Dietary Micronutrients and Risk of Chronic Kidney Disease: A Cohort Study with 12 Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
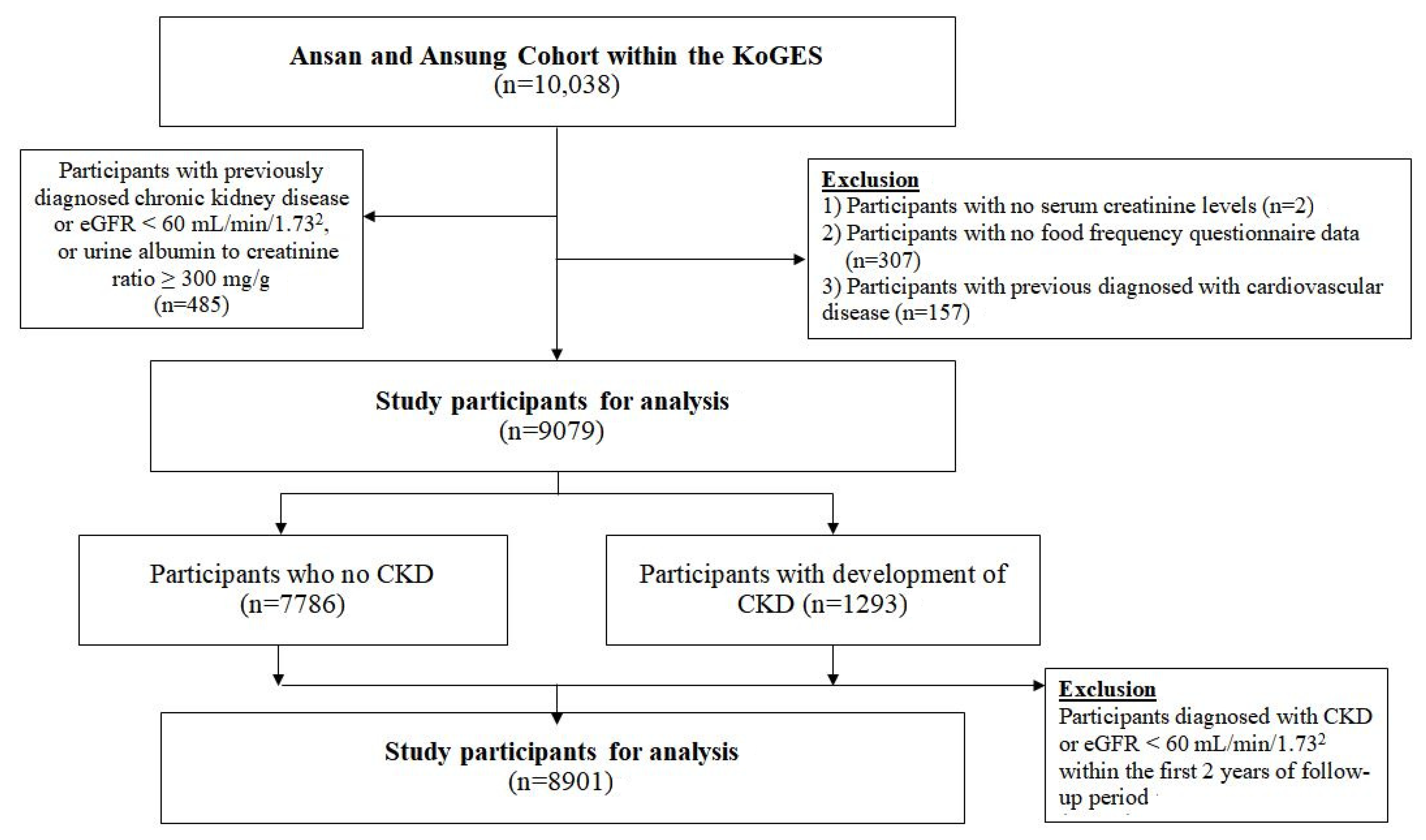
2.1. Study Population
2.2. Ascertainment of New CKD Cases over 12 Year Follow-Up
2.3. Clinical and Laboratory Measurements
2.4. Dietary Assessment
2.5. Statistical Analyses
3. Results
3.1. General Characteristics
3.2. Associations between Single-Mineral/Vitamin Intake and the Risk of Developing CKD
3.3. Discriminatory Accuracy of Models on Predicting the Risk of CKD 3B and over
3.4. Micronutrient Intake for the Risk of CKD Stage 3B and over in a Full Multivariable Clinico-Nutritional Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Park, J.I.; Baek, H.; Jung, H.H. Prevalence of chronic kidney disease in Korea: The korean national health and nutritional examination survey 2011–2013. J. Korean Med. Sci. 2016, 31, 915. [Google Scholar] [CrossRef]
- Locatelli, F.; Del Vecchio, L.; Pozzoni, P. The importance of early detection of chronic kidney disease. Nephrol. Dial. Transplant. 2002, 17, 2–7. [Google Scholar] [CrossRef]
- Haroun, M.K.; Jaar, B.G.; Hoffman, S.C.; Comstock, G.W.; Klag, M.J.; Coresh, J. Risk Factors for Chronic Kidney Disease: A Prospective Study of 23,534 Men and Women in Washington County, Maryland. J. Am. Soc. Nephrol. 2003, 14, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Kazancioğlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Farhadnejad, H.; Asghari, G.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. Micronutrient Intakes and Incidence of Chronic Kidney Disease in Adults: Tehran Lipid and Glucose Study. Nutrients 2016, 8, 217. [Google Scholar] [CrossRef]
- Huang, X.; Jiménez-Moleón, J.J.; Lindholm, B.; Cederholm, T.; Ärnlöv, J.; Risérus, U.; Sjögren, P.; Carrero, J.J. Mediterranean Diet, Kidney Function, and Mortality in Men with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1548–1555. [Google Scholar] [CrossRef]
- Lin, J.; Fung, T.T.; Hu, F.B.; Curhan, G.C. Association of Dietary Patterns with Albuminuria and Kidney Function Decline in Older White Women: A Subgroup Analysis from the Nurses’ Health Study. Am. J. Kidney Dis. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, K.-N.; Oh, K.-H.; Ahn, C.; Lee, J.; Kang, D.; Park, S.K. Association between Dietary Mineral Intake and Chronic Kidney Disease: The Health Examinees (HEXA) Study. Int. J. Environ. Res. Public Health 2018, 15, 1070. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; Group, K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A new equation to estimate glomerular filtration rate. Ann. Int. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Abutaleb, N. Why we should sub-divide CKD stage 3 into early (3a) and late (3b) components. Nephrol. Dial. Transplant. 2007, 22, 2728–2729. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.; Shoker, A. About CKD stage-3 subdivision proposal. Nephrol. Dial. Trans. 2008, 23, 1765. [Google Scholar] [CrossRef]
- Yang, J.J.; Yang, J.H.; Kim, J.; Cho, L.Y.; Park, B.; Ma, S.H.; Song, S.H.; Min, W.-K.; Kim, S.S.; Park, M.S.; et al. Reliability of Quadruplicated Serological Parameters in the Korean Genome and Epidemiology Study. Epidemiol. Health 2011, 33, e2011004. [Google Scholar] [CrossRef]
- Bellasi, A.; Mandreoli, M.; Baldrati, L.; Corradini, M.; Di Nicolò, P.; Malmusi, G.; Santoro, A. Chronic Kidney Disease Progression and Outcome According to Serum Phosphorus in Mild-to-Moderate Kidney Dysfunction. Clin. J. Am. Soc. Nephrol. 2011, 6, 883–891. [Google Scholar] [CrossRef]
- Chang, A.R.; Miller, E.R., III; Anderson, C.A.; Juraschek, S.P.; Moser, M.; White, K.; Henry, B.; Krekel, C.; Oh, S.; Charleston, J. Phosphorus additives and albuminuria in early stages of CKD: A randomized controlled trial. Am. J. Kidney Dis. 2017, 69, 200–209. [Google Scholar] [CrossRef]
- Calo, L.A.; Savica, V.; Davis, P.A. Phosphate Content of Beverages in Addition to Food Phosphate Additives: Real and Insidious Danger for Renal Patients. J. Ren. Nutr. 2012, 22, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2013, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Li, N.; Cui, L.; Zhang, X.; Liu, X.; Yu, K.; Chen, Y.; Wan, Z.; Yu, Z. Association Between Iron Status and Risk of Chronic Kidney Disease in Chinese Adults. Front. Med. 2020, 6, 303. [Google Scholar] [CrossRef] [PubMed]
- Nurko, S.; Weigelt, J.A. Anemia in chronic kidney disease: Causes, diagnosis, treatment. Clevel. Clin. J. Med. 2006, 73, 289–297. [Google Scholar] [CrossRef]
- Martines, A.M.F.; Masereeuw, R.; Tjalsma, H.; Hoenderop, J.G.; Wetzels, J.F.M.; Swinkels, D.W. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat. Rev. Nephrol. 2013, 9, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kuragano, T.; Nanami, M.; Hasuike, Y. Iron Localization and Infectious Disease in Chronic Kidney Disease Patients. Am. J. Nephrol. 2016, 43, 237–244. [Google Scholar] [CrossRef]
- Gibson, R. Assessment of the status of thiamin, riboflavin and niacin. In Principles of Nutritional Assessment; Oxford University Press: Oxford, UK, 2005; pp. 545–574. [Google Scholar]
- Skoupy, S.; Födinger, M.; Veitl, M.; Perschl, A.; Puttinger, H.; Röhrer, C.; Schindler, K.; Vychytil, A.; Hörl, W.H.; Sunder-Plassmann, G. Riboflavin Is a Determinant of Total Homocysteine Plasma Concentrations in End-Stage Renal Disease Patients. J. Am. Soc. Nephrol. 2002, 13, 1331–1337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, D.; Yuan, Y.; Guo, J.; Yang, S.; Xu, X.; Wang, Q.; Li, Y.; Qin, X.; Tang, G.; Huo, Y.; et al. Hyperhomocysteinemia predicts renal function decline: A prospective study in hypertensive adults. Sci. Rep. 2015, 5, 16268. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis 2017, 46, 265–270. [Google Scholar] [CrossRef]
- Lindner, A.; Bankson, D.D.; Stehman-Breen, C.; Mahuren, J.; Coburn, S.P. Vitamin B6 metabolism and homocysteine in end-stage renal disease and chronic renal insufficiency. Am. J. Kidney Dis. 2002, 39, 134–145. [Google Scholar] [CrossRef]
- House, A.A.; Eliasziw, M.; Cattran, D.C.; Churchill, D.N.; Oliver, M.J.; Fine, A.; Dresser, G.K.; Spence, J.D. Effect of B-vitamin therapy on progression of diabetic nephropathy: A randomized controlled trial. JAMA 2010, 303, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qin, X.; Li, Y.; Sun, D.; Wang, J.; Liang, M.; Wang, B.; Huo, Y.; Hou, F.F. Efficacy of folic acid therapy on the progression of chronic kidney disease: The renal substudy of the China Stroke Primary Prevention Trial. JAMA Intern. Med. 2016, 176, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, J.; Nair, R.; Peguero, A.; Courville, C. Vitamin C-Induced Oxalate Nephropathy. Int. J. Nephrol. 2011, 2011, 146927. [Google Scholar] [CrossRef]
- Middleton, J.P.; Lehrich, R.W. Prescriptions for dietary sodium in patients with chronic kidney disease: How will this shake out? Kidney Int. 2014, 86, 457–459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, J.; Hu, F.B.; Curhan, G.C. Associations of Diet with Albuminuria and Kidney Function Decline. Clin. J. Am. Soc. Nephrol. 2010, 5, 836–843. [Google Scholar] [CrossRef]
- Kondaiah, P.; Yaduvanshi, P.S.; Sharp, P.A.; Pullakhandam, R. Iron and Zinc Homeostasis and Interactions: Does Enteric Zinc Excretion Cross-Talk with Intestinal Iron Absorption? Nutrients 2019, 11, 1885. [Google Scholar] [CrossRef]

| Person-Years | CKD Stage 3A 1 (n = 1140) | Person-Years | CKD Stage 3B and over 1 (n = 153) | Person-Years | CKD (n = 1293) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cases n | HR (95% CI) 2 | Cases n | HR (95% CI) 2 | Cases n | HR (95% CI) 2 | ||||
| Calcium (mg) 3 | |||||||||
| <200 | 6176 | 124 | 1.0 (0.80–1.34) | 5440 | 30 | 2.0 (1.01–3.62) | 6322 | 154 | 1.1 (0.87–1.40) |
| 200–400 | 27,123 | 465 | 1.1 (0.93–1.36) | 24,181 | 52 | 1.0 (0.59–1.72) | 27,394 | 517 | 1.1 (0.92–1.32) |
| 400–600 | 22,004 | 295 | 1.0 (0.80–1.14) | 20,158 | 37 | 1.0 (0.61–1.61) | 22,207 | 332 | 1.0 (0.81–1.13) |
| ≥600 | 19,102 | 256 | 1.0 | 17,462 | 34 | 1.0 | 19,293 | 290 | 1.0 |
| Phosphorus (mg) 3 | |||||||||
| <400 | 857 | 16 | 0.9 (0.52–1.41) | 816 | 14 | 6.1 (3.22–11.58) | 920 | 30 | 1.3 (0.90–1.90) |
| 400–700 | 12,934 | 249 | 1.0 (0.84–1.18) | 11,346 | 39 | 1.7 (1.04–2.65) | 13,150 | 288 | 1.0 (0.88–1.21) |
| 700–1200 | 40,450 | 617 | 1.0 | 36,646 | 64 | 1.0 | 40,789 | 681 | 1.0 |
| ≥1200 | 20,165 | 258 | 0.8 (0.70–1.01) | 2151 | 36 | 0.9 (0.53–1.50) | 20,356 | 294 | 0.9 (0.72–1.01) |
| Sodium (mg) 3 | |||||||||
| <2000 | 16,601 | 297 | 1.0 (0.89–1.22) | 14,682 | 40 | 1.2 (0.72–1.84) | 16,808 | 337 | 1.0 (0.90–1.22) |
| 2000–2999 | 21,936 | 332 | 1.0 | 19,797 | 34 | 1.0 | 22,099 | 366 | 1.0 |
| 3000–3999 | 17,540 | 239 | 0.9 (0.78–1.09) | 15,973 | 33 | 1.1 (0.69–1.84) | 17,714 | 272 | 0.9 (0.81–1.11) |
| 4000–4999 | 9770 | 133 | 0.9 (0.75–1.14) | 1049 | 22 | 1.4 (0.83–2.52) | 9895 | 155 | 1.0 (0.80–1.17) |
| ≥5000 | 8559 | 139 | 1.0 (0.84–1.28) | 907 | 24 | 1.5 (0.87–2.71) | 8700 | 163 | 1.1 (0.88–1.30) |
| Potassium (mg) 3 | |||||||||
| <1400 | 8840 | 158 | 1.0 (0.76–1.30) | 7903 | 35 | 1.5 (0.53–4.05) | 9022 | 193 | 1.0 (0.79–1.29) |
| 1400–2400 | 29,603 | 471 | 1.1 (0.86–1.32) | 26,620 | 39 | 0.7 (0.30–1.57) | 29,810 | 510 | 1.0 (0.81–1.20) |
| 2400–3400 | 22,361 | 324 | 1.1 (0.93–1.35) | 20,383 | 46 | 1.4 (0.70–2.20) | 22,602 | 370 | 1.1 (0.93–1.32) |
| ≥3400 | 13,602 | 187 | 1.0 | 12,335 | 33 | 1.0 | 13,782 | 220 | 1.0 |
| Iron (mg) 3 | |||||||||
| <7 | 14,913 | 272 | 1.0 (0.85–1.18) | 13,250 | 48 | 1.9 (1.22–3.10) | 15,164 | 320 | 1.1 (0.91–1.24) |
| 7–10 | 21,142 | 340 | 1.0 | 18,986 | 31 | 1.0 | 21,303 | 371 | 1.0 |
| 10–15 | 26,076 | 369 | 1.0 (0.81–1.17) | 23,763 | 44 | 1.5 (0.87–2.62) | 26,315 | 413 | 1.0 (0.85–1.20) |
| ≥15 | 12,274 | 159 | 0.9 (0.69–1.12) | 11,241 | 30 | 2.1 (1.06–4.34) | 12,434 | 189 | 1.0 (0.77–1.21) |
| Zinc (mg) 3 | |||||||||
| <5 | 6484 | 143 | 0.92 (0.71–1.21) | 5620 | 32 | 2.5 (1.23–5.13) | 6637 | 175 | 1.0 (0.80–1.31) |
| 5–8 | 30,145 | 478 | 0.9 (0.69–1.05) | 27,221 | 57 | 1.3 (0.68–2.28) | 30,453 | 535 | 0.9 (0.73–1.08) |
| 8–11 | 23,234 | 339 | 1.0 | 21,044 | 38 | 1.0 | 23,448 | 377 | 1.0 |
| ≥11 | 14,543 | 180 | 0.8 (0.69–1.00) | 13,355 | 26 | 0.9 (0.56–1.58) | 14,678 | 206 | 0.8 (0.71–1.01) |
| Vitamin A (R.E) 3 | |||||||||
| <300 | 19,067 | 368 | 1.1 (0.94–1.39) | 16,828 | 54 | 1.0 (0.59–1.68) | 19,333 | 422 | 1.1 (0.93–1.35) |
| 300–500 | 24,116 | 360 | 1.1 (0.89–1.28) | 21,814 | 38 | 0.7 (0.43–1.13) | 24,309 | 398 | 1.0 (0.87–1.21) |
| 500–700 | 14,441 | 198 | 1.1 (0.89–1.31) | 13,124 | 24 | 1.0 (0.60–1.70) | 14,567 | 222 | 1.1 (0.89–1.28) |
| ≥700 | 16,783 | 214 | 1.0 | 15,475 | 37 | 1.0 | 17,006 | 251 | 1.0 |
| Retinol (µg) 3 | |||||||||
| <20 | 13,540 | 300 | 1.1 (0.86–1.31) | 11,714 | 62 | 2.2 (1.23–3.93) | 13,849 | 362 | 1.2 (0.95–1.40) |
| 20–60 | 25,553 | 402 | 1.2 (0.96–1.39) | 22,939 | 43 | 1.4 (0.79–2.46) | 25,765 | 445 | 1.2 (0.99–1.41) |
| 60–100 | 18,528 | 243 | 1.1 (0.90–1.32) | 17,034 | 29 | 1.3 (0.72–2.34) | 18,707 | 272 | 1.1 (0.93–1.34) |
| ≥100 | 16,784 | 195 | 1.0 | 15,553 | 19 | 1.0 | 16,895 | 214 | 1.0 |
| Carotene (µg) 3 | |||||||||
| <1200 | 13,517 | 248 | 1.0 (0.82–1.23) | 11,995 | 34 | 0.9 (0.53–1.58) | 13,685 | 282 | 1.0 (0.83–1.21) |
| 1200–2300 | 27,116 | 430 | 1.1 (0.91–1.27) | 24,502 | 53 | 0.8 (0.54–1.33) | 27,381 | 483 | 1.1 (0.90–1.24) |
| 2300–3400 | 15,849 | 224 | 1.0 (0.87–1.26) | 14,282 | 29 | 1.1 (0.67–1.81) | 16,007 | 253 | 1.0 (0.88–1.25) |
| ≥3400 | 17,924 | 238 | 1.0 | 16,461 | 37 | 1.0 | 18,144 | 275 | 1.0 |
| Vitamin E (mg) 3 | |||||||||
| <5 | 11,027 | 206 | 1.0 (0.81–1.33) | 9817 | 39 | 1.1 (0.52–1.94) | 11,209 | 245 | 1.0 (0.83–1.31) |
| 5–8 | 22,751 | 400 | 1.3 (1.03–1.55) | 20,213 | 35 | 0.7 (0.37–1.24) | 22,950 | 435 | 1.2 (0.97–1.43) |
| 8–11 | 19,223 | 271 | 1.1 (0.94–1.34) | 17,543 | 40 | 1.0 (0.65–1.70) | 19,429 | 311 | 1.1 (0.95–1.32) |
| ≥11 | 21,404 | 263 | 1.0 | 19,667 | 39 | 1.0 | 21,628 | 302 | 1.0 |
| Vitamin B1 (mg) 3 | |||||||||
| <0.9 | 17,773 | 339 | 1.0 (0.85–1.18) | 15,727 | 51 | 1.3 (0.79–1.96) | 18,038 | 390 | 1.0 (0.87–1.19) |
| 0.9–1.2 | 18,463 | 306 | 1.0 | 16,625 | 35 | 1.0 | 18,645 | 341 | 1.0 |
| 1.2–1.5 | 19,236 | 252 | 0.9 (0.77–1.14) | 17,620 | 32 | 1.3 (0.71–2.34) | 19,404 | 284 | 1.0 (0.79–1.15) |
| ≥1.5 | 18,934 | 243 | 0.8 (0.66–1.04) | 17,269 | 35 | 1.2 (0.58–2.42) | 19,128 | 278 | 0.9 (0.69–1.06) |
| Vitamin B2 (mg) 3 | |||||||||
| <0.7 | 17,674 | 361 | 1.0 (0.82–1.28) | 15,478 | 64 | 2.3 (1.18–4.45) | 18,004 | 425 | 1.1 (0.89–1.35) |
| 0.7–0.9 | 18,651 | 294 | 1.0 (0.85–1.26) | 16,859 | 31 | 1.4 (0.78–2.60) | 18,811 | 325 | 1.1 (0.88–1.28) |
| 0.9–1.2 | 19,248 | 263 | 1.0 | 17,502 | 25 | 1.0 | 19,381 | 288 | 1.0 |
| ≥1.2 | 18,833 | 222 | 0.9 (0.78–1.13) | 17,401 | 33 | 1.2 (0.69–2.02) | 19,021 | 255 | 1.0 (0.82–1.16) |
| Niacin (mg) 3 | |||||||||
| <10 | 17,808 | 345 | 0.7 (0.58–1.00) | 10,508 | 44 | 1.1 (0.58–2.28) | 18,116 | 404 | 0.8 (0.61–0.98) |
| 10–14 | 18,611 | 300 | 0.9 (0.69–1.06) | 19,311 | 42 | 0.8 (0.44–1.50) | 18,786 | 333 | 0.9 (0.70–1.06) |
| 14–19 | 18,891 | 271 | 1.0 | 20,904 | 40 | 1.0 | 19,059 | 303 | 1.0 |
| ≥19 | 19,095 | 224 | 0.9 (0.76–1.09) | 16,517 | 27 | 1.0 (0.57–1.60) | 19,255 | 253 | 0.9 (0.77–1.09) |
| Folate (µg) 3 | |||||||||
| <100 | 3555 | 57 | 1.1 (0.75–1.54) | 3248 | 22 | 3.0 (1.04–8.39) | 3663 | 79 | 1.2 (0.89–1.68) |
| 100–200 | 25,961 | 433 | 1.2 (0.97–1.60) | 23,253 | 47 | 1.1 (0.45–2.63) | 26,224 | 480 | 1.2 (0.93–1.49) |
| 200–300 | 26,083 | 396 | 1.2 (0.96–1.51) | 23,536 | 36 | 0.7 (0.35–1.45) | 26,255 | 432 | 1.1 (0.91–1.40) |
| 300–400 | 11,099 | 153 | 1.2 (0.94–1.56) | 10,177 | 28 | 1.4 (0.75–2.50) | 11,244 | 181 | 1.2 (0.98–1.55) |
| ≥400 | 7708 | 101 | 1.0 | 7027 | 20 | 1.0 | 7830 | 121 | 1.0 |
| Vitamin B6 (mg) 3 | |||||||||
| <1.0 | 5946 | 120 | 1.0 (0.80–1.25) | 5292 | 30 | 2.7 (1.52–4.93) | 6078 | 150 | 1.1 (0.91–1.37) |
| 1.0–1.3 | 12,436 | 215 | 1.0 (0.86–1.25) | 11,058 | 22 | 1.2 (0.65–2.25) | 12,567 | 237 | 1.0 (0.87–1.24) |
| 1.3–1.6 | 15,251 | 244 | 1.0 | 13,729 | 19 | 1.0 | 15,349 | 263 | 1.0 |
| ≥1.6 | 40,773 | 561 | 1.0 (0.82–1.20) | 37,161 | 82 | 2.3 (1.24–4.14) | 41,222 | 643 | 1.1 (0.89–1.28) |
| Vitamin C (mg) 3 | |||||||||
| <60 | 13,034 | 215 | 0.9 (0.77–1.14) | 11,815 | 30 | 1.4 (0.77–2.61) | 13,176 | 245 | 1.0 (0.81–1.17) |
| 60–75 | 8369 | 145 | 1.0 (0.84–1.29) | 7459 | 17 | 1.7 (0.83–3.33) | 8472 | 162 | 1.1 (0.87–1.31) |
| 75–100 | 13,717 | 208 | 1.0 | 12,418 | 16 | 1.0 | 13,802 | 224 | 1.0 |
| ≥100 | 39,286 | 572 | 0.9 (0.79–1.11) | 35,547 | 90 | 1.9 (1.07–3.22) | 39,766 | 662 | 1.0 (0.85–1.17) |
| Entire Cohort | Restricted Cohort 3 | |||
|---|---|---|---|---|
| HR (95% CI) 2 | p-Value | HR (95% CI) 2 | p-Value | |
| Age | 1.17 (1.14–1.21) | <0.01 | 1.17 (1.14–1.22) | <0.01 |
| Sex (Female) | 2.14 (1.21–3.79) | <0.01 | 1.96 (1.06–3.64) | 0.03 |
| Baseline eGFR | 0.94 (0.93–0.95) | <0.01 | 0.95 (0.94–0.94) | <0.01 |
| Smoker | 1.96 (1.11–3.45) | 0.02 | 1.54 (0.83–2.84) | 0.17 |
| Physical activity | 1.20 (0.85–1.68) | 0.29 | 1.08 (0.12–2.05) | 0.67 |
| BMI (≥25) | 1.27 (0.90–1.79) | 0.17 | 1.19 (0.82–1.72) | 0.36 |
| Hypertension | 1.71 (1.23–2.38) | <0.01 | 1.79 (1.24–2.56) | <0.01 |
| Diabetes | 5.01 (3.51–7.15) | <0.01 | 5.46 (3.71–8.03) | <0.01 |
| Diet energy intake | 0.94 (0.56–1.59) | 0.82 | 0.94 (0.54–1.65) | 0.83 |
| Diet protein intake | 1.41 (0.71–2.78) | 0.32 | 1.43 (0.69–2.95) | 0.33 |
| Calcium (mg) 4 | ||||
| <200 | 0.68 (0.21–2.20) | 0.51 | 0.77 (0.21–2.85) | 0.69 |
| 200–400 | 0.84 (0.34–2.05) | 0.69 | 0.98 (0.37–2.60) | 0.97 |
| 400–600 | 1.06 (0.54–2.08) | 0.86 | 1.21 (0.58–2.55) | 0.60 |
| ≥600 | 1.0 | - | 1.0 | - |
| Phosphorus (mg) 4 | ||||
| <400 | 6.78 (2.18–21.11) | <0.01 | 8.51 (2.33–31.08) | <0.01 |
| 400–700 | 1.89 (0.95–4.11) | 0.09 | 2.42 (1.02–5.74) | 0.04 |
| 700–1200 | 1.0 | - | 1.0 | - |
| ≥1200 | 0.62 (0.29–1.36) | 0.23 | 0.60 (0.26–1.37) | 0.22 |
| Sodium (mg) 4 | ||||
| <2000 | 0.95 (0.54–1.69) | 0.86 | 0.95 (0.50–1.82) | 0.88 |
| 2000–2999 | 1.0 | - | 1.0 | - |
| 3000–3999 | 1.18 (0.70–1.99) | 0.54 | 1.14 (0.64–2.04) | 0.65 |
| 4000–4999 | 1.25 (0.67–2.32) | 0.47 | 1.45 (0.75–2.83) | 0.26 |
| ≥5000 | 1.38 (0.69–2.76) | 0.36 | 1.76 (0.84–3.68) | 0.13 |
| Iron (mg) 4 | ||||
| <7 | 1.00 (0.46–2.17) | 0.99 | 0.93 (0.41–2.15) | 0.87 |
| 7–10 | 1.0 | - | 1.0 | - |
| 10–15 | 1.11 (0.58–2.13) | 0.74 | 1.22 (0.60–2.51) | 0.57 |
| ≥15 | 2.05 (0.72–5.82) | 0.17 | 2.06 (0.66–6.46) | 0.21 |
| Retinol (µg) 4 | ||||
| <20 | 1.78 (0.81–3.92) | 0.15 | 1.78 (0.76–4.16) | 0.18 |
| 20–60 | 1.28 (0.63–2.62) | 0.49 | 1.24 (0.57–2.68) | 0.59 |
| 60–100 | 1.25 (0.67–2.34) | 0.47 | 1.33 (0.68–2.61) | 0.40 |
| ≥100 | 1.0 | - | 1.0 | - |
| Vitamin B2 (mg) 4 | ||||
| <0.7 | 2.90 (1.01–8.33) | 0.04 | 2.69 (0.95–8.10) | 0.08 |
| 0.7–0.9 | 1.81 (0.87–3.74) | 0.11 | 2.13 (0.98–4.64) | 0.05 |
| 0.9–1.2 | 1.0 | - | 1.0 | - |
| ≥1.2 | 0.92 (0.43–1.95) | 0.81 | 1.11 (0.49–2.55) | 0.06 |
| Folate (µg) 4 | ||||
| <100 | 2.57 (0.66–10.08) | 0.17 | 6.72 (1.40–32.16) | 0.02 |
| 100–200 | 1.39 (0.48–4.04) | 0.54 | 1.35 (0.44–4.19) | 0.60 |
| 200–300 | 0.74 (0.33–1.70) | 0.48 | 0.60 (0.25–1.44) | 0.25 |
| 300–400 | 1.38 (0.71–2.70) | 0.34 | 1.09 (0.53–2.23) | 0.75 |
| ≥400 | 1.0 | - | 1.0 | - |
| Vitamin B6 (mg) 4 | ||||
| <1.0 | 0.90 (0.32–2.56) | 0.84 | 0.43 (0.12–1.55) | 0.19 |
| 1.0–1.3 | 0.87 (0.42–1.81) | 0.71 | 0.97 (0.44–2.14) | 0.93 |
| 1.3–1.6 | 1.0 | - | 1.0 | - |
| ≥1.6 | 2.71 (1.26–5.81) | 0.01 | 3.08 (1.34–7.09) | <0.01 |
| Vitamin C (mg) 4 | ||||
| <60 | 0.64 (0.31–1.32) | 0.23 | 0.45 (0.19–1.04) | 0.06 |
| 60–75 | 1.39 (0.69–2.80) | 0.35 | 1.45 (0.68–3.06) | 0.33 |
| 75–100 | 1.0 | - | 1.0 | - |
| ≥100 | 1.83 (1.00–3.33) | 0.05 | 1.79 (0.98–3.41) | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Oh, K.-H.; Park, S.-K. Dietary Micronutrients and Risk of Chronic Kidney Disease: A Cohort Study with 12 Year Follow-Up. Nutrients 2021, 13, 1517. https://doi.org/10.3390/nu13051517
Lee J, Oh K-H, Park S-K. Dietary Micronutrients and Risk of Chronic Kidney Disease: A Cohort Study with 12 Year Follow-Up. Nutrients. 2021; 13(5):1517. https://doi.org/10.3390/nu13051517
Chicago/Turabian StyleLee, Juyeon, Kook-Hwan Oh, and Sue-Kyung Park. 2021. "Dietary Micronutrients and Risk of Chronic Kidney Disease: A Cohort Study with 12 Year Follow-Up" Nutrients 13, no. 5: 1517. https://doi.org/10.3390/nu13051517
APA StyleLee, J., Oh, K.-H., & Park, S.-K. (2021). Dietary Micronutrients and Risk of Chronic Kidney Disease: A Cohort Study with 12 Year Follow-Up. Nutrients, 13(5), 1517. https://doi.org/10.3390/nu13051517






