Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards
Abstract
:1. Introduction
1.1. Definition and Epidemiology of AP
1.2. Classification of AP
1.3. Pathomechanism of AP, the Role of Oxidative Stress in AP
1.4. Assessment of the Severity of AP
1.5. Disturbances of the Nutritional Status in SAP
1.6. Comparison of Management and Nutritional Support in Acute and Chronic Pancreatitis
2. The Literature Searching and Review
3. European Society for Clinical Nutrition and Metabolism (ESPEN), American Gastroenterological Association (AGA), and UK Guidelines on Clinical Nutrition in Severe Acute Pancreatitis
4. Gut Rousing, But Not Resting, and No “Pancreatic Rest” in SAP Patients
5. The Optimal Route of Nutritional Support in SAP Patients: Enteral versus Parenteral Nutrition
6. The Optimal Timing of Nutritional Support in SAP Patients
| Author | Findings | Type of Analysis | Outcomes |
|---|---|---|---|
| Sun et al. [48] | Lower CD4+ T-lymphocyte %, CD4 +/CD8+ ratio, CRP Higher IgG and HLA-DR in EEN Lower SIRS, MODS, and pancreatic infection rates Lower duration of hospitalization in the ICU in EEN | Randomized controlled trial including 60 patients Comparison of EEN (48 h) and DEN (8th day) in SAP | EEN improves the course, but not decreases mortality compared to DEN in SAP patients |
| Sun et al. [49] | EEN does not increase IAP Decreased AP severity and clinical course, but did not decreased mortality in EEN | Randomized controlled trial including 60 patients Comparison of EEN (48 h) and DEN (8th day): impact on IAP and disease severity in SAP | EEN improves the course, but does not decrease mortality compared to DEN, EEN does not increase IAP in SAP patients |
| Zou et al. [50] | Lower hospital mortality, duration of hospitalization, % of patients requiring mechanical ventilation, surgery, continuous renal replacement therapy Lower incidence of local and systemic septic complications, acute kidney injury EEN | Retrospective analysis of 93 patients Comparison of EEN (within 72 h) and DEN (later than 72 h, within 7 days) in SAP | EEN should be started within 72 h of SAP onset |
| Vaughn et al. [51] | Systematic review including 11 RCTs (11 RCTs on SAP) (948 patients) Comparison of EEN (≤48 h) and DEN (>48 h) in all severity degrees of AP | No difference in outcomes between EEN and DEN in SAP patients | |
| Bakker et al. [52] | Lower rate of complications in EEN | Meta-analysis of 8 RCTs (165 patients) Comparison of EEN (≤24 h) and DEN (>24 h) in all severity degrees of AP | EEN is associated with a reduction of complications |
| Bakker et al. [53] | Comparable rates of complications and mortality | Multicenter RCT including 208 patients Comparison of EEN EEN with an oral diet at 72 h of admission in SAP | EEN is not superior to an oral diet after 77 h in SAP patients |
| Wereszczyńska-Siemiątkowska et al. [54] | Lower mortality rate, frequency of infected necrosis/fluid collections, respiratory failure, and a need for ICU hospitalization in EEN | Retrospective analysis of 197 patients Comparison of EEN (≤48 h) and DEN (>48 h) in pSAP | EE in SAP should be started within 48 h after admission to hospital |
| Song et al. [55] | Lower mortality, MOF, surgery, systemic and local infection rates in EEN Comparable SIRS and other local complication rates in EEN | Meta-analysis including 10 RCTs (1051 patients) Comparison of EEN (≤48 h) and DEN (>48 h) or PN in pSAP, SAP | EEN is efficient and safe in pSAP and SAP patients |
| Li et al. [56] | Lower rate of overall infectious, catheter-related septic and local infectious complications lower hyperglycemia, shorter length of hospital stay, decreased mortality in EEN Comparable pulmonary complications | Meta-analysis of 11 studies (775 patients) Comparison of EEN (≤48 h) and DEN (>48 h) in pSAP | EEN improves the outcome and reduces complication rate in pSAP and SAP patients |
| Qi et al. [57] | Lower number of local infectious complications and MODS only in EEN in pSAP and SAP | Meta-analysis including 8 studies (727 patients) Comparison of EEN (<24 h) with DEN, PN in with all AP severity degrees | EEN should be used only in pSAP and SAP patients (not lower degrees) No advantages of EEN in MAP and MSAP patients |
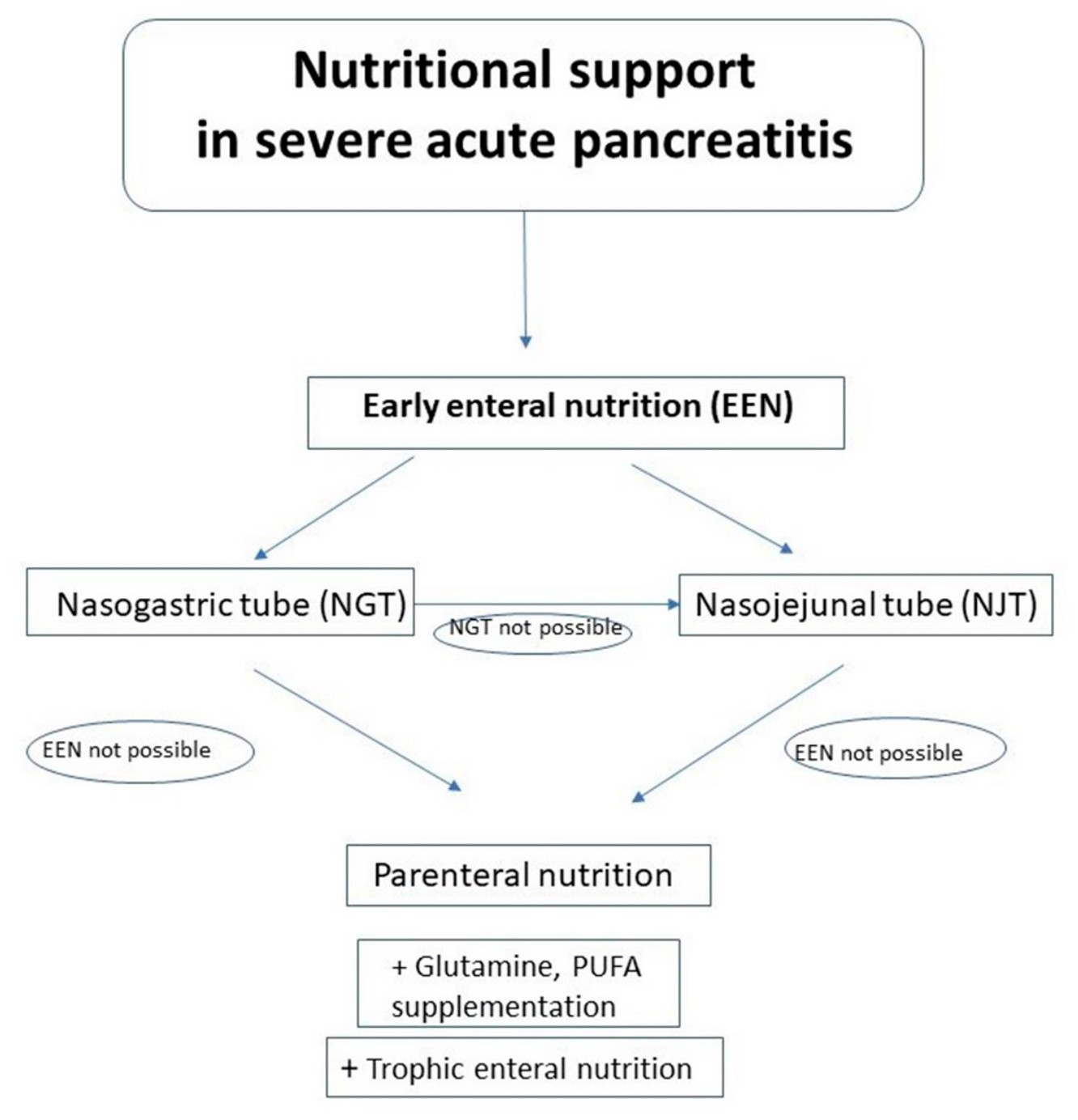
7. The Nasogastric versus Nasojejunal Tube in Enteral Nutrition of SAP Patients
| Author | Findings | Type of Analysis | Outcomes |
|---|---|---|---|
| Eatock et al. [58] | Comparable outcome in NGT and NJT Mortality (18.5%) in NGT and (30.4%) in NJT patients | Pilot Randomized control trial including 50 patients Comparison of NGT and NJT in EE in SAP | EN via NGT was easier and equally effective compared to EN via NJT in SAP patients |
| Singh et al. [59] | Comparable rate of infectious complications, abdominal pain during refeeding, bowel permeability, and endotoxemia in both groups | Randomized control trial including 78 patients Comparison of NGT and NJT in EE in SAP | EE via NGT comparable to EE via NJT in SAP patients |
| Petrov et al. [60] | Comparable effects including mortality and feeding intolerance in both groups | Meta-analysis including 4 trials (92 patients) Comparison of NGT and NJT in EE in pSAP | EE via NGT safe and well tolerated in pSAP patients |
| Chang et al. [61] | Comparable mortality, and complications (tracheal aspiration, diarrhea, increased abdominal pain), covering of energy requirement in both groups | Meta-analysis including 3 trials (157 patients) Comparison of NGT and NJT in EE in pSAP | EE via NGT safe and well tolerated in pSAP patients |
| Nally et al. [62] | Comparable covering of the energy requirement, tolerance of enteral feeding, increase of abdominal pain and tube displacement was similar in both groups | Meta-analysis including 4 RCT Comparison of NGT and NJT in EE in SAP | NGT feeding is efficacious in 90% of SAP patients |
| Dutta et al. [63] | Comparable mortality, MODS, infectious complications, tube insertion and enteral feeding related complications, indications for surgery, intolerance of enteral feeding with necessity of PN administration, increased abdominal pain in both groups | Meta-analysis including 5 RCT (220 patients) Comparison of NGT and NJT in EE in SAP | Insufficient evidence regarding superiority/inferiority/equivalence between NGT and NJT in EE in SAP patients |
8. Composition of Enteral Nutrition Formulas in SAP Patients
9. Immunomodulating Nutrition (IN) in SAP Patients
9.1. Immunonutrients
9.2. Probiotics
| Author | Findings | Type of Analysis | Outcomes |
|---|---|---|---|
| Enteral Immunonutrition | |||
| Petrov et al. [70] | Comparable risk of infectious complications and mortality, duration of hospitalization in both groups | A meta-analysis including 3 RCTs (78 patients) Comparison of IN and standard enteral formula in AP patients (from MAP to SAP) | No benefits of IN in EE in AP patients (including SAP patients |
| Poropat et al. [71] | Comparable overall mortality and SIRS rate in both groups | A meta-analysis including 3 RCTs (78 patients) Comparison of IN and standard enteral formula in AP patients (from MAP to SAP) | No benefits of IN in AP patients |
| Pearce et al. [72] | Comparable decreased CRP in both groups | Randomized controlled trial including 31 patients Comparison of EIN and control feeding in pSAP patients | The cause of the unexpectedly higher CRP values in the study group is unclear |
| Huang et al. [73] | Comparable APACHE II score, duration of hospitalization, costs in both groups | Randomized controlled trial including 32 patients Comparison of EIN and control feeding in pSAP patients | EIN (Gln, Arg) improves the gut barrier function by reducing the gastrointestinal permeability and decreasing plasma endotoxin level in the early SAP phase |
| Singh et al. [74] | Comparable infectious complications, prealbumin level, total duration of hospitalization/duration of hospitalization in ICU, and mortality in both groups | Randomized controlled trial including 80 patients Comparison of EIN (Gln) and control feeding in pSAP patients | No significant impact of Gln on gut permeability in SAP patients |
| Arutla et al. [75] | Comparable rated of infected necrosis and in-hospital mortality in both groups Higher increase of serum Gln, lower polyethylene glycol, higher decrease of Il-6 in Gln group | Randomized controlled trial including 40 patients Comparison of standard nutrition and standard nutrition supplemented with enteral Gln in SAP and pSAP patients | Enteral Gln supplementation improves the gut permeability and oxidative stress in SAP and pSAP patients |
| Parenteral immunonutrition | |||
| Jafari et al. [76] | Lower mortality rate, shorter duration of hospitalization PIN group | Meta-analysis including 7 RCTS on PIN supplemented with Gln and/or PUFA | PIN (Gln, PUFA) can improve prognoses in patients with AP |
| Fuentes-Orozco et al. [81] | Increased IL-10, total lymphocyte and lymphocyte subpopulation counts, and albumin levels, improvement of nitrogen balance, lower rate of infectious complications in Gln group Comparable duration of hospitalization and mortality rate in both groups | Randomized controlled trial including 44 patients Comparison of standard PN (n = 22) and Gln-supplemented PN in SAP patients | PIN (Gln) may decrease infectious morbidity rate |
| Xu et al. [82] | Shorter duration of acute respiratory distress syndrome, renal insufficiency, acute hepatitis, shock, encephalopathy, and paralytic ileus, and hospitalization, lower APACHE II score, lower infection, surgery and mortality rates in early group | Randomized controlled trial including 80 patients Comparison of 2 groups of intravenous Gln (early treatment group) or 5 d after (late treatment group) admission in SAP patients | Early Gln supplementation superior to delayed in SAP patients |
| Wang et al. [83] | Higher eicosatetraenoic acid (EPA), lower CRP level, better oxygenation index, shorter duration of continuous renal replacement therapy in PUFA group | Randomized controlled trial including 40 patients Comparison of standard PN and PN supplemented with omega-3-fatty acids | PN supplemented with PUFA diminished the hyperinflammatory response by the EPA increase and the proinflammatory cytokine decrease in SAP patients |
| Probiotics | |||
| Gou et al. [85] | No impact of probiotics on pancreatic infection, total infections, operation, mortality rates, duration of hospitalization | Meta-analysis including 6 trials (536 patients) Analysis of advantages and disadvantages of probiotics on the outcome in pSAP patients | No sufficient data to draw conclusions on the role of probiotics in nutrition in pSAP patients |
| Besselink et al. [86] | Higher infectious complications, mortality, bowel ischemia rates in probiotics group | Multicenter randomized controlled trial including 298 pSAP patients Comparison of probiotic sand placebo groups | Probiotics do not decrease a risk of septic complications in pSAP patients Use the probiotic prophylaxis is not recommended in SAP patients |
| Wang et al. [87] | Lowest pancreatic infectious complications, MODS, mortality rate, TNF-α and IL-6 levels, highest Il-10 as well as APACHE II scores in EN + EcoIN | Randomized controlled trial including 183 SAP patients Comparison of receiving PN, EN, or EN + EcoIN | Combination of EcoIN with EN has got more advantages compared to exclusive EN in SAP patients |
10. Antisecretory Management
11. Summary
12. The Other Clinical Considerations and Practical Tips Regarding Nutritional Support in Patients with SAP
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Kozieł, D.; Głuszek, S. Epidemiology of acute pancreatitis in Poland—Selected problems. Med. Stud 2016, 1, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical evaluation of the antioxidant effects of hydroxytyrosol on pancreatitis-associated gut injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Phillip, V.; Steiner, J.M.; Algül, H. Early phase of acute pancreatitis: Assessment and management. World. J. Gastrointest. Pathophysiol. 2014, 5, 158–168. [Google Scholar] [CrossRef]
- Khanna, A.K.; Meher, S.; Prakash, S.; Tiwary, S.K.; Singh, U.; Srivastava, A.; Dixit, V.K. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. Hpb Surg. 2013, 2013, 367581. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, Y.; Xie, R.; Yang, K.; Xu, D.; Li, L.; Lin, J.; Zheng, L.; Zhang, C.; Xu, X.; et al. Prognostic value of the creatinine-albumin ratio in acute pancreatitis debridement. BMC Surg. 2020, 20, 322. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayan, G.; Garg, P.K.; Prasad, H.; Tandon, R.K. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 2007, 22, 550–554. [Google Scholar] [CrossRef]
- Chen, C.C.; Wang, S.S.; Lee, F.Y.; Chang, F.Y.; Lee, S.D. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am. J. Gastroenterol. 1999, 94, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Exley, A.R.; Leese, T.; Holliday, M.P.; Swann, R.A.; Cohen, J. Endotoxemia and serum tumor necrosis factor as prognostic markers in sever acute pancreatitis. Gut 1992, 33, 1126–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brivet, F.G.; Emilie, D.; Galanaud, P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: An early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit. Care Med. 1999, 27, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Paajanen, H.; Laato, M.; Jaakkola, M.; Pulkki, K.; Niinikoski, J.; Nordback, I. Serum tumour necrosis factor compared with C-reactive protein in the early assessment of severity of acute pancreatitis. Br. J. Surg. 1995, 82, 271–273. [Google Scholar] [CrossRef]
- Simovic, M.O.; Bonham, M.J.; Abu-Zidan, F.M.; Windsor, J.A. Anti-inflammatory cytokine response and clinical outcome in acute pancreatitis. Crit. Care Med. 1999, 27, 2662–2665. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, Z.; Chi, C.; Wu, C.; Su, L.; Zhang, Y.; Zhu, J.; Liu, Y. Angiopoietin-2 Is an early predictor for acute gastrointestinal injury and intestinal barrier dysfunction in patients with acute pancreatitis. Dig. Dis. Sci. 2021, 66, 114–120. [Google Scholar] [CrossRef]
- Gomes, C.A.; Di Saverio, S.; Sartelli, M.; Segallini, E.; Cilloni, N.; Pezzilli, R.; Pagano, N.; Gomes, F.C.; Catena, F. Severe acute pancreatitis: Eight fundamental steps revised according to the ‘PANCREAS’ acronym. Ann. R. Coll. Surg. Engl. 2020, 102, 555–559. [Google Scholar] [CrossRef]
- Ramanathan, M.; Aadam, A.A. Nutrition management in acute pancreatitis. Nutr. Clin. Pract. 2019, 34, S7–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakananurak, N.; Gramlich, L. Nutrition management in acute pancreatitis: Clinical practice consideration. World J. Clin. Cases 2020, 8, 1561–1573. [Google Scholar] [CrossRef]
- Murphy, A.E.; Codner, P.A. Acute pancreatitis: Exploring nutrition implications. Nutr. Clin. Pract. 2020, 35, 807–817. [Google Scholar] [CrossRef]
- Yang, A.L. Nutrition and Acute Pancreatitis. J. Clin. Med. 2021, 10, 836. [Google Scholar] [CrossRef]
- Khaliq, A.; Dutta, U.; Kochhar, R.; Singh, K. Management of acute pancreatitis: “PANCREAS” contains eight easy steps to remember the treatment. JOP 2010, 11, 492–493. [Google Scholar] [PubMed]
- O’Brien, S.J.; Omer, E. Chronic Pancreatitis and Nutrition Therapy. Nutr. Clin. Pract. 2019, 34, S13–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S.; Bhutiani, N.; Adamson, D.T.; Jones, C.M. Pancreatectomy, islet cell transplantation, and nutrition considerations. Nutr. Clin. Pract. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, S.; Forsmark, C.E. Exocrine pancreatic insufficiency following acute pancreatitis: True association or EPIphenomenon? Dig. Dis. Sci. 2019, 64, 1731–1733. [Google Scholar] [CrossRef] [Green Version]
- Andersson, E.; Andersson, R. Exocrine insufficiency in acute pancreatitis. Scand J. Gastroenterol. 2004, 39, 1035–1039. [Google Scholar] [CrossRef]
- Arvanitakis, M.; Ockenga, J.; Bezmarevic, M.; Gianotti, L.; Krznarić, Ž.; Lobo, D.N.; Löser, C.; Madl, C.; Meier, R.; Phillips, M.; et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin. Nutr. 2020, 39, 612–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollemans, R.A.; Hallensleben, N.D.L.; Mager, D.J.; Kelder, J.C.; Besselink, M.G.; Bruno, M.J.; Verdonk, R.C.; van Santvoort, H.C.; Dutch Pancreatitis Study Group. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology 2018, 18, 253–262. [Google Scholar] [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; American gastroenterological association institute clinical guidelines committee. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut 2005, 54, iii1–iii9. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.M.; Sankaran, S.J.; Plank, L.D.; Windsor, J.A.; Petrov, M.S. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br. J. Surg. 2014, 101, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, K.A.; Akshintala, V.S.; Singh, V.K. Nutritional Management of Acute Pancreatitis. Gastroenterol. Clin. 2021, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, M.; Albalawi, Z.H.; Tashkandi, M.F.; Al-Ansary, L.A. Enteral versus parenteral nutrition for acute pancreatitis. Cochr. Database Syst. Rev. 2010, CD002837. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Ge, L.; Zhao, J.; Lei, Y.; Zhou, F.; Chen, Z.; Zhu, Y.; Xia, B. Meta-analysis: Total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern. Med. 2012, 51, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; He, C.; Deng, L.; Liao, G. Enteral versus parenteral nutrition in critically ill patients with severe pancreatitis: A meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 66–68. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Chang, W.K.; Dhaliwal, R.; Heyland, D.K. Nutrition support in acute pancreatitis: A systematic review of the literature. J. Parenter. Enteral. Nutr. 2006, 30, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kotani, J.; Usami, M.; Nomura, H.; Iso, A.; Kasahara, H.; Kuroda, Y.; Oyanagi, H.; Saitoh, Y. Enteral nutrition prevents bacterial translocation but does not improve survival during acute pancreatitis. Arch. Surg. 1999, 134, 287–292, Erratum in 1999, 134, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.M.; Nahikian-Nelms, M.; Ukleja, A.; Lara, L.F. Nutritional aspects of acute pancreatitis. Gastroenterol. Clin. 2018, 47, 77–94. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Y.; Lu, T.; Gao, F.; Mo, Z. Meta-analysis of enteral nutrition versus total parenteral nutrition in patients with severe acute pancreatitis. Ann. Nutr. Metab. 2008, 53, 268–275. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, S.; Li, J. Safety and efficacy of total parenteral nutrition versus total enteral nutrition for patients with severe acute pancreatitis: A meta-analysis. J. Int. Med. Res. 2018, 46, 3948–3958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Li, L.; Sun, W. Efficacy comparisons of enteral nutrition and parenteral nutrition in patients with severe acute pancreatitis: A meta-analysis from randomized controlled trials. Biosci. Rep. 2018, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Tang, C.; Feng, W.; Bao, Y.; Yu, H. Early nasogastric feeding versus parenteral nutrition in severe acute pancreatitis: A retrospective study. Pak. J. Med. Sci. 2016, 32, 1517–1521. [Google Scholar] [CrossRef]
- Gupta, R.; Patel, K.; Calder, P.C.; Yaqoob, P.; Primrose, J.N.; Johnson, C.D. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II > or =6). Pancreatology 2003, 3, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Felbinger, T.W. Gastrointestinal dysmotility in the critically ill: A role for nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 353–359. [Google Scholar] [CrossRef]
- Liang, H.; Huang, Z.; Wang, T.; Lin, N.; Liu, W.; Cui, J.; Yan, H.; Tang, L. Abdominal paracentesis drainage improves tolerance of enteral nutrition in acute pancreatitis: A randomized controlled trial. Scand. J. Gastroenterol. 2017, 52, 389–395. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S.; American College of Gastroenterology. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415, Erratum in 2014, 109, 302. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enteral. Nutr. 2016, 44, 390–438. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Mu, X.W.; Li, W.Q.; Tong, Z.H.; Li, J.; Zheng, S.Y. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J. Gastroenterol. 2013, 19, 917–922. [Google Scholar] [CrossRef]
- Sun, J.K.; Li, W.Q.; Ke, L.; Tong, Z.H.; Ni, H.B.; Li, G.; Zhang, L.Y.; Nie, Y.; Wang, X.Y.; Ye, X.H.; et al. Early enteral nutrition prevents intra-abdominal hypertension and reduces the severity of severe acute pancreatitis compared with delayed enteral nutrition: A prospective pilot study. World J. Surg. 2013, 37, 2053–2060. [Google Scholar] [CrossRef]
- Zou, L.; Ke, L.; Li, W.; Tong, Z.; Wu, C.; Chen, Y.; Li, G.; Li, N.; Li, J. Enteral nutrition within 72 h after onset of acute pancreatitis versus delayed initiation. Eur. J. Clin. Nutr. 2014, 68, 1288–1293. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, V.M.; Shuster, D.; Rogers, M.A.M.; Mann, J.; Conte, M.L.; Saint, S.; Chopra, V. Early versus delayed feeding in patients with acute pancreatitis: A systematic review. Ann. Intern. Med. 2017, 166, 883–892. [Google Scholar] [CrossRef]
- Bakker, O.J.; van Brunschot, S.; Farre, A.; Johnson, C.D.; Kalfarentzos, F.; Louie, B.E.; Oláh, A.; O’Keefe, S.J.; Petrov, M.S.; Powell, J.J.; et al. Timing of enteral nutrition in acute pancreatitis: Meta-analysis of individuals using a single-arm of randomised trials. Pancreatology. 2014, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Bakker, O.J.; van Brunschot, S.; van Santvoort, H.C.; Besselink, M.G.; Bollen, T.L.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Bosscha, K.; Ahmed Ali, U.; et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N. Engl. J. Med. 2014, 371, 1983–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wereszczynska-Siemiatkowska, U.; Swidnicka-Siergiejko, A.; Siemiatkowski, A.; Dabrowski, A. Early enteral nutrition is superior to delayed enteral nutrition for the prevention of infected necrosis and mortality in acute pancreatitis. Pancreas 2013, 42, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhong, Y.; Lu, X.; Kang, X.; Wang, Y.; Guo, W.; Liu, J.; Yang, Y.; Pei, L. Enteral nutrition provided within 48 h after admission in severe acute pancreatitis: A systematic review and meta-analysis. Medicine (Baltimore) 2018, 97, e11871. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, T.; Chen, G.C.; Yuan, Y.H.; Zhong, W.; Zhao, L.N.; Chen, Q.K. Enteral nutrition within 48 h of admission improves clinical outcomes of acute pancreatitis by reducing complications: A meta-analysis. PLoS ONE 2013, 8, e64926. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Yu, B.; Huang, J.; Peng, M. Meta-analysis of early enteral nutrition provided within 24 hours of admission on clinical outcomes in acute pancreatitis. J. Parenter. Enteral. Nutr. 2018, 42, 1139–1147. [Google Scholar] [CrossRef]
- Eatock, F.C.; Chong, P.; Menezes, N.; Murray, L.; McKay, C.J.; Carter, C.R.; Imrie, C.W. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am. J. Gastroenterol. 2005, 100, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sharma, B.; Sharma, M.; Sachdev, V.; Bhardwaj, P.; Mani, K.; Joshi, Y.K.; Saraya, A. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: A noninferiority randomized controlled trial. Pancreas 2012, 41, 153–159. [Google Scholar] [CrossRef]
- Petrov, M.S.; Correia, M.I.; Windsor, J.A. Nasogastric tube feeding in predicted severe acute pancreatitis. A systematic review of the literature to determine safety and tolerance. JOP 2008, 9, 440–448. [Google Scholar]
- Chang, Y.S.; Fu, H.Q.; Xiao, Y.M.; Liu, J.C. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: A meta-analysis. Crit. Care 2013, 17, R118. [Google Scholar] [CrossRef] [Green Version]
- Nally, D.M.; Kelly, E.G.; Clarke, M.; Ridgway, P. Nasogastric nutrition is efficacious in severe acute pancreatitis: A systematic review and meta-analysis. Br. J. Nutr. 2014, 112, 1769–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, A.K.; Goel, A.; Kirubakaran, R.; Chacko, A.; Tharyan, P. Nasogastric versus nasojejunal tube feeding for severe acute pancreatitis. Cochr. Database Syst. Rev. 2020, 3, 1–47. [Google Scholar] [CrossRef]
- Tiengou, L.E.; Gloro, R.; Pouzoulet, J.; Bouhier, K.; Read, M.H.; Arnaud-Battandier, F.; Plaze, J.M.; Blaizot, X.; Dao, T.; Piquet, M.A. Semi-elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? Randomized comparative study. J. Parenter. Enteral. Nutr. 2006, 30, 1–5. [Google Scholar] [CrossRef]
- Petrov, M.S.; Loveday, B.P.; Pylypchuk, R.D.; McIlroy, K.; Phillips, A.R.; Windsor, J.A. Systematic review and meta-analysis of enteral nutrition formulations in acute pancreatitis. Br. J. Surg. 2009, 96, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Shiraishi, A.; Fushimi, K.; Murata, K.; Otomo, Y. Comparative effectiveness of elemental formula in the early enteral nutrition management of acute pancreatitis: A retrospective cohort study. Ann. Intensive Care 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Liang, Z.Y.; Yu, W.Q.; Wang, Z.E.; Chen, Z.B.; Zhang, Y. Early enteral nutrition with polymeric feeds was associated with chylous ascites in patients with severe acute pancreatitis. Pancreas 2014, 43, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Mrowiec, S. The Role of Immunonutrition in Patients Undergoing Pancreaticoduodenectomy. Nutrients 2020, 12, 2547. [Google Scholar] [CrossRef]
- Brewczyński, A.; Jabłońska, B.; Mrowiec, S.; Składowski, K.; Rutkowski, T. Nutritional Support in Head and Neck Radiotherapy Patients Considering HPV Status. Nutrients 2020, 13, 57. [Google Scholar] [CrossRef]
- Petrov, M.S.; Atduev, V.A.; Zagainov, V.E. Advanced enteral therapy in acute pancreatitis: Is there a room for immunonutrition? A meta-analysis. Int. J. Surg. 2008, 6, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poropat, G.; Giljaca, V.; Hauser, G.; Štimac, D. Enteral nutrition formulations for acute pancreatitis. Cochr. Database Syst. Rev. 2015, 3, 1–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, C.B.; Sadek, S.A.; Walters, A.M.; Goggin, P.M.; Somers, S.S.; Toh, S.K.; Johns, T.; Duncan, H.D. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP 2006, 7, 361–371. [Google Scholar]
- Huang, X.X.; Wang, X.P.; Ma, J.J.; Jing, D.D.; Wang, P.W.; Wu, K. Effects of enteral nutrition supplemented with glutamine and arginine on gut barrier in patients with severe acute pancreatitis: A prospective randomized controlled trial. Zhonghua Yi Xue Za Zhi 2008, 88, 2407–2409. [Google Scholar] [PubMed]
- Singh, N.; Mishra, S.K.; Sachdev, V.; Sharma, H.; Upadhyay, A.D.; Arora, I.; Saraya, A. Effect of oral glutamine supplementation on gut permeability and endotoxemia in patients with severe acute pancreatitis: A randomized controlled trial. Pancreas 2014, 43, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Arutla, M.; Raghunath, M.; Deepika, G.; Jakkampudi, A.; Murthy, H.V.V.; Rao, G.V.; Reddy, D.N.; Talukdar, R. Efficacy of enteral glutamine supplementation in patients with severe and predicted severe acute pancreatitis- A randomized controlled trial. Indian J. Gastroenterol. 2019, 38, 338–347. [Google Scholar] [CrossRef]
- Jafari, T.; Feizi, A.; Askari, G.; Fallah, A.A. Parenteral immunonutrition in patients with acute pancreatitis: A systematic review and meta-analysis. Clin. Nutr. 2015, 34, 35–43. [Google Scholar] [CrossRef] [PubMed]
- De Beaux, A.C.; O’Riordain, M.G.; Ross, J.A.; Jodozi, L.; Carter, D.C.; Fearon, K.C. Glutamine-supplemented total parenteral nutrition reduces blood mononuclearcell interleukin-8 release in severe acute pancreatitis. Nutrition 1998, 14, 261–265. [Google Scholar] [CrossRef]
- Ockenga, J.; Borchert, K.; Rifai, K.; Manns, M.P.; Bischoff, S.C. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin. Nutr. 2002, 21, 409–416. [Google Scholar] [CrossRef]
- He, X.L.; Ma, Q.J.; Lu, J.G.; Chu, Y.K.; Du, X.L. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation onoutcome in severe acute pancreatitis (SAP). Clin. Nutr. Suppl. 2004, 1, 43–47. [Google Scholar]
- Sahin, H.; Mercanligil, S.M.; Inanc, N.; Ok, E. Effects of glutamine-enriched totalparenteral nutrition on acute pancreatitis. Eur. J. Clin. Nutr. 2007, 61, 1429–1434. [Google Scholar] [CrossRef]
- Fuentes-Orozco, C.; Cervantes-Guevara, G.; Muciño-Hernández, I.; López-Ortega, A.; Ambriz-González, G.; Gutiérrez-de-la-Rosa, J.L.; Gómez-Herrera, E.; Hermosillo-Sandoval, J.M.; González-Ojeda, A. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. J. Parenter. Enteral. Nutr. 2008, 32, 403–411. [Google Scholar] [CrossRef]
- Xue, P.; Deng, L.H.; Xia, Q.; Zhang, Z.D.; Hu, W.M.; Yang, X.N.; Song, B.; Huang, Z.W. Impact of alanyl-glutamine dipeptide on severe acute pancreatitis in early stage. World J. Gastroenterol. 2008, 14, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Li, N.; Li, J. Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: A randomized and controlled study. J. Parenter. Enteral. Nutr. 2008, 32, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Madden, A.M.; Gurusamy, K.S. Nutritional supplementation in enteral and parenteral nutrition for people with acute pancreatitis. Cochrane Database Syst. Rev. 2019, 2019, CD013250. [Google Scholar] [CrossRef]
- Gou, S.; Yang, Z.; Liu, T.; Wu, H.; Wang, C. Use of probiotics in the treatment of severe acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Crit. Care 2014, 18, R57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wen, J.; Xu, L.; Zhou, S.; Gong, M.; Wen, P.; Xiao, X. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J. Surg. Res. 2013, 183, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Beglinger, C.; Layer, P.; Gullo, L.; Keim, V.; Laugier, R.; Friess, H.; Schweitzer, M.; Macfie, J.; ESPEN Consensus Group. ESPEN guidelines on nutrition in acute pancreatitis. European Society of Parenteral and Enteral Nutrition. Clin. Nutr. 2002, 21, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska, B. Standard akredytacyjny. Zasady Żywienia Dojelitowego i Pozajelitowego; UCK SUM: Katowice, Poland, 2021; pp. 1–16. [Google Scholar]
| Severity of Acute Pancreatitis | Description |
|---|---|
| Mild acute pancreatitis | No organ failure No local/systemic complications |
| Moderate severe acute pancreatitis | Organ failure < 48 h |
| Severe acute pancreatitis | Organ failure > 48 h Local/systemic complications |
| Author | Findings | Type of Analysis | Outcomes |
|---|---|---|---|
| Cao et al. [39] | Lower rate of infectious complications, local complications, organ failure, MODS and mortality in EN Complication rates comparable | Meta-analysis including 6 RCTs (224 patients) Comparison of EN and PN in SAP | EN safer compared to PN in SAP patients |
| Al-Omran et al. [33] | Lower rate of mortality, MODS, septic complications, and indications for surgery than in EN | Meta-analysis including 8 (5 regarding SAP) RCTs (348 patients) Comparison of EN and PN in AP | EN should be standard nutritional intervention in AP |
| Yi et al. [34] | Lower mortality, infections, MODS, and surgery rates in EE. Comparable duration of hospitalization and nutrition | Meta-analysis including 8 RCTs (381 patients) Comparison of EN and PN in SAP | EE superior to TPN in SAP patients |
| Li et al. [40] | Lower rate of mortality, complications, MODS and surgery, shorter duration of hospitalization in EN | Meta-analysis including 9 RCTs (500 patients) Comparison of EN and PN in SAP | EE is preferred rather than TPN in SAP patients |
| Wu et al. [41] | Lower rate of mortality and infectious complications, shorter duration of hospitalization in EN. Comparable MODS rate in EE and PN. | Meta-analysis including 11 RCTs (562 patients) (348 patients) Comparison of EN and PN in SAP | EN recommended as an initial treatment for patients with SAP |
| Yao et al. [35] | Lower rate of mortality and MODS in EE. | Meta-analysis including 5 RCTs (348 patients) Comparison of EN and PN in SAP | EN should be recommended as the preferred route of nutrition for critically ill patients with SAP |
| Tao et al. [42] | Lower rate of infectious complications, MODS and mortality, shorter total duration of hospitalization and duration of hospitalization in the ICU. | Retrospective analysis of 185 patients Comparison of EE and PN in SAP | EE superior to PN in SAP patients |
| Gupta et al. [43] | Lower rate of respiratory and non-respiratory organ failure, shorter duration of hospitalization, lower cost of hospitalization in EE | Randomized controlled trial (17 patients) Comparison of EE and PN in SAP | EE safer and less expensive than PN in SAP patients |
| Author | Findings | Type of Analysis | Outcomes |
|---|---|---|---|
| Tiengou et al. [64] | Comparable feeding tolerance in both groups Shorter duration of hospitalization, lower loss of weight in patients receiving a semi-elemental formula. | Randomized controlled trials including 30 patients Comparison semi-elemental and polymeric formula in AP patients stratified according to severity | Comparable food tolerance in both groups, but better clinical outcome in patients receiving a semi-elemental formula |
| Petrov et al. [65] | Comparable feeding tolerance, infectious complications and mortality rates in both groups | Meta-analysis including trials (1070 patients) Comparison of semi-elemental or polymeric formula Comparison of semi-elemental and polymeric formula | The use of polymeric formula, compared to semi-elemental formula, does not lead to increased feeding intolerance, infectious complications or mortality |
| Endo et al. [66] | Comparable mortality, sepsis rates, hospital-free duration, total health-care costs in both groups. | Retrospective cohort study including 382 patients Comparison of elemental or control formula | Comparable results of EN with the use elemental, semi-elemental and polymeric formulas |
| Aspect of Nutritional Support in SAP | Our Recommendations |
|---|---|
| The optimal route of feeding | EN is feeding of choice in SAP patients in whom oral nutrition is impossible Parenteral nutrition is reserved for patients with intolerance or impossibility of EE |
| The optimal timing of nutrition | EEN (<48 h of admission) is superior to DEN EN should be started within 48 h of admission |
| NGT versus NJT | NGT is the route of choice NJT is preferred in patients with GOO |
| Immunonutrition | IN supplementation (including Gln in dose 0.3–0.5 g/kg/d) is recommended in PN |
| Probiotics | Not recommended |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, B.; Mrowiec, S. Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards. Nutrients 2021, 13, 1498. https://doi.org/10.3390/nu13051498
Jabłońska B, Mrowiec S. Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards. Nutrients. 2021; 13(5):1498. https://doi.org/10.3390/nu13051498
Chicago/Turabian StyleJabłońska, Beata, and Sławomir Mrowiec. 2021. "Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards" Nutrients 13, no. 5: 1498. https://doi.org/10.3390/nu13051498
APA StyleJabłońska, B., & Mrowiec, S. (2021). Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards. Nutrients, 13(5), 1498. https://doi.org/10.3390/nu13051498






