Glycerol Monocaprylate Modulates Gut Microbiota and Increases Short-Chain Fatty Acids Production without Adverse Effects on Metabolism and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Glucose Metabolism
2.3. Biochemical Analysis
2.4. H&E Staining and Histology Analysis
2.5. Gene Expression Analysis by Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blot
2.7. Gut Microbiota Analysis with 16S rRNA Gene Sequencing
2.8. Short-Chain Fatty Acids (SCFAs) Composition Analysis
2.9. Statistical Analysis
3. Results
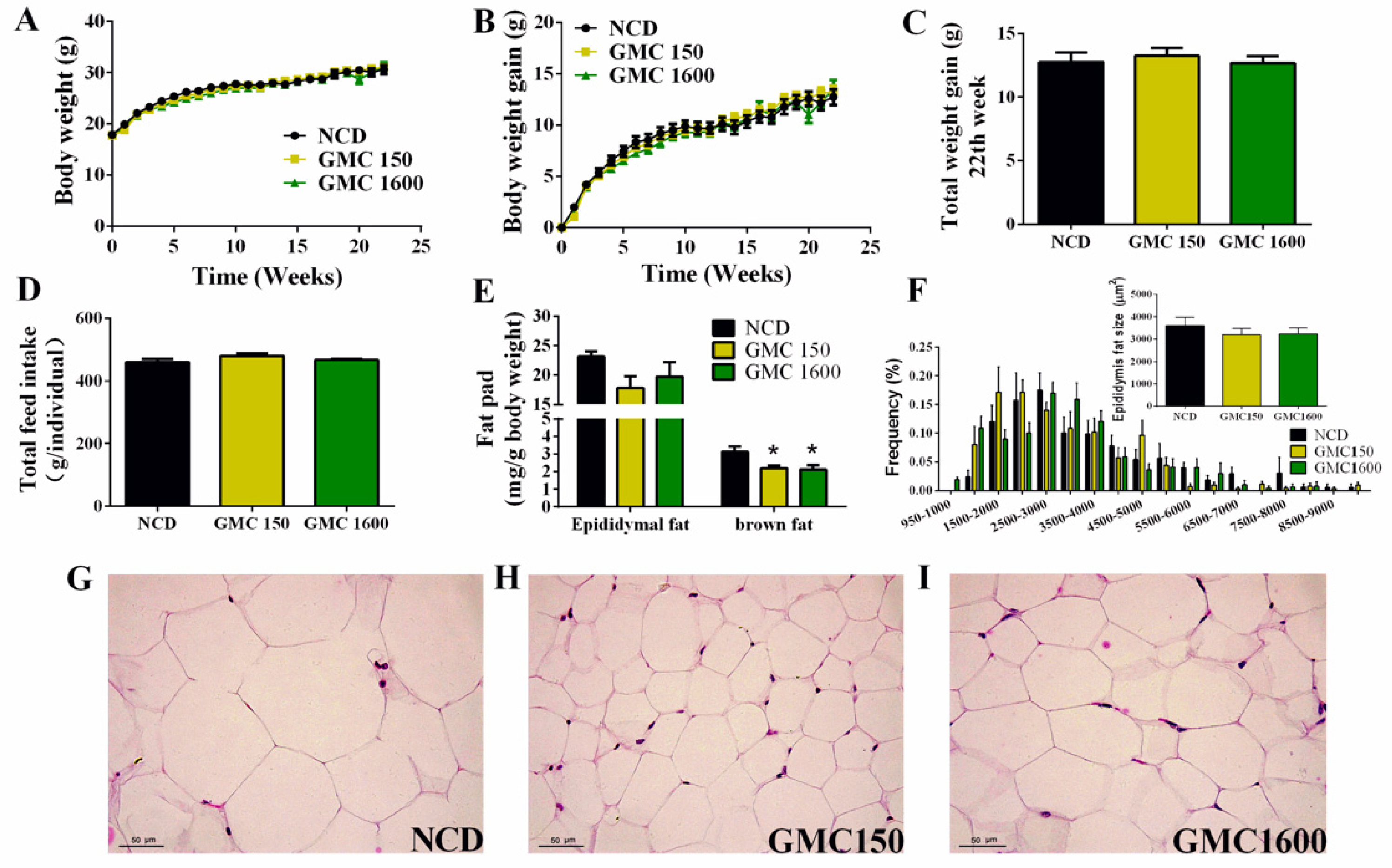
3.1. Effects of GMC Supplementation on Body Weight, Feed Intake, Adipocyte Size, and Liver Histology
3.2. Effects of GMC Supplementation on Glucose and Lipid Metabolism
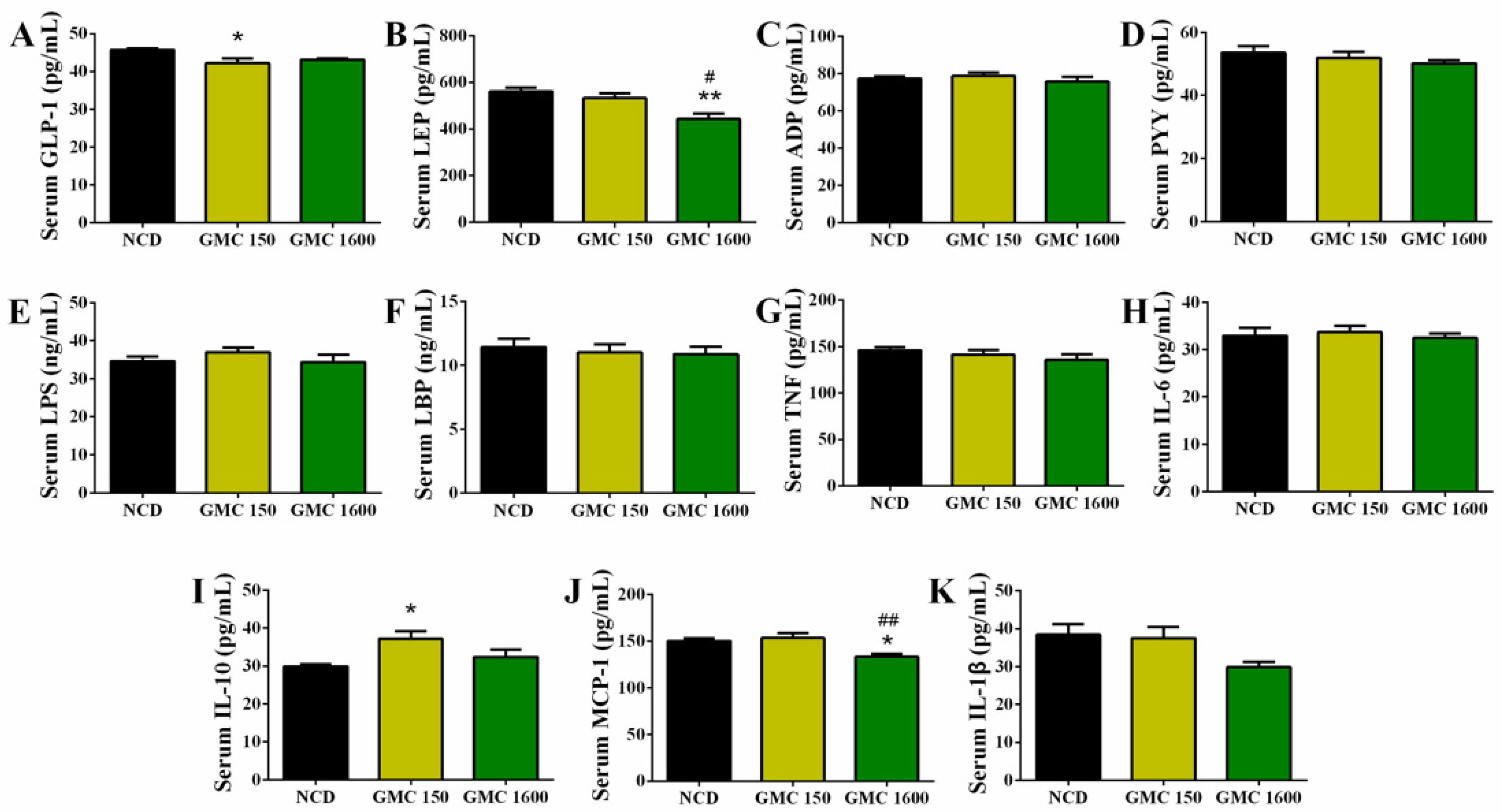
3.3. Effects of GMC Supplementation on Appetite-Hormone Level and Inflammation-Related Cytokines in Serum
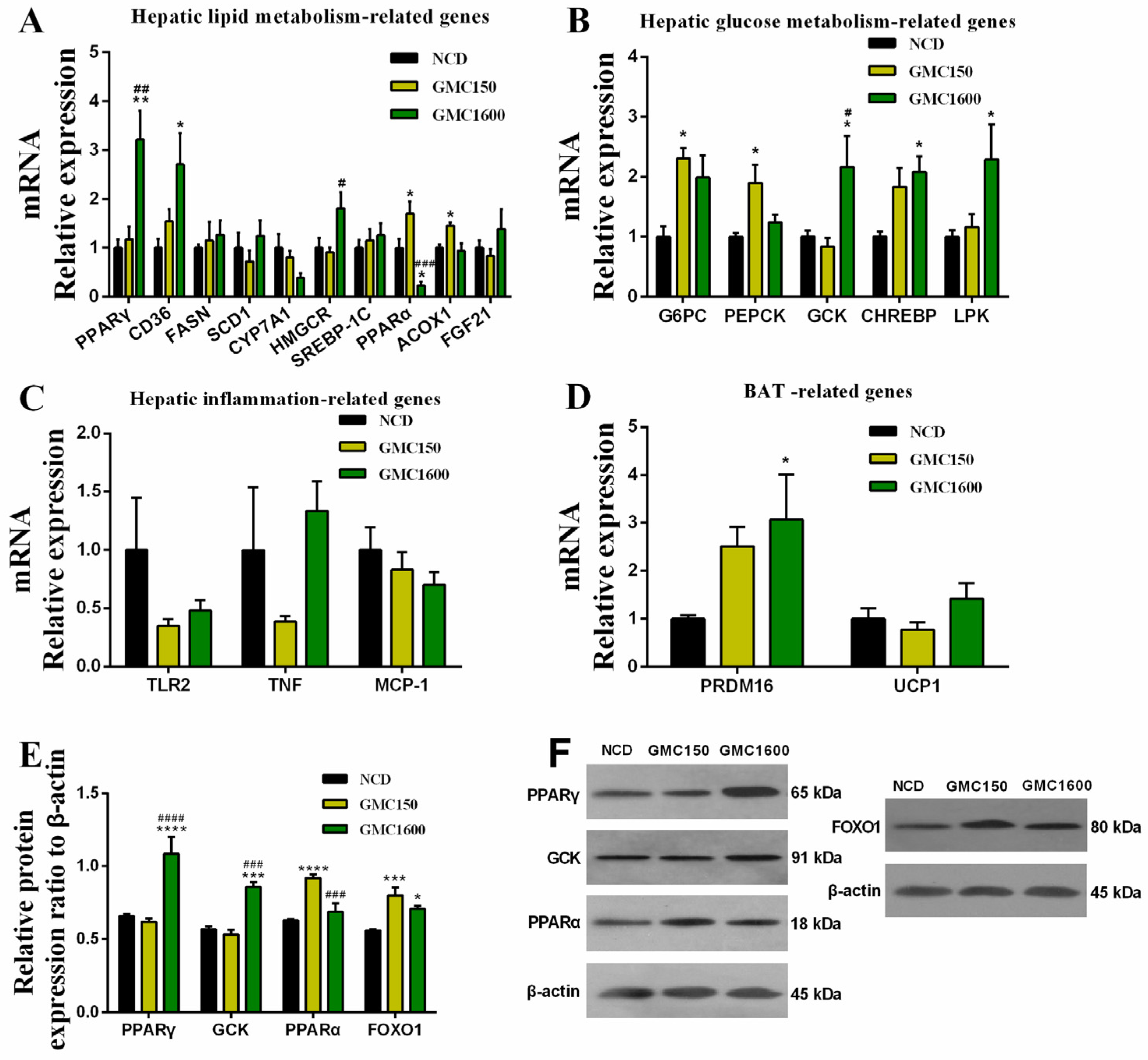
3.4. Effects of GMC Supplementation on the Expressions of Genes and Proteins Related with Glucose and Lipid Metabolism and Inflammation
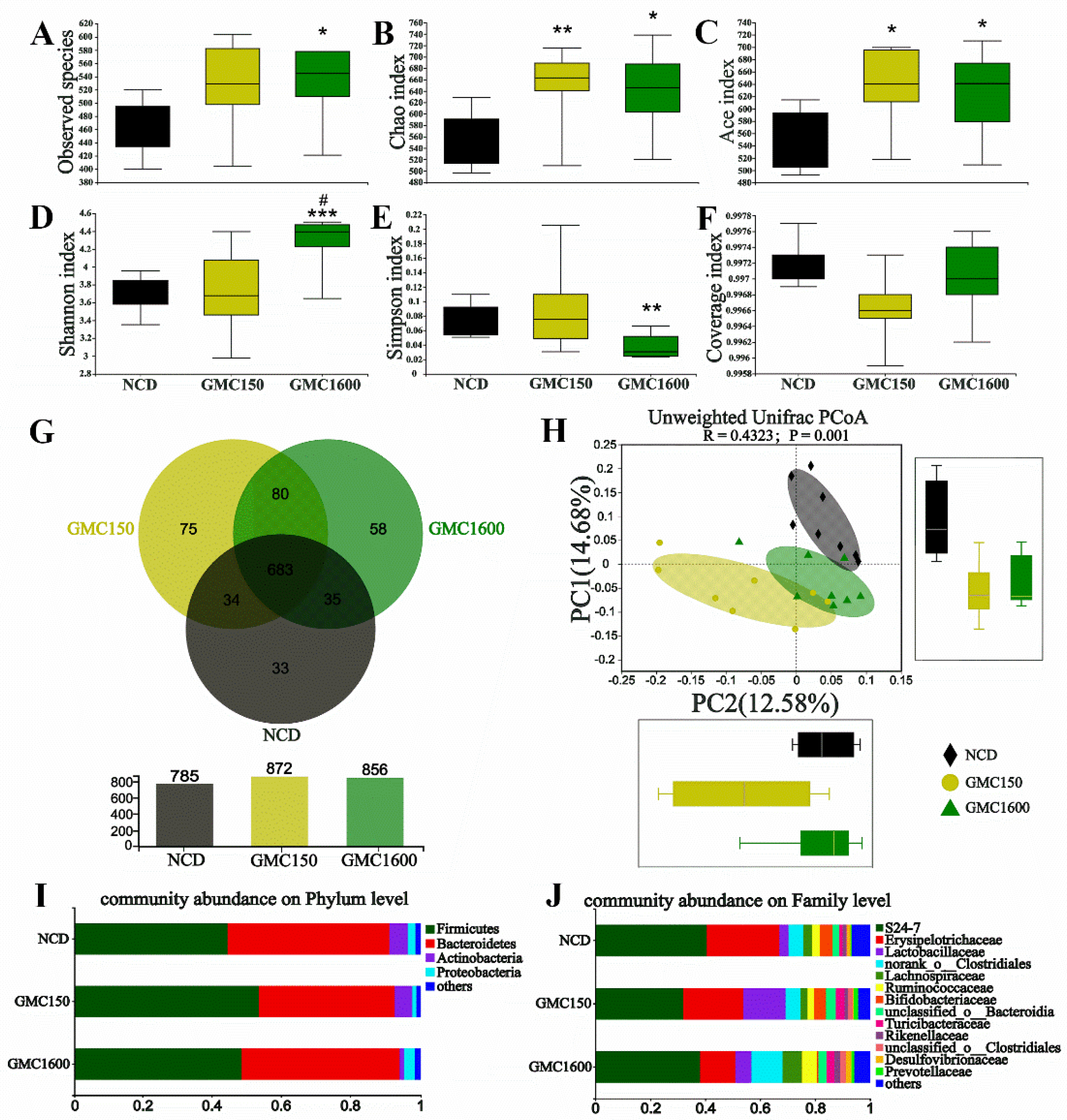
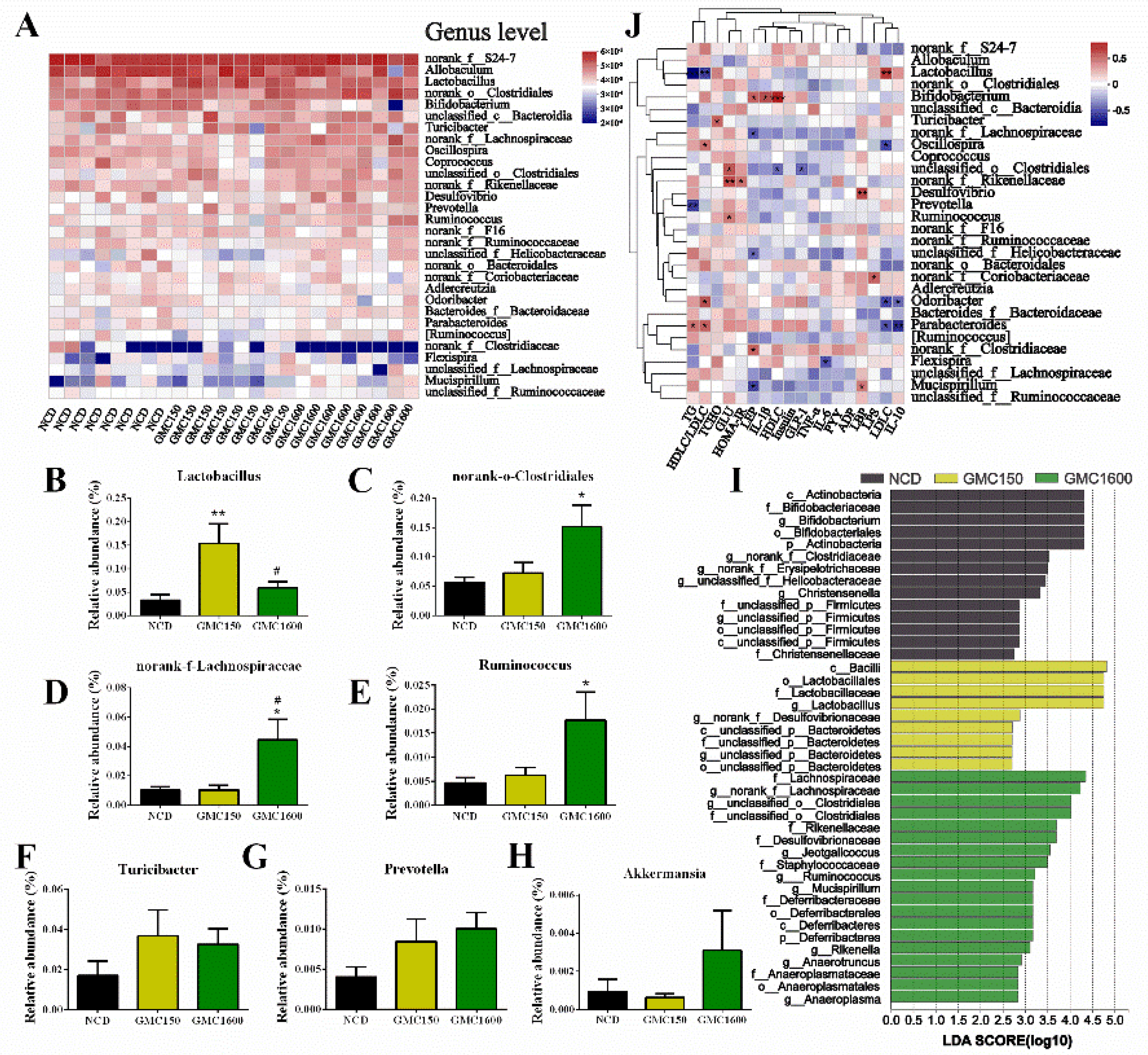
3.5. Effects of GMC Supplementation on the Diversity and Composition of Gut Microbiota
3.6. Gut Microbiota Composition at Genus Level and Correlation Analysis of Blood Biochemical Criterion
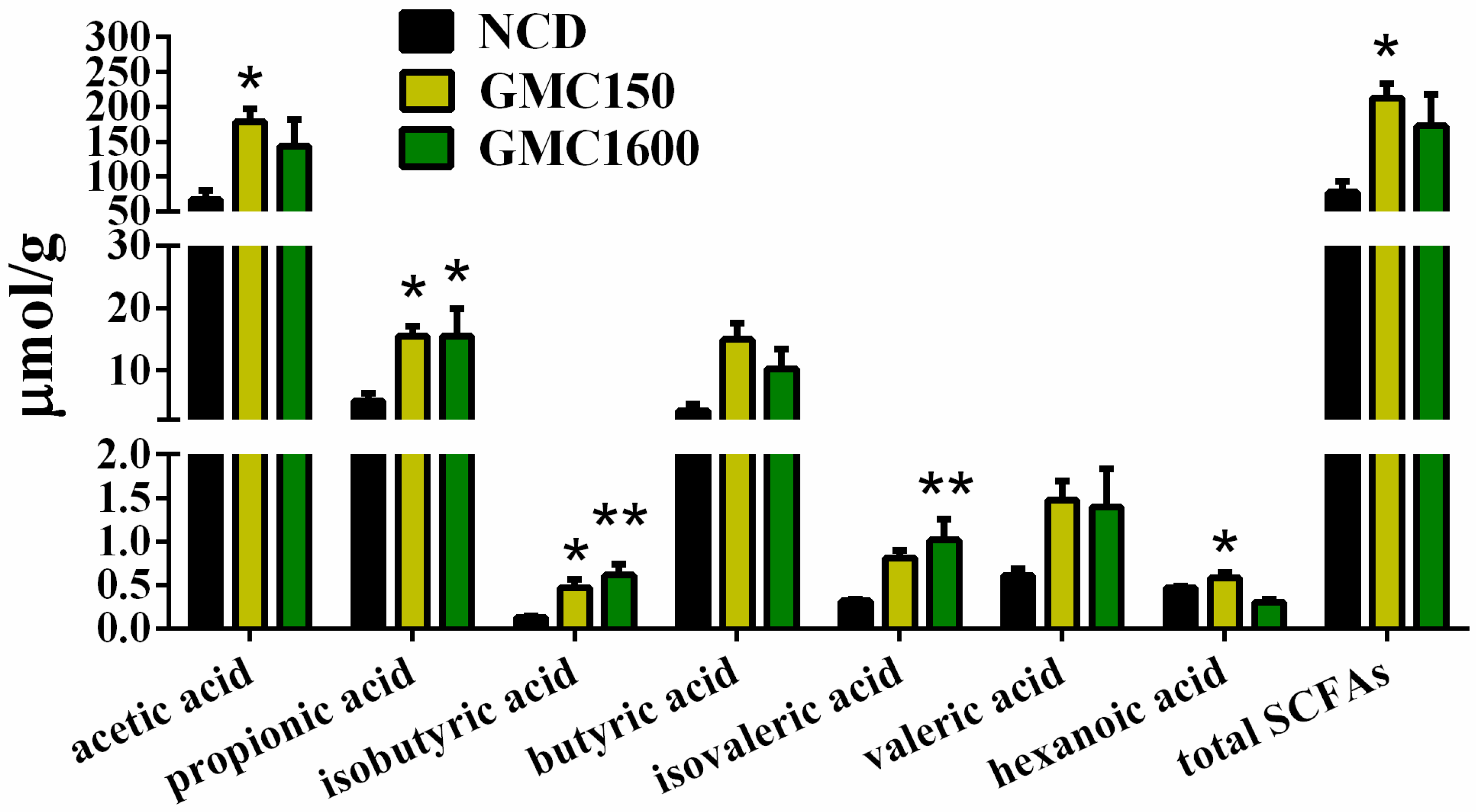
3.7. GMC Supplementation Increased the Content of SCFAs in Feces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GMC | glycerol monocaprylate |
| MCFA | medium-chain fatty acid |
| SCFA | short-chain fatty acid |
| GML | glycerol monolaurate |
| TG | triglyceride |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| FFA | free fatty acid |
| LEP | leptin |
| PYY | peptide YY |
| GLP-1 | glucagon like peptide 1 |
| ADP | adiponectin |
| LBP | lipopolysaccharide binding protein |
| LPS | lipopolysaccharide |
| IL-1β | interleukin 1beta |
| IL-6 | interleukin 6 |
| IL-10 | interleukin 10 |
| TNF | tumor necrosis factor |
| MCP-1 | monocyte chemotactic proteins 1 |
| H&E | haematoxylin and eosin |
| PPARα | peroxisome proliferators activate receptor alpha |
| PPARγ2 | peroxisome proliferators activate receptor gamma2 |
| SCD1 | stearoyl-CoA desaturase 1 |
| CYP7A1 | cholesterol 7 alpha-hydroxylase A1 |
| SREBP-1C | sterol regulatory element-binding protein-1c |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase |
| G6PC | glucose-6-phosphatase catalytic subunit |
| PEPCK | phosphoenolpyruvate carboxykinase |
| GCK | glucokinase |
| TLR2 | toll-like receptor 2 |
| MCP-1 | monocyte chemotactic protein-1 |
| CD36 | cluster of differentiation 36 |
| FASN | fatty acid synthase |
| ACOX1 | acyl-coenzyme A oxidase 1 |
| FGF21 | fibroblast growth factor 21 |
| CHREBP | carbohydrate responsive element binding protein |
| LPK | L-typepyruvatekinase |
| PRDM16 | PR domain-containing 16 |
| UCP1 | uncoupling protein 1 |
| FOXO1 | forkhead box transcription factor O1 |
| YWHAZ | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta |
References
- Pantoja-Romero, W.S.; Estrada-Lopez, E.D.; Picciani, P.H.S.; Oliveira, O.N.; Lachter, E.R.; Pimentel, A.S. Efficient molecular packing of glycerol monostearate in Langmuir monolayers at the air-water interface. Colloid Surf. A Physicochem. Eng. Asp. 2016, 508, 85–92. [Google Scholar] [CrossRef]
- Ge, H.; Zang, Y.; Cao, Z.; Ye, X.; Chen, J. Rheological properties, textural and compound preservative of kelp recombination noodles. LWT Food Sci. Technol. 2020, 118, 108729. [Google Scholar] [CrossRef]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [Green Version]
- Gulick, T.; Cresci, S.; Caira, T.; Moore, D.D.; Kelly, D.P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. USA 1994, 91, 11012–11016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, S.; Lech, W. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar]
- Li, H.; Liu, Y.; Zhang, X.; Xu, Q.; Zhang, Y.; Xue, C.; Guo, C. Medium-chain fatty acids decrease serum cholesterol via reduction of intestinal bile acid reabsorption in C57BL/6J mice. Nutr. Metab. 2018, 15, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Geng, Z.; Dai, X.; Zhang, X.; Wei, W.; Wang, X.; Jin, Q. Triacylglycerol containing medium-chain fatty acids: Comparison of human milk and infant formulas on lipolysis during in vitro digestion. J. Agric. Food Chem. 2020, 68, 4187–4195. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Fredrik, B. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats feda cholesterol-rich diet. Br. J. Nutr. 2006, 95, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial emulsifier-glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis, and systemic low-grade inflammation in low-fat diet fed mice. Mol. Nutr. Food Res. 2018, 62, 1700547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X.; et al. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated amelioration of obesity in mice fed a high-fat diet. mBio 2020, 11, e00190-20. [Google Scholar] [CrossRef] [Green Version]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: New insights into its antimicrobial potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-monolaurate-mediated attenuation of metabolic syndrome is associated with the modulation of gut microbiota in high-fat-diet-fed mice. Mol. Nutr. Food Res. 2019, 63, 1801417. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, J.; Feng, F. Medium-chain alpha-monoglycerides improves productive performance and egg quality in aged hens associated with gut microbiota modulation. Poult. Sci. 2020, 99, 7122–7132. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hamilton, J.A.; Kirkland, J.L.; Corkey, B.E.; Guo, W. Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes. Res. 2003, 11, 734–744. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Jones, P.J.H. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity (Reprinted from vol 132, pg 329, 2002). J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Li, J.J.; Feng, J.; Ji, J.; Yu, Q.; Li, Y.; Zheng, Y.Y.; Dai, W.Q.; Wu, J.Y.; Guo, C.Y. Crosstalk between PPARs and gut microbiota in NAFLD. Biomed. Pharmacother. 2021, 136, 8. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPAR alpha). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauca, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liu, C.; Zhao, M.; Zhang, Q.; Lu, Y.; Liu, P.; Yang, H.; Yang, J.; Chen, X.; Yao, Y. The pharmacodynamic and differential gene expression analysis of PPAR alpha/delta agonist GFT505 in CDAHFD-induced NASH model. PLoS ONE 2020, 15, e243911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 367–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPAR alpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef]
- Ip, E.; Farrell, G.; Hall, P.; Robertson, G.; Leclercq, I. Administration of the potent PPAR alpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 2004, 39, 1286–1296. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Candelaresi, C.; Saccomanno, S.; Ferretti, G.; Bachetti, T.; Marzioni, M.; De Minicis, S.; Nobili, L.; Salzano, R.; Omenetti, A.; et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats-Role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am. J. Pathol. 2006, 169, 846–860. [Google Scholar] [CrossRef] [Green Version]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chechi, K.; Yasui, N.; Ikeda, K.; Yamori, Y.; Cheema, S.K. Flax oil-mediated activation of PPAR-gamma correlates with reduction of hepatic lipid accumulation in obese spontaneously hypertensive/NDmcr-cp rats, a model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1313–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.A.; Thomazy, V.A.; MEvans, R. PPAR gamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Memon, R.A.; Tecott, L.H.; Nonogaki, K.; Beigneux, A.; Moser, A.H.; Grunfeld, C.; Feingold, K.R. Up-regulation of peroxisome proliferator-activated receptors (PPAR-alpha) and PPAR-gamma messenger ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of PPAR-gamma-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 2000, 141, 4021–4031. [Google Scholar] [PubMed]
- Wupper, S.; Fischer, A.; Luersen, K.; Lucius, R.; Okamoto, H.; Ishida, Y.; Terao, K.; Rimbach, G. High Dietary Kuding Tea Extract Supplementation Induces Hepatic Xenobiotic-Metabolizing Enzymes-A 6-Week Feeding Study in Mice. Nutrients 2020, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, S.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.J.; Guan, Z.J.; Gao, Y.; Xing, J.L.; Zhou, X.X.; Wang, C.Y.; Xu, J.; Li, W.M. Huang-Qi San ameliorates hyperlipidemia with obesity rats via activating brown adipocytes and converting white adipocytes into brown-like adipocytes. Phytomedicine 2020, 78, 10. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, Y.J.; Zhang, J.; Yao, Q.Y.; Song, Y.F.; Shen, Q.W.; Lin, J.; Gao, Y.X.; Wang, X.Y.; Zhang, L.; et al. Cold-Inducible Klf9 Regulates Thermogenesis of Brown and Beige Fat. Diabetes 2020, 69, 2603–2618. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Vasquez, D.S.; Ravnskjaer, K.; Denechaud, P.-D.; Yu, R.T.; Alvarez, J.G.; Downes, M.; Evans, R.M.; Montminy, M.; Shaw, R.J. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 2011, 145, 607–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, Z.A.; Cusumano, A.; Yan, Z.; Remedi, M.S.; Moley, K.H. Reduced islet function contributes to impaired glucose homeostasis in fructose-fed mice. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E109–E116. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1 alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Unterman, T.G. Regulation of Hepatic Glucose Metabolism by FoxO Proteins, an Integrated Approach. Curr. Top Dev. Biol. 2018, 127, 119–147. [Google Scholar] [PubMed]
- Kuhre, R.E.; Gribble, F.M.; Hartmann, B.; Reimann, F.; Windelov, J.A.; Rehfeld, J.F.; Holst, J.J. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Gastroint. Liver Physiol. 2014, 306, G622–G630. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.H.; Lu, J.Y.; Chen, H.Y.; Wei, C.C.; Xu, X.F.; Li, H.; Bai, Q.F.; Xia, F.Z.; Lam, S.M.; Zhang, H.; et al. Liver ChREBP Protects Against Fructose-Induced Glycogenic Hepatotoxicity by Regulating L-Type Pyruvate Kinase. Diabetes 2020, 69, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Luo, Y.; Gong, S.; Zhou, X.; Li, Y.; Liu, W.; Zhang, S.; Cai, X.; Ren, Q.; Zhou, L.; et al. Low-Frequency Genetic Variant in the Hepatic Glucokinase Gene Is Associated With Type 2 Diabetes and Insulin Resistance in Chinese Population. Diabetes 2021, 70, 809–816. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, C.; Xie, Q.; Xu, J.; Fei, G. TLR2-melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front. Immunol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012, 61, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serino, M. SCFAs-the thin microbial metabolic line between good and bad. Nat. Rev. Endocrinol. 2019, 15, 318–319. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, N.; Fang, H.; Chen, X.; Guo, Y.; Gong, S.; Niu, M.; Zhou, H.; Jiang, Y.; Chang, P.; et al. Enteric dysbiosis is associated with sepsis in patients. FASEB J. 2019, 33, 12299–12310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Vargas, H.E.; Reddy, K.R.; Lai, J.C.; O’Leary, J.G.; Tandon, P.; Wong, F.; Mitrani, R.; White, M.B.; Kelly, M.; et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Xu, R.; Tan, C.; He, Y.; Wu, Q.; Wang, H.; Yin, J. Dysbiosis of gut microbiota and short-chain fatty acids in encephalitis: A Chinese pilot study. Front. Immunol. 2020, 11, 1994. [Google Scholar] [CrossRef]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179. [Google Scholar] [CrossRef]
- Madsen, K.L.; Doyle, J.S.; Jewell, L.D.; Tavernini, M.M.; Fedorak, R.N. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 1999, 116, 1107–1114. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Abdullah; Deng, L.; Zhao, M.; Tang, J.; Liu, T.; Zhang, H.; Feng, F. Probiotic-fermented blueberry juice prevents obesity and hyperglycemia in high fat diet-fed mice in association with modulating the gut microbiota. Food Funct. 2020, 11, 9192–9207. [Google Scholar] [CrossRef] [PubMed]
- Tye, H.; Yu, C.-H.; Simms, L.A.; de Zoete, M.R.; Kim, M.L.; Zakrzewski, M.; Penington, J.S.; Harapas, C.R.; Souza-Fonseca-Guimaraes, F.; Wockner, L.F.; et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat. Commun. 2018, 9, 3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayama, G.; Priya, S.; Niccum, D.E.; Khoruts, A.; Blekhman, R. Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Med. 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitsuji, K.; Xiao, J.; Nagatomo, R.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; Tsuneyama, K. Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci. Rep. 2017, 7, 15876. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wong, F.S. Dietary short-chain fatty acids protect against type 1 diabetes. Nat. Immunol. 2017, 18, 484–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Feng, F.; Zhao, M. Glycerol Monocaprylate Modulates Gut Microbiota and Increases Short-Chain Fatty Acids Production without Adverse Effects on Metabolism and Inflammation. Nutrients 2021, 13, 1427. https://doi.org/10.3390/nu13051427
Zhang J, Feng F, Zhao M. Glycerol Monocaprylate Modulates Gut Microbiota and Increases Short-Chain Fatty Acids Production without Adverse Effects on Metabolism and Inflammation. Nutrients. 2021; 13(5):1427. https://doi.org/10.3390/nu13051427
Chicago/Turabian StyleZhang, Junhui, Fengqin Feng, and Minjie Zhao. 2021. "Glycerol Monocaprylate Modulates Gut Microbiota and Increases Short-Chain Fatty Acids Production without Adverse Effects on Metabolism and Inflammation" Nutrients 13, no. 5: 1427. https://doi.org/10.3390/nu13051427
APA StyleZhang, J., Feng, F., & Zhao, M. (2021). Glycerol Monocaprylate Modulates Gut Microbiota and Increases Short-Chain Fatty Acids Production without Adverse Effects on Metabolism and Inflammation. Nutrients, 13(5), 1427. https://doi.org/10.3390/nu13051427








