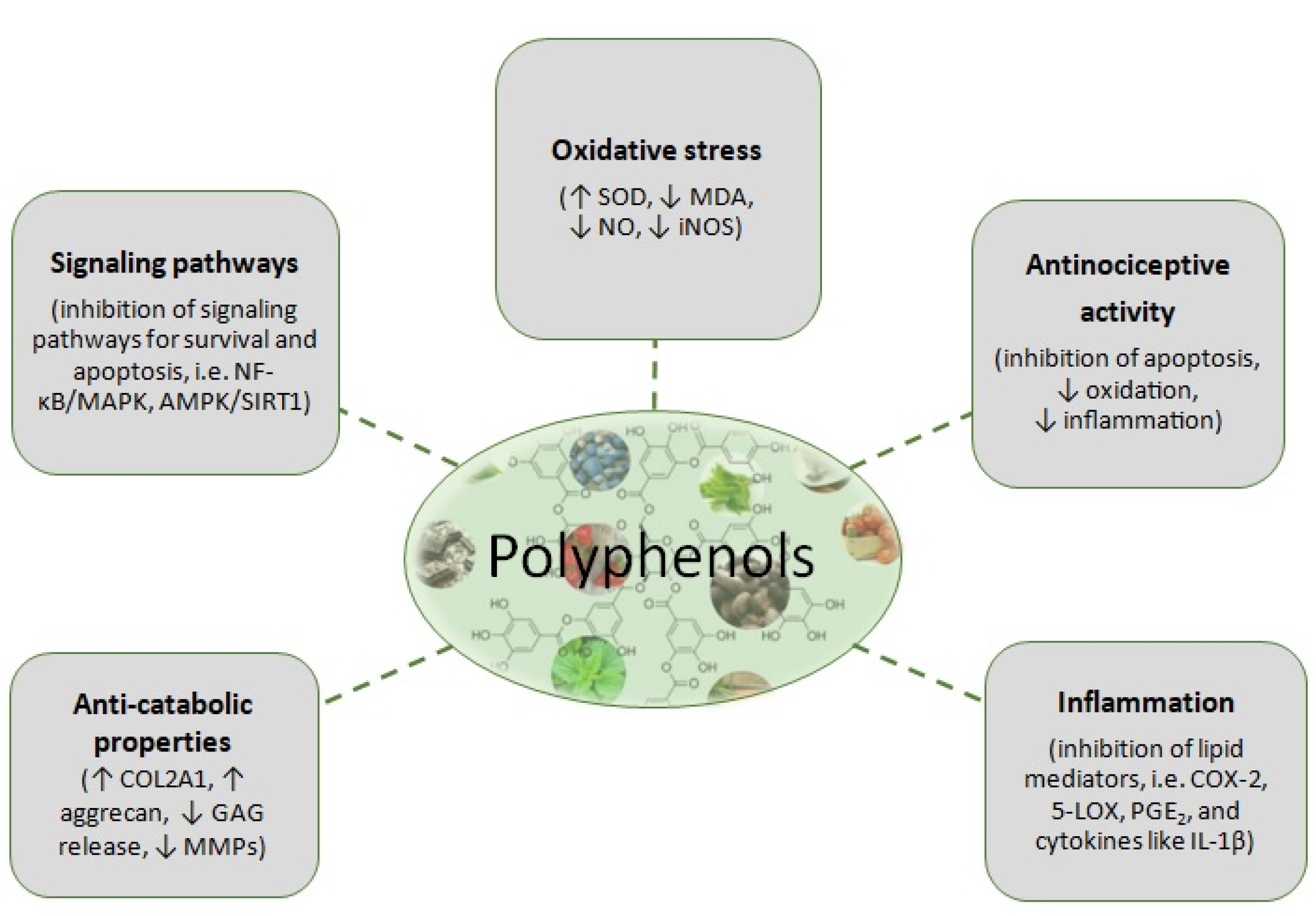
3. Polyphenols
4. Synergy/Pharmaceuticals
5. Preclinical and Clinical Studies
5.1. Grapeseed and Olive Extracts
5.2. Astragalus membranaceus and Lithospermum erythrorhizon Extracts (ALM16)
5.3. Terminalia chebula, Curcuma longa, and Boswellia serrata Extracts (LI73014F2)
5.4. Combinations with Curcumin
5.5. Kaempferol and Apigenin
5.6. Turmeric Extract, Black Pepper, and Ginger
6. Conclusive Remarks and Future Perspectives
| First Author, Year | Composition of Pharmaceuticals | Experimental Design | Results |
|---|---|---|---|
| Grapeseed and olive extract (OPCO) | |||
| Wauquier et al. 2019 [48] (in vitro, ex vivo) | (1) PHO extract (Olivex®, Grap’sud, Cruviers-Lascours, France), derived from olive, 6.5% of hydroxytyrosol/21% total polyphenol content; (2) OPC extract (Exgrape® SEED, Grap’sud, Cruviers-Lascours, France), derived from grape seed, 30% of procyanidines/90% total polyphenol content; and (3) OPCO extract (Oleogrape® SEED, Grap’sud, Cruviers-Lascours, France), derived from grapeseed and olive, 6.5% of hydroxytyrosol, 30% of procyanidines/90% total polyphenol content | HACs pretreated with grape extract, olive extract, or OPCO (10 µg/mL) for 24 h and then cotreated with IL-1β (1 ng/mL) for 24 h Fasted volunteers (n = 20) received 3.2 g of OPCO extract. Enriched serum with OPCO metabolites was collected at 100 min postingestion (peak absorption) HACs pretreated with 10% of human-enriched serum and then cotreated with IL-1β (1 ng/mL) for 24 h | Treatment with OPCO extract: 🖗IL-1β induced production of MMP-13, NO, PGE2 (anti-inflammatory effect) 🖗IL-1β mediated activation of the NF-κB p65 signaling pathway by reducing p65 translocation to the nucleus Treatment with human-enriched sera: 🖗IL-1β induced production of MMP-13, NO, PGE2 |
| Astragalus membranaceus and Lithospermum erythrorhizon extracts (ALM16) | |||
| Choi et al. 2019 [49] (in vitro, in vivo) | A.membranaceus and L. erythrorhizon extracts powder mixed together at a ratio of 7:3 (w/w) to prepare the final extract mixture (ALM16). Active compounds of ALM16 as quantified by HPLC-DAD were calycosin (0.571 mg/g), calycosin-7-O-β-D-glucoside (0.809 mg/g), and lithospermic acid (0.168 mg/g). | Human SW1353 chondrosarcoma cells pretreated with A. membranaceus extract (A), L. erythrorhizon extract (L) and a herbal mixture (7:3) of ethanol extracts of A & L (ALM16) (0, 25, 50, 100, 200 μg/mL) for 30 min and co-treated with IL-1β (20 ng/mL) for 24 h Male ICR mice (5 groups, n = 8/group) were administered: A (400 mg/kg b.w.), L (400 mg/kg b.w.), ALM16 (100, 200 and 400 mg/kg b.w.), or celecoxib™ (100 mg/kg b.w.) 1 h prior to the acetic acid injection MIA-injected rats (5 groups, n = 8/group) were treated once daily for 14 days with: 1. Normal, 2. control, 3. JOINS™ (extracts of oriental herbs as a positive control, 200 mg/kg b.w.), 4. A extract (400 mg/kg b.w.), 5. L extract (400 mg/kg b.w.), and 6–8. ALM16 (100, 200 and 400 mg/kg b.w., respectively) | In cells treated with ALM16: 🖗IL-1β induced production of MMPs (−1, −3 and −13) in a dose-dependent manner 🖗IL-1β induced GAG releasing In mice receiving ALM16: 🖗 Acetic acid-induced writhing response in mice (anti-analgesic effect) 🖗Carrageenan-induced paw edema in mice in ALM16 group in a dose-dependent manner (anti-edematous effect) In rats receiving ALM16: 🖞Paw withdrawal thresholds in MIA-induced OA rats in ALM16 group (change of mechanical allodynia) 🖗Histopathological changes (arthritis score) in MIA induced OA rats in 200 mg/kg ALM16 group |
| Terminalia chebula, Curcuma longa, and Boswellia serrata extracts (LI73014F2) | |||
| Karlapudi et al. 2018 [50] (in vivo) | LI73014F2 comprises of the aqueous extract of T. chebula fruit and the alcohol extract of C. longa rhizome, and B. serrata extract at 2:1:2 ratio. Through HPLC, the major components were identified to be 1% gallic acid, 0.5% ellagic acid, 2.0% total curcuminoids, and 0.6% AKBA. For the purpose of the study, LI73014F2 was encapsulated in hard gelatin capsules with excipients microcrystalline cellulose powder (8%) and syloid (2%). | MIA induced OA in Sprague Dawley rats (n = 30, 5 groups): 1. Normal control, 2. MIA induced, 3. LI73014F2 (250 mg/kg), 4. LI73014F2 (500 mg/kg), 5. tramadol hydrochloride (10 mg/kg), 28 days A 90-day RCT, n = 105 randomized into 3 groups: Placebo (n = 35), 200 mg/day LI73014F2 (n = 35), 400 mg/day LI73014F2 (n = 35) | In rats receiving LI73014F2: 🖞Weight-bearing capacity (pain relief) 🖗Thermal hyperalgesia (pain perception) In humans receiving LI73014F2: 🖗WOMAC scores (pain, stiffness, physical function), VAS scores, LFI |
| Kim et al. 2020, a [51] | LI73014F2 and the individual extracts were obtained from Laila Nutraceuticals | HACs cotreated with individual extracts of T. chebula (TCE; 50 μg/mL), C. longa (CLE; 50 μg/mL), B. serrata (BSE; 50 μg/mL), LI73014F2 (50 µg/mL) and IL-1β (10 ng/mL) for 24 h HACs co-treated with LI73014F2 (0, 12.5, 25, 50 μg/mL) and IL-1β (10 ng/mL) for 24 h HACs pretreated with LI73014F2 (12.5, 25, 50 μg/mL) for 24 h and then co-treated IL-1β (10 ng/mL) for 30 min | In LI73014F2 treated chondrocytes: 🖗IL-1β induced expression of COX-2, mPGES-1, PGE2, 5-LOX and LTB4 🖗IL-1β induced expression of TNF-α, IL-6 🖗IL-1β induced production of MMP-2, -3, -9, -13 🖗IL-1β induced expression of Bax/Bcl-2, caspase-3,-9, PARP (apoptotic markers) 🖗IL-1β induced phosphorylation of NF-κB p65 and p38 MAPK |
| Kim et al. 2020, b [52] | Through HPLC, the composition of LI73014F2 was determined to be gallic acid (18 mg/g), total curcuminoids (35 mg/g), and AKBA (9 mg/g). | MIA induced OA in Sprague Dawley rats (6 groups, n = 8 rats/group); 1. control + vehicle, 2. MIA-induced control + vehicle, 3. MIA + 25 mg/kg LI73014F2, 4. MIA + 50 mg/kg LI73014F2, 5. MIA + 100 mg/kg LI73014F2, 6. MIA + 20 mg/kg ibuprofen for 21 days | In LI73014F2 groups: 🖞Weight-bearing capabilities (pain relief) 🖗Arthritis index In synovial fluid from LI73014F2 groups: 🖗Production of IL-1β In articular cartilage from LI73014F2 groups: 🖗Expression of IL-1β, IL-6, TNF-α, COX-2, 5-LOX, PGE2, LTB4, MMP-2, -3, -9 🖗Mankin score (structural OA related morphological changes) |
| Curcumin+ | |||
| D’Ascola et al. 2019 [53] (in vitro) | Flavocoxid, a FDA-regulated medical food known as Limbrel® of Primus Pharmaceuticals, is a mixed extract of Scutellaria baicalensis and Acacia catechu rich in the flavonoids baicalin and catechin | HACs treated with LPS (2 μg/mL) or with IL-1β (10 ng/mL) alone or in combination with curcumin (0.65, 1.25, 2.5, 5, and 10 μg/mL) and with flavocoxid (4, 8, 16, 32, and 64 μg/mL) HACs treated with LPS (2 μg/mL) or with IL-1β (10 ng/mL) alone or in combination with curcumin at the same doses reported above and with BCP (1.25, 2.5, 5, 10, or 20 μg/mL) | Curcumin + Flavocoxid, Curcumin + BCP 🖗LPS induced expression of NF-κB and STAT3 mRNA 🖗IL-1β or LPS induced decrease of expression of COL2A1 |
| Kaempferol & Apigenin | |||
| Estakhri et al. 2020 [54] (in vivo) | - | ACTL-induced OA in Sprague Dawley rats (6 groups, n = 10/group); 1. Νο ACLT, 2. ACTL (Negative control), 3. Positive control: ACLT + treatment with Hyaluronic acid (H), 4. OA + treatment with MSCs, 5. OA + kaempferol (K) 10 μM, 6. OA + MSCs and K 10 μM, 7. OA + K 20 μM, 8. OA + MSCs and K 20 μM, 9. OA + K 10 μM + apigenin (A) 0.1 μM, 10. OA + MSCs + K 10 μM + A 0.1 μM | Greatest results in cartilage homogenate: 🖗IL-1β and TNF-α in OA + MSCs + K 10 and 20 μM groups 🖞SOD in OA + MSCs + K 10 μM and K 20 μM, OA + K 20 μM and OA + A 0.1 μM + K 10 μM groups 🖗OA score as seen in radiographs in OA + H, OA + MSCs and OA + MSCs + K 10 μM groups Improvement in histopathologic scores in OA + MSCs and OA + MSCs + K + A groups 🖗safranin O staining score in OA + MSCs + K + A group 🖗MDA in OA + MSCs + K 10 μM and K20 μM and OA + MSCs + K + A groups 🖗IL-1β, TNF-α, iNOS, MMP-3, -13 expression in OA + MSCs + K 20 μM and OA + MSCs + K + A groups 🖞COL2A1, SOX-9, aggrecan expression in OA + MSCs + K + A group |
| Turmeric extract, black pepper, and ginger | |||
| Heidari-Beni et al. 2020 [55] In vivo (clinical) | Mixodin is a dietary supplement that consists of turmeric extract (curcumin; 300 mg/g), ginger extract (gingerol; 7.5 mg/g), and black pepper extract (piperine; 3.75 mg/g). | A randomized, double-blinded, controlled clinical trial, 60 volunteers with mild knee OA received Mixodin (n = 30) which contained curcumin (300 mg), gingerols (7.5 mg) and piperine (3.75 mg) or Naproxen (n = 30)for 4 weeks twice a day after meals | 🖗PGE2 in both groups |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACLT | cruciate ligament transection |
| ACR | American College of Rheumatology |
| ACs | articular condrocytes |
| ADAMTS | disintegrin and metalloprotease with thrombospondin motifs |
| BCP | β-caryophyllene |
| CI | combination index |
| COL2A1 | collagen type II alpha 1 |
| COX-2 | cyclooxygenase-2 |
| DAMPs | damage associated molecular patterns |
| ECM | extracellular matrix |
| EGCG | epigallocatechin gallate |
| GAG | glycosaminoglycans |
| HACs | human articular chondrocytes |
| HT | hydroxytyrosol |
| iNOS | inducible nitric oxide synthase |
| LFI | Lequesne Functional Index |
| 5-LOX | 5-lipoxygenase |
| LPFI | Lequesne’s Pain-Function Index |
| LPS | lipopolysaccharide |
| MDA | malondialdehyde |
| MIA | monoiodoacetate |
| MMPs | matrix metalloproteinases |
| MSCs | mesenchymal stem cells |
| MyD88 | myeloid differentiation factor 88 |
| NF-κΒ | nuclear factor kappa-Β |
| NLRs | NOD-like receptors |
| NO | nitric oxide |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| OPC | grape extract |
| OPCO | grape seed and olive extract |
| OS | oxidative stress |
| PCy | procyanidins |
| PGA | patient’s global assessment |
| PGAD | physician’s global assessment of disease activity |
| PGE2 | prostaglandin E2 |
| PHO | olive extract |
| PRRs | pattern recognition receptors |
| PTOA | post-traumatic OA animal models |
| PWT | paw withdrawal threshold |
| RCT | randomized controlled trial |
| ROS | reactive oxygen species |
| RosA | rosmarinic acid |
| SGAD | subject’s global assessment of disease activity |
| SGADc | subject’s global assessment of disease related discomfort |
| SIRT1/AMPK | sirtuin1/adenosine monophosphate-activated protein kinase |
| SMMSCs | synovial membrane-derived mesenchymal stem cells |
| SOD | superoxide dismoutase |
| TLRs | toll-like receptors |
| TRIF | toll/interferon response factor |
| VAS | Visual Analog Scale |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Safiri, S.; Kolahi, A.A.; Smith, Ε.; Hill, C.; Bettampadi, D.; Mansournia, Μ.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Deveza, L.A.; Nelson, A.E.; Loeser, R.F. Phenotypes of osteoarthritis: Current state and future implications. Clin. Exp. Rheumatol. 2019, 37, 64–72. [Google Scholar] [PubMed]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay-Jensen, A.C.; Mobasheri, A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Prodromos, C.; Finkle, S.; Rumschlag, T.; Lotus, J. Autologous Mesenchymal Stem Cell Treatment is Consistently Effective for the Treatment of Knee Osteoarthritis: The Results of a Systematic Review of Treatment and Comparison to a Placebo Group. Medicines 2020, 7, 42. [Google Scholar] [CrossRef]
- Leong, D.; Choudhury, M.; Hirsh, D.; Hardin, J.; Cobelli, N.; Sun, H. Nutraceuticals: Potential for Chondroprotection and Molecular Targeting of Osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Santoni, G.; Cardinali, C.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Amantini, C. Danger- and pathogen-associated molecular patterns recognition receptors and ion channels of the transient receptor potential family triggers the inflamasome activation in immune cells and sensory neurons. J. Neuroinflamm. 2015, 12, 21. [Google Scholar] [CrossRef]
- Jimenez-Dalaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors—from microbial recognition to autoimmunity: A comprehensive review. Autoimmun. Rev. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tukhvatulin, A.I.; Logunov, D.Y.; Gitlin, I.I.; Shmarov, M.M.; Kudan, P.V.; Adzhieva, C.A.; Moroz, A.F.; Kostyukova, N.N.; Burdelya, L.G.; Naroditsky, B.S.; et al. In Vitro and In Vivo Study of the Ability of NOD1 Ligands to Activate the Transcriptional Factor NF-kB. Acta Naturae 2011, 3, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Woodell-May, J.E.; Sommerfeld, S.V. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Shen, C.L.; Smith, B.J.; Lo, D.F.; Chyu, M.C.; Dunn, D.M.; Chen, C.H.; Kwun, I.S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef]
- Walzer, S.M.; Weinmann, D.; Toegel, S. Medical Plant Extracts for Treating Knee Osteoarthritis: A Snapshot of Recent Clinical Trials and Their Biological Background. Curr. Rheumatol. Rep. 2015, 17, 54. [Google Scholar] [CrossRef]
- Guan, V.X.; Mobasheri, A.; Probst, Y.C. A systematic review of osteoarthritis prevention and management with dietary phytochemicals from foods. Maturitas 2019, 122, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food. Argic. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018, 17, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef]
- Ghoochani, N.; Karandish, M.; Mowla, K.; Haghighizadeh, M.H.; Jalali, M.T. The effect of pomegranate juice on clinical signs, matrix metalloproteinases and antioxidant status in patients with knee osteoarthritis. J. Sci. Food Agric. 2016, 96, 4377–4381. [Google Scholar] [CrossRef] [PubMed]
- Connelly, A.E.; Tucker, A.J.; Tulk, H.; Catapang, M.; Chapman, L.; Sheikh, N.; Yurchenko, S.; Fletcher, R.; Kott, L.S.; Duncan, A.M.; et al. High-rosmarinic acid spearmint tea in the management of knee osteoarthritis symptoms. J. Med. Food 2014, 17, 1361–1367. [Google Scholar] [CrossRef]
- Schumacher, H.R.; Pullman-Mooar, S.; Gupta, S.R.; Dinella, J.E.; Kim, R.; McHugh, M.P. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 2013, 21, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Alishiri, G.H.; Bayat, N.; Hosseini, S.M.; Sahebkar, A. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: A randomized controlled trial. EXCLI J. 2016, 15, 203–210. [Google Scholar]
- Stebbings, S.; Beattie, E.; McNamara, D.; Hunt, S. A pilot randomized, placebo-controlled clinical trial to investigate the efficacy and safety of an extract of Artemisia annua administered over 12 weeks, for managing pain, stiffness, and functional limitation associated with osteoarthritis of the hip and knee. Clin. Rheumatol. 2016, 35, 1829–1836. [Google Scholar]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose—effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Greco, W.R.; Faessel, H.; Levasseur, L. The search for cytotoxic synergy between anticancer agents: A case of Dorothy and the ruby slippers? J. Natl. Cancer Inst. 1996, 88, 699–700. [Google Scholar] [CrossRef]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol) a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef]
- Grimm, T.; Chovanova, Z.; Muchova, J.; Sumegova, K.; Liptakova, A.; Durackova, Z.; Högger, P. Inhibition of NF-kappaB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J. Inflamm. 2006, 3, 1. [Google Scholar] [CrossRef]
- Mülek, M.; Seefried, L.; Genest, F.; Högger, P. Distribution of constituents and metabolites of maritime pine bark extract (Pycnogenol) into serum, blood cells, and synovial fluid of patients with severe osteoarthritis: A randomized controlled trial. Nutrients 2017, 9, 443. [Google Scholar] [CrossRef]
- Farid, R.; Mirfeizi, Z.; Mirheidari, M.; Rezaieyazdi, Z.; Mansouri, H.; Esmaelli, H.; Zibadi, S.; Rohdewald, P.; Watchon, R.R. Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee arthritis. Nutr. Res. 2007, 27, 692–697. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Duggal, M.; et al. Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol. Redox Rep. 2008, 13, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtassák, J.; Duraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Saikovsky, R.; Shmidt, E.; Khokhlov, A.; Burnett, B.P. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: A short-term randomized, double-blind pilot study. Nutr. Res. 2009, 29, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Khokhlov, A.; Kopenkin, S.; Bart, B.; Ermolova, T.; Kantemirova, R.; Mazurov, V.; Bell, M.; Caldron, P.; Pillai, L.; et al. Efficacy and Safety of Flavocoxid, a Novel Therapeutic, Compared with Naproxen: A Randomized Multicenter Controlled Trial in Subjects with Osteoarthritis of the Knee. Adv. Ther. 2010, 27, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Minutoli, L.; David, A.; Irrera, N.; Rinaldi, M.; Ventuti, F.S.; Squadrito, F.; Altavilla, D. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit. Care 2012, 16, R32. [Google Scholar] [CrossRef]
- Burnett, B.P.; Bitto, A.; Altavilla, D.; Squadrito, F.; Levy, R.M.; Pillai, L. Flavocoxid inhibits phospholipase A2, peroxidase moieties of the cyclooxygenases (COX), and 5-lipoxygenase, modifies COX-2 gene expression, and acts as an antioxidant. Mediat. Inflamm. 2011, 2011, e385780. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Squadrito, F.; Bitto, A.; Polito, F.; Burnett, B.P.; Di Stefano, V.; Minutoli, L. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts proinflammatory phenotype activation in endotoxin-stimulated macrophages. Br. J. Pharmacol. 2009, 157, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Owensby, J.K.; Xie, F. Comparative safety of flavocoxid vs prescription NSAIDs among osteoarthritis patients. Osteoarthr. Cartil. 2020, 28, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Gala, J.; Bhasale, S.; Naik, S.; Modak, M.; Thakur, H.; Deo, N.; Miller, M.J. Comparison of glucosamine sulfate and a polyherbal supplement for the relief of osteoarthritis of the knee: A randomized controlled trial [ISRCTN25438351]. BMC Complement Altern. Med. 2007, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Mével, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, S.I.; Choi, B.R.; Lee, Y.S.; Lee, D.Y.; Kim, G.S. Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats. BMC Complement Altern. Med. 2019, 19, 325. [Google Scholar] [CrossRef]
- Karlapudi, V.; Prasad Mungara, A.V.V.; Sengupta, K.; Davis, B.A.; Raychaudhuri, S.P. A Placebo-Controlled Double-Blind Study Demonstrates the Clinical Efficacy of a Novel Herbal Formulation for Relieving Joint Discomfort in Human Subjects with Osteoarthritis of Knee. J. Med. Food 2018, 21, 511–520. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, H.J.; Lee, D.R.; Choi, B.K.; Yang, S.H. Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes. Molecules 2020, 25, 2033. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, H.J.; Lee, D.R.; Choi, B.K.; Yang, S.H. Herbal Composition LI73014F2 Alleviates Articular Cartilage Damage and Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Molecules 2020, 25, 5467. [Google Scholar] [CrossRef]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Vincenzo, A.; et al. Exploiting Curcumin Synergy with Natural Products Using Quantitative Analysis of Dose-Effect Relationships in an Experimental In Vitro Model of Osteoarthritis. Front. Pharmacol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Estakhri, F.; Panjehshahin, M.R.; Tanideh, N.; Gheisari, R.; Mahmoodzadeh, A.; Azarpira, N.; Gholijani, N. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee 2020, 27, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Heidary-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Roell, K.R.; Reif, D.M.; Motsinger, A.A. An introduction to terminology of chemical synergy-Perspectives from across disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Loewe, S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 1953, 3, 285–290. [Google Scholar] [PubMed]
- Wooten, D.J.; Meyer, C.T.; Quaranta, V.; Lopez, C. A consensus framework unifies multi-drug synergy metrics. BioRxiv 2019. [Google Scholar] [CrossRef]
- Demidenko, E.; Miller, T.W. Statistical determination of synergy based on Bliss definition of drugs independence. PLoS ONE 2019, 14, e0224137. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-κB signal pathway. Drug Dev. Res. 2019, 80, 353–359. [Google Scholar] [CrossRef]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol. Med. Rep. 2018, 17, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Bek, Z.A.; Sarıbaş, G.S.; Özoğul, C.; Goker, B.; Bitik, B.; Aktekin, C.N.; Karasu, Ç. TLR4, RAGE, and p-JNK/JNK mediated inflammatory aggression in osteoathritic human chondrocytes are counteracted by redox-sensitive phenolic olive compounds: Comparison with ibuprofen. J. Tissue Eng. Regen. Med. 2020, 14, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Gu, H.; Jiang, M.; Huang, Y.; Liu, X.; Liu, L. Resveratrol inhibits the development of obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling pathways. Connect Tissue Res. 2019, 60, 571–582. [Google Scholar] [CrossRef]
- Gu, H.; Jiao, Y.; Yu, X.; Li, X.; Wang, W.; Ding, L.; Liu, L. Resveratrol inhibits the IL-1β-induced expression of MMP-13 and IL-6 in human articular chondrocytes via TLR4/MyD88-dependent and -independent signaling cascades. Int. J. Mol. Med. 2017, 39, 734–740. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, J.; Zhao, D.; Wang, C.; Zhang, Y.; Wang, Y.; Li, T. Therapeutic effect and mechanism of action of quercetin in a rat model of osteoarthritis. J. Int. Med. Res. 2020, 48, 300060519873461. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharm. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valsamidou, E.; Gioxari, A.; Amerikanou, C.; Zoumpoulakis, P.; Skarpas, G.; Kaliora, A.C. Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies. Nutrients 2021, 13, 1420. https://doi.org/10.3390/nu13051420
Valsamidou E, Gioxari A, Amerikanou C, Zoumpoulakis P, Skarpas G, Kaliora AC. Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies. Nutrients. 2021; 13(5):1420. https://doi.org/10.3390/nu13051420
Chicago/Turabian StyleValsamidou, Evdokia, Aristea Gioxari, Charalampia Amerikanou, Panagiotis Zoumpoulakis, George Skarpas, and Andriana C. Kaliora. 2021. "Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies" Nutrients 13, no. 5: 1420. https://doi.org/10.3390/nu13051420
APA StyleValsamidou, E., Gioxari, A., Amerikanou, C., Zoumpoulakis, P., Skarpas, G., & Kaliora, A. C. (2021). Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies. Nutrients, 13(5), 1420. https://doi.org/10.3390/nu13051420









