Association of Zinc Deficiency with Development of CVD Events in Patients with CKD
Abstract
1. Introduction
2. Zinc and Nutrition
3. Zinc Deficiency in CKD
3.1. Urinary Zinc Excretion in CKD
3.2. Taste Change Associated with CKD
3.3. Albumin and Zinc in CKD
3.4. Other Factors of Zinc Deficiency in CKD
4. Vascular Calcification and CVD in CKD
5. Vascular Calcification and Vascular Smooth Muscle Cells
5.1. Vascular Smooth Muscle Cells
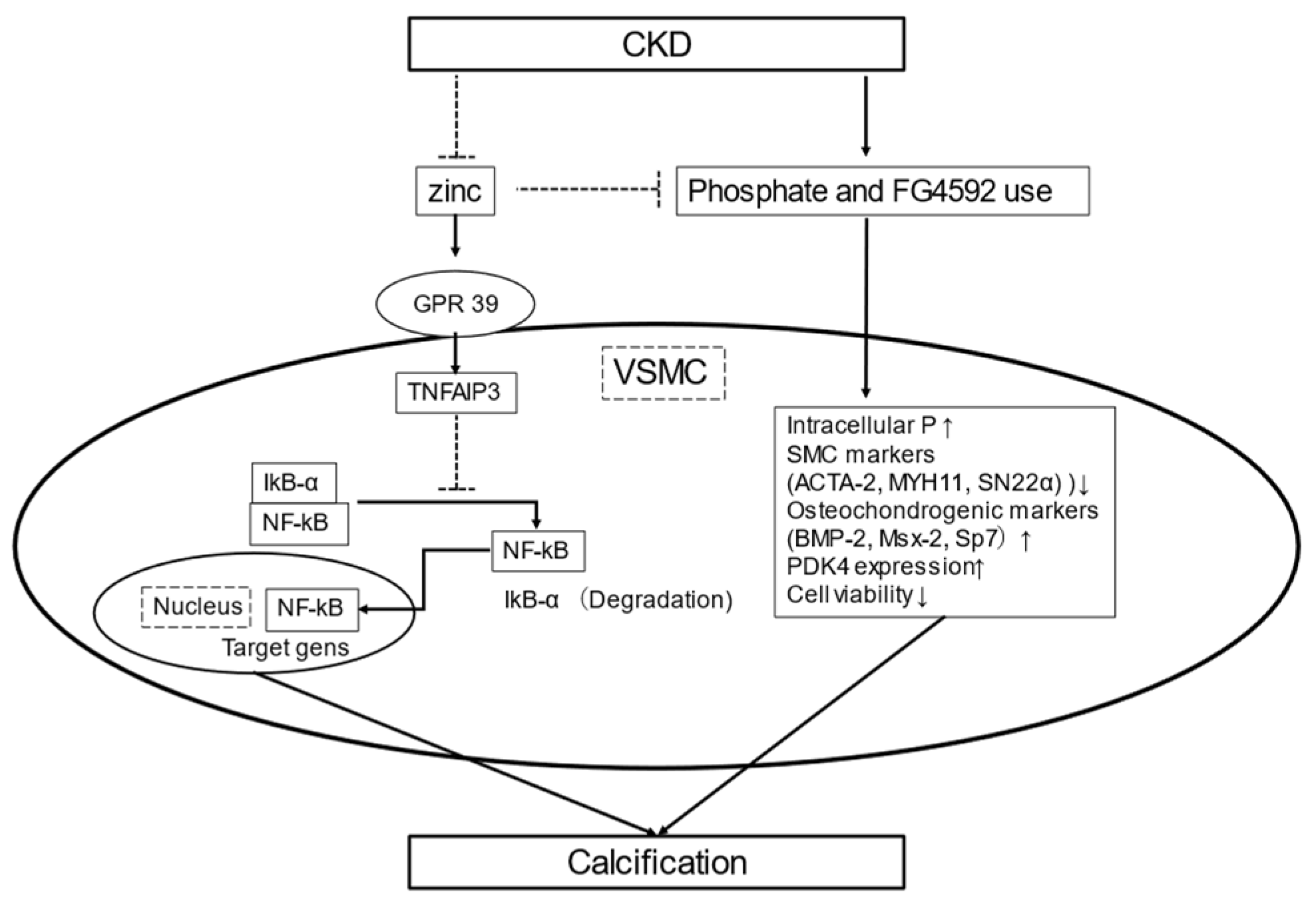
5.2. Zinc Inhibits Phosphate-Induced VSMC Calcification
6. Zinc and Calcification Propensity in Serum
6.1. Serum Calcification Propensity (T50)
6.2. Association of Zinc and Serum Calcification Propensity
7. Zinc and Vascular Change
7.1. Zinc and Abdominal Aortic Calcification
7.2. Zinc and Carotid Intima-Media Thickness
8. Zinc Deficiency and Risk Factors for CVD
8.1. Zinc Deficiency and Blood Pressure
8.2. Zinc Deficiency and Dyslipidemia
8.3. Zinc Deficiency and Type 2 Diabetes
8.4. Zinc Deficiency and Inflammation
8.5. Zinc Deficiency and Oxidative Stress
9. Zinc Levels and CVD Events
10. Zinc and CVD Mortality
10.1. Blood Zinc Level and CVD Mortality
10.2. Dietary Zinc Intake and CVD Mortality
11. Zinc and Progression of CKD
12. Zinc Supplementation in Patients with CKD
13. Optimal Serum Zinc Level
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | abdominal aortic calcification |
| ACTA-2 | smooth muscle a-2 actin |
| BMI | body mass index |
| BMP2 | bone morphogenetic protein-2 |
| CIMT | carotid intima-media thickness |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| eGFR | estimated glomerular rate |
| HIF | hypoxia-inducible factor |
| LDL | low-density lipoprotein |
| MGP | matrix Gla protein |
| Msx-2 | Msh homeobox 2 |
| MYH11 | smooth muscle myosin heavy chain 11; |
| NCC | Na+-Cl− cotransporter |
| NF-k B | nuclear factor kappa light chain enhancer of activated B |
| NLRP3 | nucleotide-binding domain and leucine-rich repeat-containing family, pyrin domain-containing-3 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PDK4 | pyruvate dehydrogenase kinase 4 |
| PHI | prolyl hydroxylase inhibitors |
| RCT | randomized controlled trial |
| RDI | recommended dietary intake |
| ROS | reactive oxygen species |
| Runx2 | runt-related transcription factor 2 |
| SOD | chronic kidney disease |
| T50 | serum calcification propensity |
| TNF | tumor necrosis factor |
| TNFAIP3 | TNFa-induced protein 3 |
| VSMC | vasculature smooth muscle cell |
References
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef]
- Dvornik, S.; Cuk, M.; Racki, S.; Zaputovic, L. Serum zinc concentrations in the maintenance hemodialysis patients. Coll. Antropol. 2006, 30, 125–129. [Google Scholar]
- Lee, S.H.; Huang, J.W.; Hung, K.Y.; Leu, L.J.; Kan, Y.T.; Yang, C.S.; Chung Wu, D.; Huang, C.L.; Chen, P.Y.; Chen, J.S.; et al. Trace Metals’ abnormalities in hemodialysis patients: Relationship with medications. Artif. Organs. 2000, 24, 841–844. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Golden, M.H.; Golden, B.E. Effect of zinc supplementation on the dietary intake, rate of weight gain, and energy cost of tissue deposition in children recovering from severe malnutrition. Am. J. Clin. Nutr. 1981, 34, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Walravens, P.A.; Hambidge, K.M.; Koepfer, D.M. Zinc supplementation in infants with a nutritional pattern of failure to thrive: A double-blind, controlled study. Pediatrics 1989, 83, 532–538. [Google Scholar]
- Henkin, R.I. Zinc in taste function: A critical review. Biol. Trace Elem. Res. 1984, 6, 263–280. [Google Scholar] [CrossRef]
- Lask, B.; Fosson, A.; Rolfe, U.; Thomas, S. Zinc deficiency and childhood-onset anorexia nervosa. J. Clin. Psychiatry 1993, 54, 63–66. [Google Scholar]
- Gray, N.A.; Dhana, A.; Stein, D.J.; Khumalo, N.P. Zinc and atopic dermatitis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1042–1050. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985, 5, 341–363. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wu, M.L.; Chou, Y.Y.; Li, S.Y.; Deng, J.F.; Yang, W.C.; Ng, Y.Y. Essential trace element status and clinical outcomes in long-term dialysis patients: A two-year prospective observational cohort study. Clin. Nutr. 2012, 31, 630–636. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants (Basel) 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Yang, L.; Wang, J.; Hu, Y.; Bian, A.; Liu, J.; Ma, J. Zinc inhibits high glucose-induced NLRP3 inflammasome activation in human peritoneal mesothelial cells. Mol. Med. Rep. 2017, 16, 5195–5202. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Petho, D.; Gall, T.; Zavaczki, E.; Nyitrai, M.; Posta, J.; Zarjou, A.; Agarwal, A.; Balla, G.; Balla, J. Zinc Inhibits HIF-Prolyl Hydroxylase Inhibitor-Aggravated VSMC Calcification Induced by High Phosphate. Front. Physiol. 2019, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro, O.M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-kappaB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef]
- Gorriz, J.L.; Molina, P.; Cerveron, M.J.; Vila, R.; Bover, J.; Nieto, J.; Barril, G.; Martinez-Castelao, A.; Fernandez, E.; Escudero, V.; et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin. J. Am. Soc. Nephrol. 2015, 10, 654–666. [Google Scholar] [CrossRef]
- Levin, A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin. Dial. 2003, 16, 101–105. [Google Scholar] [CrossRef]
- Pasch, A.; Farese, S.; Graber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Nakatani, S.; Mori, K.; Sonoda, M.; Nishide, K.; Uedono, H.; Tsuda, A.; Emoto, M.; Shoji, T. Association between Serum Zinc and Calcification Propensity (T50) in Patients with Type 2 Diabetes Mellitus and In Vitro Effect of Exogenous Zinc on T50. Biomedicines 2020, 8, 337. [Google Scholar] [CrossRef]
- Chen, W.; Eisenberg, R.; Mowrey, W.B.; Wylie-Rosett, J.; Abramowitz, M.K.; Bushinsky, D.A.; Melamed, M.L. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial. Transplant. 2020, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y. [Dietary reference intakes of trace elements for Japanese and problems in clinical fields]. Nihon Rinsho 2016, 74, 1066–1073. [Google Scholar]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s Practical Guidelines for Zinc Deficiency with a Particular Focus on Taste Disorders, Inflammatory Bowel Disease, and Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Craig, W.J.; Baines, S.K. Zinc and vegetarian diets. Med. J. Aust. 2013, 199, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Grungreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency-An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478S–1483S. [Google Scholar] [CrossRef]
- Wessels, I.; Rink, L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020, 77, 108240. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.K.; Bowersox, E.M.; Rye, D.L.; Abu-Hamdan, D.K.; Prasad, A.S.; McDonald, F.D.; Biersack, K.L. Factors underlying abnormal zinc metabolism in uremia. Kidney Int. Suppl. 1989, 27, S269–S273. [Google Scholar] [PubMed]
- Cardozo, L.; Mafra, D. Don’t forget the zinc. Nephrol. Dial. Transplant. 2020, 35, 1094–1098. [Google Scholar] [CrossRef]
- Pisano, M.; Hilas, O. Zinc and Taste Disturbances in Older Adults: A Review of the Literature. Consult. Pharm. 2016, 31, 267–270. [Google Scholar] [CrossRef]
- Chen, Y.H.; Jeng, S.S.; Hsu, Y.C.; Liao, Y.M.; Wang, Y.X.; Cao, X.; Huang, L.J. In anemia zinc is recruited from bone and plasma to produce new red blood cells. J. Inorg. Biochem. 2020, 210, 111172. [Google Scholar] [CrossRef]
- Tavares, A.; Mafra, D.; Leal, V.O.; Gama, M.D.S.; Vieira, R.; Brum, I.; Borges, N.A.; Silva, A.A. Zinc Plasma Status and Sensory Perception in Nondialysis Chronic Kidney Disease Patients. J. Ren. Nutr. 2020. [Google Scholar] [CrossRef]
- Shen, Y.; Yin, Z.; Lv, Y.; Luo, J.; Shi, W.; Fang, J.; Shi, X. Plasma element levels and risk of chronic kidney disease in elderly populations (>/= 90 Years old). Chemosphere 2020, 254, 126809. [Google Scholar] [CrossRef]
- Damianaki, K.; Lourenco, J.M.; Braconnier, P.; Ghobril, J.P.; Devuyst, O.; Burnier, M.; Lenglet, S.; Augsburger, M.; Thomas, A.; Pruijm, M. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol. Dial. Transplant. 2020, 35, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.F.; Lin, C.J.; Chen, S.H.; Huang, C.F.; Lee, C.C. Association between trace element concentrations and anemia in patients with chronic kidney disease: A cross-sectional population-based study. J. Investig. Med. 2019, 67, 995–1001. [Google Scholar] [CrossRef]
- Aziz, M.A.; Majeed, G.H.; Diab, K.S.; Al-Tamimi, R.J. The association of oxidant-antioxidant status in patients with chronic renal failure. Ren. Fail. 2016, 38, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Cuppari, L.; Cozzolino, S.M. Iron and zinc status of patients with chronic renal failure who are not on dialysis. J. Ren. Nutr. 2002, 12, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Hasanato, R.M. Assessment of trace elements in sera of patients undergoing renal dialysis. Saudi Med. J. 2014, 35, 365–370. [Google Scholar] [PubMed]
- Lobo, J.C.; Stockler-Pinto, M.B.; Farage, N.E.; Faulin Tdo, E.; Abdalla, D.S.; Torres, J.P.; Velarde, L.G.; Mafra, D. Reduced plasma zinc levels, lipid peroxidation, and inflammation biomarkers levels in hemodialysis patients: Implications to cardiovascular mortality. Ren. Fail. 2013, 35, 680–685. [Google Scholar] [CrossRef]
- Guo, C.H.; Wang, C.L.; Chen, P.C.; Yang, T.C. Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in patients undergoing either hemodialysis or peritoneal dialysis. Perit. Dial. Int. 2011, 31, 583–591. [Google Scholar] [CrossRef]
- Dashti-Khavidaki, S.; Khalili, H.; Vahedi, S.M.; Lessan-Pezeshki, M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity. Saudi. J. Kidney Dis. Transpl. 2010, 21, 641–645. [Google Scholar]
- Kiziltas, H.; Ekin, S.; Erkoc, R. Trace element status of chronic renal patients undergoing hemodialysis. Biol. Trace Elem. Res. 2008, 124, 103–109. [Google Scholar] [CrossRef]
- Batista, M.N.; Cuppari, L.; de Fatima Campos Pedrosa, L.; Almeida, M.; de Almeida, J.B.; de Medeiros, A.C.; Canziani, M.E. Effect of end-stage renal disease and diabetes on zinc and copper status. Biol. Trace Elem. Res. 2006, 112, 1–12. [Google Scholar] [CrossRef]
- Barnett, J.P.; Blindauer, C.A.; Kassaar, O.; Khazaipoul, S.; Martin, E.M.; Sadler, P.J.; Stewart, A.J. Allosteric modulation of zinc speciation by fatty acids. Biochim. Biophys. Acta. 2013, 1830, 5456–5464. [Google Scholar] [CrossRef]
- Melichar, B.; Malir, F.; Jandik, P.; Malirova, E.; Vavrova, J.; Mergancova, J.; Voboril, Z. Increased urinary zinc excretion in cancer patients is linked to immune activation and renal tubular cell dysfunction. Biometals 1995, 8, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.F.; Fons, C.; Fussellier, M.; Bardet, L.; Orsetti, A. Urinary zinc and its relationships with microalbuminuria in type I diabetics. Biol. Trace Elem. Res. 1992, 32, 317–323. [Google Scholar] [CrossRef]
- Marreiro, D.N.; do Perpetuo Socorro, C.M.M.; de Sousa, S.S.; Ibiapina, V.; Torres, S.; Pires, L.V.; do Nascimento Nogueira, N.; Lima, J.M.; do Monte, S.J. Urinary excretion of zinc and metabolic control of patients with diabetes type 2. Biol. Trace Elem. Res. 2007, 120, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pruijm, M.; Ponte, B.; Ackermann, D.; Paccaud, F.; Guessous, I.; Ehret, G.; Pechere-Bertschi, A.; Vogt, B.; Mohaupt, M.G.; Martin, P.Y.; et al. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin. J. Am. Soc. Nephrol 2016, 11, 70–80. [Google Scholar] [CrossRef]
- Manley, K.J. Saliva composition and upper gastrointestinal symptoms in chronic kidney disease. J. Ren. Care. 2014, 40, 172–179. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Wiese, G.; Moorthi, R.N.; Moe, S.M.; Hill Gallant, K.; Running, C.A. Characterizing Dysgeusia in Hemodialysis Patients. Chem. Senses 2019, 44, 165–171. [Google Scholar] [CrossRef]
- Dawson, J.; Brennan, F.P.; Hoffman, A.; Josland, E.; Li, K.C.; Smyth, A.; Brown, M.A. Prevalence of Taste Changes and Association with Other Nutrition-Related Symptoms in End-Stage Kidney Disease Patients. J. Ren. Nutr. 2021, 31, 80–84. [Google Scholar] [CrossRef]
- Naganuma, M.; Ikeda, M.; Tomita, H. Changes in soft palate taste buds of rats due to aging and zinc deficiency--scanning electron microscopic observation. Auris. Nasus. Larynx. 1988, 15, 117–127. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tomita, H. Electron microscopic observation of vallate taste buds of zinc-deficient rats with taste disturbance. Auris. Nasus. Larynx. 1986, 13 Suppl 1, S25–31. [Google Scholar] [CrossRef]
- Sekine, H.; Takao, K.; Yoshinaga, K.; Kokubun, S.; Ikeda, M. Effects of zinc deficiency and supplementation on gene expression of bitter taste receptors (TAS2Rs) on the tongue in rats. Laryngoscope 2012, 122, 2411–2417. [Google Scholar] [CrossRef]
- Yoshida, S.; Endo, S.; Tomita, H. A double-blind study of the therapeutic efficacy of zinc gluconate on taste disorder. Auris. Nasus. Larynx. 1991, 18, 153–161. [Google Scholar] [CrossRef]
- Heckmann, S.M.; Hujoel, P.; Habiger, S.; Friess, W.; Wichmann, M.; Heckmann, J.G.; Hummel, T. Zinc gluconate in the treatment of dysgeusia--a randomized clinical trial. J. Dent. Res. 2005, 84, 35–38. [Google Scholar] [CrossRef]
- Sakai, F.; Yoshida, S.; Endo, S.; Tomita, H. Double-blind, placebo-controlled trial of zinc picolinate for taste disorders. Acta Otolaryngol. 2002. [Google Scholar] [CrossRef]
- Sakagami, M.; Ikeda, M.; Tomita, H.; Ikui, A.; Aiba, T.; Takeda, N.; Inokuchi, A.; Kurono, Y.; Nakashima, M.; Shibasaki, Y.; et al. A zinc-containing compound, Polaprezinc, is effective for patients with taste disorders: Randomized, double-blind, placebo-controlled, multi-center study. Acta Otolaryngol. 2009, 129, 1115–1120. [Google Scholar] [CrossRef]
- Scott, B.J.; Bradwell, A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.K.; Prasad, A.S.; Rabbani, P.; Briggs, W.A.; McDonald, F.D. Zinc metabolism in uremia. J. Lab. Clin. Med. 1979, 94, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Takagi, K.; Masuda, K.; Yamazaki, M.; Kiyohara, C.; Itoh, S.; Wasaki, M.; Inoue, H. Metal ion and vitamin adsorption profiles of phosphate binder ion-exchange resins. Clin. Nephrol. 2010, 73, 30–35. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- van der Velde, M.; Matsushita, K.; Coresh, J.; Astor, B.C.; Woodward, M.; Levey, A.; de Jong, P.; Gansevoort, R.T.; Chronic Kidney Disease Prognosis, C.; van der Velde, M.; et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011, 79, 1341–1352. [Google Scholar] [CrossRef]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 2014, 307, F891–S900. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, I.; Holt, S.G.; Hewitson, T.D.; Smith, E.R.; Toussaint, N.D. Current and potential therapeutic strategies for the management of vascular calcification in patients with chronic kidney disease including those on dialysis. Semin Dial. 2018, 31, 487–499. [Google Scholar] [CrossRef]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Houben, E.; Neradova, A.; Schurgers, L.J.; Vervloet, M. The influence of phosphate, calcium and magnesium on matrix Gla-protein and vascular calcification: A systematic review. G. Ital. Nefrol. 2016, 33. [Google Scholar]
- Lang, F.; Ritz, E.; Voelkl, J.; Alesutan, I. Vascular calcification--is aldosterone a culprit? Nephrol. Dial. Transplant. 2013, 28, 1080–1084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cozzolino, M.; Gallieni, M.; Brancaccio, D. Vascular calcification in uremic conditions: New insights into pathogenesis. Semin. Nephrol. 2006, 26, 33–37. [Google Scholar] [CrossRef]
- Jablonski, K.L.; Chonchol, M. Vascular calcification in end-stage renal disease. Hemodial Int. 2013, 17 Suppl 1, S17–21. [Google Scholar] [CrossRef]
- Demer, L.L.; Tintut, Y. Mineral exploration: Search for the mechanism of vascular calcification and beyond: The 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc. Biol. 2003, 23, 1739–1743. [Google Scholar] [CrossRef]
- Wallin, R.; Wajih, N.; Greenwood, G.T.; Sane, D.C. Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med. Res. Rev. 2001, 21, 274–301. [Google Scholar] [CrossRef]
- Tanaka, T.; Sato, H.; Doi, H.; Yoshida, C.A.; Shimizu, T.; Matsui, H.; Yamazaki, M.; Akiyama, H.; Kawai-Kowase, K.; Iso, T.; et al. Runx2 represses myocardin-mediated differentiation and facilitates osteogenic conversion of vascular smooth muscle cells. Mol. Cell Biol. 2008, 28, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.E.; Chen, T.M.; Wallingford, M.C.; Nguyen, N.B.; Yamada, S.; Sawangmake, C.; Zhang, J.; Speer, M.Y.; Giachelli, C.M. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc. Res. 2016, 112, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xu, M.J.; Zhao, M.M.; Dai, X.Y.; Kong, W.; Wilson, G.M.; Guan, Y.; Wang, C.Y.; Wang, X. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012, 82, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Besarab, A.; Chernyavskaya, E.; Motylev, I.; Shutov, E.; Kumbar, L.M.; Gurevich, K.; Chan, D.T.; Leong, R.; Poole, L.; Zhong, M.; et al. Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients. J. Am. Soc. Nephrol. 2016, 27, 1225–1233. [Google Scholar] [CrossRef]
- Besarab, A.; Provenzano, R.; Hertel, J.; Zabaneh, R.; Klaus, S.J.; Lee, T.; Leong, R.; Hemmerich, S.; Yu, K.H.; Neff, T.B. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial. Transplant. 2015, 30, 1665–1673. [Google Scholar] [CrossRef]
- Mokas, S.; Lariviere, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, W.Q.; Han, X.Q.; Wang, Y.; Wang, X.; Liu, N.F. Advanced glycation end products accelerate calcification in VSMCs through HIF-1alpha/PDK4 activation and suppress glucose metabolism. Sci. Rep. 2018, 8, 13730. [Google Scholar] [CrossRef]
- Heiss, A.; Jahnen-Dechent, W.; Endo, H.; Schwahn, D. Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases 2007, 2, 16–20. [Google Scholar] [CrossRef]
- Heiss, A.; DuChesne, A.; Denecke, B.; Grotzinger, J.; Yamamoto, K.; Renne, T.; Jahnen-Dechent, W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 2003, 278, 13333–13341. [Google Scholar] [CrossRef]
- Pasch, A.; Block, G.A.; Bachtler, M.; Smith, E.R.; Jahnen-Dechent, W.; Arampatzis, S.; Chertow, G.M.; Parfrey, P.; Ma, X.; Floege, J. Blood Calcification Propensity, Cardiovascular Events, and Survival in Patients Receiving Hemodialysis in the EVOLVE Trial. Clin. J. Am. Soc. Nephrol. 2017, 12, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; de Borst, M.H.; van den Berg, E.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016, 27, 239–248. [Google Scholar] [CrossRef]
- Dahle, D.O.; Asberg, A.; Hartmann, A.; Holdaas, H.; Bachtler, M.; Jenssen, T.G.; Dionisi, M.; Pasch, A. Serum Calcification Propensity Is a Strong and Independent Determinant of Cardiac and All-Cause Mortality in Kidney Transplant Recipients. Am. J. Transplant. 2016, 16, 204–212. [Google Scholar] [CrossRef]
- Silaghi, C.N.; Ilyes, T.; Van Ballegooijen, A.J.; Craciun, A.M. Calciprotein Particles and Serum Calcification Propensity: Hallmarks of Vascular Calcifications in Patients with Chronic Kidney Disease. J. Clin. Med. 2020, 9, 1287. [Google Scholar] [CrossRef]
- Wilson, P.W.; Kauppila, L.I.; O’Donnell, C.J.; Kiel, D.P.; Hannan, M.; Polak, J.M.; Cupples, L.A. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001, 103, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.J.; van den Brand, J.A.; van Zuilen, A.D.; Koster, Y.; Bots, M.L.; Vervloet, M.G.; Blankestijn, P.J.; Wetzels, J.F.; Group, M.S. Abdominal aortic calcification in patients with CKD. J. Nephrol. 2017, 30, 109–118. [Google Scholar] [CrossRef]
- Yang, C.W.; Guo, Y.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Liu, C.H.; Wang, M.C.; Yang, S.Y.; Li, T.C.; Lin, C.C. Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. Int. J. Environ. Res. Public Health 2020, 17, 4745. [Google Scholar] [CrossRef]
- Yang, Y.J.; Choi, B.Y.; Chun, B.Y.; Kweon, S.S.; Lee, Y.H.; Park, P.S.; Kim, M.K. Dietary zinc intake is inversely related to subclinical atherosclerosis measured by carotid intima-media thickness. Br. J. Nutr. 2010, 104, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Ari, E.; Kaya, Y.; Demir, H.; Asicioglu, E.; Keskin, S. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol. Trace Elem. Res. 2011, 144, 351–359. [Google Scholar] [CrossRef]
- Sato, M.; Yanagisawa, H.; Nojima, Y.; Tamura, J.; Wada, O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: Possible role of Cu/Zn-superoxide dismutase. Clin. Exp. Hypertens 2002, 24, 355–370. [Google Scholar] [CrossRef]
- Dimitrova, A.A.; Strashimirov, D.; Betova, T.; Russeva, A.; Alexandrova, M. Zinc content in the diet affects the activity of Cu/ZnSOD, lipid peroxidation and lipid profile of spontaneously hypertensive rats. Acta Biol. Hung. 2008, 59, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Mistry, M.; Cheriyan, A.M.; Williams, J.M.; Naraine, M.K.; Ellis, C.L.; Mallick, R.; Mistry, A.C.; Gooch, J.L.; Ko, B.; et al. Zinc deficiency induces hypertension by promoting renal Na(+) reabsorption. Am. J. Physiol. Renal Physiol. 2019, 316, F646–F653. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Laukkanen, J.A. Serum zinc concentrations and incident hypertension: New findings from a population-based cohort study. J. Hypertens 2016, 34, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Bergomi, M.; Rovesti, S.; Vinceti, M.; Vivoli, R.; Caselgrandi, E.; Vivoli, G. Zinc and copper status and blood pressure. J. Trace Elem. Med. Biol. 1997, 11, 166–169. [Google Scholar] [CrossRef]
- Beattie, J.H.; Gordon, M.J.; Rucklidge, G.J.; Reid, M.D.; Duncan, G.J.; Horgan, G.W.; Cho, Y.E.; Kwun, I.S. Aorta protein networks in marginal and acute zinc deficiency. Proteomics 2008, 8, 2126–2135. [Google Scholar] [CrossRef]
- Reiterer, G.; MacDonald, R.; Browning, J.D.; Morrow, J.; Matveev, S.V.; Daugherty, A.; Smart, E.; Toborek, M.; Hennig, B. Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J. Nutr. 2005, 135, 2114–2118. [Google Scholar] [CrossRef]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Tako, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-gamma-linolenic acid (LA:DGLA) ratio; a sensitive physiological marker of zinc status in vivo (Gallus gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef]
- Knez, M.; Stangoulis, J.C.R.; Glibetic, M.; Tako, E. The Linoleic Acid: Dihomo-gamma-Linolenic Acid Ratio (LA:DGLA)-An Emerging Biomarker of Zn Status. Nutrients 2017, 9, 825. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Wathurapatha, W.S.; Ishara, M.H.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Effects of Zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr. Metab. (Lond) 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.C.; Torres, J.P.; Fouque, D.; Mafra, D. Zinc deficiency in chronic kidney disease: Is there a relationship with adipose tissue and atherosclerosis? Biol. Trace Elem. Res. 2010, 135, 16–21. [Google Scholar] [CrossRef]
- Kalkan Ucar, S.; Coker, M.; Sozmen, E.; Goksen Simsek, D.; Darcan, S. An association among iron, copper, zinc, and selenium, and antioxidative status in dyslipidemic pediatric patients with glycogen storage disease types IA and III. J. Trace Elem. Med. Biol 2010, 24, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Argani, H.; Mahdavi, R.; Ghorbani-haghjo, A.; Razzaghi, R.; Nikniaz, L.; Gaemmaghami, S.J. Effects of zinc supplementation on serum zinc and leptin levels, BMI, and body composition in hemodialysis patients. J. Trace Elem. Med. Biol 2014, 28, 35–38. [Google Scholar] [CrossRef]
- Roozbeh, J.; Hedayati, P.; Sagheb, M.M.; Sharifian, M.; Hamidian Jahromi, A.; Shaabani, S.; Jalaeian, H.; Raeisjalali, G.A.; Behzadi, S. Effect of zinc supplementation on triglyceride, cholesterol, LDL, and HDL levels in zinc-deficient hemodialysis patients. Ren. Fail. 2009, 31, 798–801. [Google Scholar] [CrossRef]
- Chausmer, A.B. Zinc, insulin and diabetes. J. Am. Coll. Nutr. 1998, 17, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Farooq, D.M.; Alamri, A.F.; Alwhahabi, B.K.; Metwally, A.M.; Kareem, K.A. The status of zinc in type 2 diabetic patients and its association with glycemic control. J. Family Community Med. 2020, 27, 29–36. [Google Scholar] [CrossRef]
- Keller, S.R. Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes. Biol. Pharm. Bull. 2004, 27, 761–764. [Google Scholar] [CrossRef][Green Version]
- Tang, X.; Shay, N.F. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J. Nutr. 2001, 131, 1414–1420. [Google Scholar] [CrossRef]
- Chabosseau, P.; Rutter, G.A. Zinc and diabetes. Arch. Biochem. Biophys. 2016, 611, 79–85. [Google Scholar] [CrossRef]
- Fernandez-Cao, J.C.; Warthon-Medina, M.; Horan, V.H.; Arija, V.; Doepking, C.; Serra-Majem, L.; Lowe, N.M. Zinc Intake and Status and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Attia, J.; Ali, L.; McEvoy, M.; Selim, S.; Sibbritt, D.; Akhter, A.; Akter, S.; Peel, R.; Faruque, O.; et al. Zinc supplementation for improving glucose handling in pre-diabetes: A double blind randomized placebo controlled pilot study. Diabetes Res. Clin. Pract. 2016, 115, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Ho, E. Zinc and its role in age-related inflammation and immune dysfunction. Mol. Nutr. Food Res. 2012, 56, 77–87. [Google Scholar] [CrossRef]
- Beattie, J.H.; Gordon, M.J.; Duthie, S.J.; McNeil, C.J.; Horgan, G.W.; Nixon, G.F.; Feldmann, J.; Kwun, I.S. Suboptimal dietary zinc intake promotes vascular inflammation and atherogenesis in a mouse model of atherosclerosis. Mol. Nutr. Food Res. 2012, 56, 1097–1105. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Mlyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-kappaB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Djafarian, K.; Mojtahed, A.; Varkaneh, H.K.; Shab-Bidar, S. The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2018, 834, 10–16. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Cortese, M.M.; Suschek, C.V.; Wetzel, W.; Kroncke, K.D.; Kolb-Bachofen, V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic. Biol. Med. 2008, 44, 2002–2012. [Google Scholar] [CrossRef]
- Ha, K.N.; Chen, Y.; Cai, J.; Sternberg, P., Jr. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: Implication for protection against oxidative stress. Invest. Ophthalmol Vis. Sci 2006, 47, 2709–2715. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef]
- Pedruzzi, L.M.; Cardozo, L.F.; Daleprane, J.B.; Stockler-Pinto, M.B.; Monteiro, E.B.; Leite, M., Jr.; Vaziri, N.D.; Mafra, D. Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J. Nephrol. 2015, 28, 495–501. [Google Scholar] [CrossRef]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef]
- Eide, D.J. The oxidative stress of zinc deficiency. Metallomics 2011, 3, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef]
- Chu, A.; Foster, M.; Samman, S. Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus-A Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 707. [Google Scholar] [CrossRef] [PubMed]
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyorala, K.; Lehto, S.; Ronnemaa, T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Winklhofer-Roob, B.M.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; Marz, W. Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br. J. Nutr. 2009, 101, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Toida, T.; Toida, R.; Ebihara, S.; Takahashi, R.; Komatsu, H.; Uezono, S.; Sato, Y.; Fujimoto, S. Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients. Nutrients 2020, 12, 3187. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Marniemi, J.; Jarvisalo, J.; Toikka, T.; Raiha, I.; Ahotupa, M.; Sourander, L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int. J. Epidemiol. 1998, 27, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kogirima, M.; Kurasawa, R.; Kubori, S.; Sarukura, N.; Nakamori, M.; Okada, S.; Kamioka, H.; Yamamoto, S. Ratio of low serum zinc levels in elderly Japanese people living in the central part of Japan. Eur. J. Clin. Nutr. 2007, 61, 375–381. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Chen, F.; Du, M.; Blumberg, J.B.; Ho Chui, K.K.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association Among Dietary Supplement Use, Nutrient Intake, and Mortality Among U.S. Adults: A Cohort Study. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chu, A.; Zhen, S.; Taylor, A.W.; Dai, Y.; Riley, M.; Samman, S. Association between dietary zinc intake and mortality among Chinese adults: Findings from 10-year follow-up in the Jiangsu Nutrition Study. Eur. J. Nutr. 2018, 57, 2839–2846. [Google Scholar] [CrossRef]
- Lee, D.H.; Folsom, A.R.; Jacobs, D.R., Jr. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: The Iowa Women’s Health Study. Am. J. Clin. Nutr. 2005, 81, 787–791. [Google Scholar] [CrossRef]
- Sarukura, N.; Kogirima, M.; Takai, S.; Kitamura, Y.; Kalubi, B.; Yamamoto, S.; Takeda, N. Dietary zinc intake and its effects on zinc nutrition in healthy Japanese living in the central area of Japan. J. Med. Investig. 2011, 58, 203–209. [Google Scholar] [CrossRef]
- Quintana Pacheco, D.A.; Sookthai, D.; Wittenbecher, C.; Graf, M.E.; Schubel, R.; Johnson, T.; Katzke, V.; Jakszyn, P.; Kaaks, R.; Kuhn, T. Red meat consumption and risk of cardiovascular diseases-is increased iron load a possible link? Am. J. Clin. Nutr. 2018, 107, 113–119. [Google Scholar] [CrossRef]
- Ascherio, A.; Willett, W.C.; Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J. Dietary iron intake and risk of coronary disease among men. Circulation 1994, 89, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Ayala-Macedo, G.; Sakihara, G.; Peralta, S.; Almaraz-Gomez, A.; Barrado, E.; Marugan-Miguelsanz, J.M. Effects of Zinc Supplementation on Nutritional Status in Children with Chronic Kidney Disease: A Randomized Trial. Nutrients 2019, 11, 2671. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Thompson, S.; Kinniburgh, D.; Klarenbach, S.W.; Walsh, M.; Bello, A.K.; Faruque, L.; Field, C.; Manns, B.J.; et al. Trace element supplementation in hemodialysis patients: A randomized controlled trial. BMC Nephrol. 2015, 16, 52. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, M.Q.; Hu, R.; Yang, Y.; Huang, Y.S.; Xian, S.X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.N.; Ibrahim, S.A.; El-Mashad, G.M.; Sabry, J.H.; Sherbini, N.S. Effect of zinc supplementation on body mass index and serum levels of zinc and leptin in pediatric hemodialysis patients. Int. J. Nephrol. Renovasc. Dis. 2015, 8, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Shahroodi, J.R.; Zolgadr, A.A.; Larie, H.A.; Nikoo, M.H.; Malekmakan, L. Effects of zinc supplement on plasma homocysteine level in end-stage renal disease patients: A double-blind randomized clinical trial. Biol. Trace Elem. Res. 2013, 153, 11–15. [Google Scholar] [CrossRef]
- Mazani, M.; Argani, H.; Rashtchizadeh, N.; Ghorbanihaghjo, A.; Hamdi, A.; Estiar, M.A.; Nezami, N. Effects of zinc supplementation on antioxidant status and lipid peroxidation in hemodialysis patients. J. Ren. Nutr. 2013, 23, 180–184. [Google Scholar] [CrossRef]
- Guo, C.H.; Wang, C.L. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Rahimi-Ardabili, B.; Argani, H.; Ghorbanihaghjo, A.; Rashtchizadeh, N.; Naghavi-Behzad, M.; Ghorashi, S.; Nezami, N. Paraoxonase enzyme activity is enhanced by zinc supplementation in hemodialysis patients. Ren. Fail. 2012, 34, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.A.; Salehi, M.; Piroozmand, A.; Sagheb, M.M. Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J. Ren. Nutr. 2009, 19, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Nava, H.J.; Amato, D. Effect of zinc supplements on the levels of pre-albumin and transferrin in patients with dialysis. Rev. Investig. Clín. 2005, 57, 123–125. [Google Scholar]
- Matson, A.; Wright, M.; Oliver, A.; Woodrow, G.; King, N.; Dye, L.; Blundell, J.; Brownjohn, A.; Turney, J. Zinc supplementation at conventional doses does not improve the disturbance of taste perception in hemodialysis patients. J. Ren. Nutr. 2003, 13, 224–228. [Google Scholar] [CrossRef]
- Chevalier, C.A.; Liepa, G.; Murphy, M.D.; Suneson, J.; Vanbeber, A.D.; Gorman, M.A.; Cochran, C. The effects of zinc supplementation on serum zinc and cholesterol concentrations in hemodialysis patients. J. Ren. Nutr. 2002, 12, 183–189. [Google Scholar] [CrossRef]
- Candan, F.; Gultekin, F.; Candan, F. Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc-deficient haemodialysis patients. Cell. Biochem. Funct. 2002, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jern, N.A.; VanBeber, A.D.; Gorman, M.A.; Weber, C.G.; Liepa, G.U.; Cochran, C.C. The effects of zinc supplementation on serum zinc concentration and protein catabolic rate in hemodialysis patients. J. Ren. Nutr. 2000, 10, 148–153. [Google Scholar] [CrossRef]
- Brodersen, H.P.; Holtkamp, W.; Larbig, D.; Beckers, B.; Thiery, J.; Lautenschlager, J.; Probst, H.J.; Ropertz, S.; Yavari, A. Zinc supplementation and hepatitis B vaccination in chronic haemodialysis patients: A multicentre study. Nephrol. Dial. Transpl. 1995, 10, 1780. [Google Scholar]
- Group, K.W. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am. J. Kidney Dis. 2009, 53, S11–S104. [Google Scholar] [CrossRef]
- Abraham, A.G.; Mak, R.H.; Mitsnefes, M.; White, C.; Moxey-Mims, M.; Warady, B.; Furth, S.L. Protein energy wasting in children with chronic kidney disease. Pediatr. Nephrol. 2014, 29, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee; European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Qu, X.; Yang, H.; Yu, Z.; Jia, B.; Qiao, H.; Zheng, Y.; Dai, K. Serum zinc levels and multiple health outcomes: Implications for zinc-based biomaterials. Bioact. Mater. 2020, 5, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Nishime, K.; Kondo, M.; Saito, K.; Miyawaki, H.; Nakagawa, T. Zinc Burden Evokes Copper Deficiency in the Hypoalbuminemic Hemodialysis Patients. Nutrients 2020, 12, 577. [Google Scholar] [CrossRef]

| Author, Year [30] | Country | Number of CKD/HD Patients | Number of Healthy Subjects | Sample | Zinc level, CKD vs. Control † |
|---|---|---|---|---|---|
| CKD | |||||
| Tavares et al. 2020 [40] | Brazil | 21 | 22 | Plasma | 70.1 ± 19.2 vs. 123.2 ± 24.6 (μg/dL) |
| Shen et al. 2020 [41] | China | 193 | 173 | Plasma | 188 vs. 229 (μg/dL) |
| Damianaki et al. 2020 [42] | Switzerland | 108 | 42 | Plasma | 60.6 ± 10.6 vs. 66.4 ± 10.1 (μg/dL) |
| Pan et al. 2019 [43] | Taiwan | 204 | 2853 | Serum | 76.9 ± 1.29 vs. 82.8 ± 0.67 (μg/dL) |
| Aziz et al. 2016 [44] | Iraq | 49 | 42 | Plasma | 83 ± 10 vs. 112 ± 19 (μg/dL) |
| Mafra et al. 2002 [45] | Brazil | 29 | 19 | Plasma | 74 ± 17.7 vs. 82.1 ± 15.5 (μg/dL) |
| HD | |||||
| Hasanato 2014 [46] | Saudi Arabia | 42 | 18 | Plasma | 9.5 vs. 13.2 (nmol/L) |
| Lobo et al. 2013 [47] | Brazil | 45 | 20 | Plasma | 54.9 ± 16.1 vs. 78.8 ± 9.4 (μg/dL) |
| Guo et al. 2011 [48] | Taiwan | 20 | 20 | Plasma | 68 ± 3 vs. 76 ± 8 (μg/L) |
| Dashti-Khavidaki et al. 2010 [49] | Iran | 94 | 47 | Serum | 69.2 ± 17.3 vs. 82.9 ± 14.8 (μg/dL) |
| Kiziltas et al. 2008 [50] | Turkey | 30 | 30 | Serum | 15.7 ± 1.25 vs. 21.2 ± 1.44 (μmol/L) |
| Batista et al. 2006 [51] | Brazil | 30 | 20 | Plasma | 81.2 ± 19.8 vs. 93.3 ± 12.1 (μg/dL) |
| Author, Year, (Reference) | Country | Number of Subjects | Age (Years) † | Follow-Up Period (years) ‡ | Number of CVD Deaths | Association of Lower Blood Zinc Levels with Higher CVD Mortality |
|---|---|---|---|---|---|---|
| Bates et al. 2011 [25] | UK | 1054 (general population) | ≥65 years old Male: 75.8 ± 6.9 Female: 77.3 ± 7.9 | n/a | 189 | Yes (HR 0.79; 95% CI 0.72–0.87) |
| Pilz et al. 2009 [140] | Germany | 3316 (patients referred for coronary angiopathy) | Male: 62 ± 11 Female: 65 ± 10 | 7.75 | 484 | Yes (HR 1.10; 95% CI 1.01–1.21) (Reference: high serum zinc group) |
| Leone et al. 2006 [142] | France | 4035 males (general population) | 30–60 years old 43 ± 5 (alive) 44 ± 4 (dead) | 18 ± 2.9 | 56 | No (RR 0.7; 95% CI 0.3–1.5) |
| Marniemi et al. 1998 [143] | Finland | 344 (general population) | ≥65 years old 65–69 (n = 99), 70–74 (n = 98) 75–80 (n = 84), ≥80−(n = 63) | 13 | 142 | No (HR 0.77; 95% CI 0.42–1.41) |
| Author, Year (Reference) | Country | Number of Subjects | Age (Years) † | Follow-Up Period (years) | Number of CVD Deaths | Outcomes |
|---|---|---|---|---|---|---|
| Chen et al. 2019 [146] | USA | 30,899 | 46.9 | 6.1 | 945 | Adequate nutrient intake of zinc associated with lower CVD mortality (RR = 0.50; 95% CI 0.36–0.71). |
| Shi et al. 2018 [147] | China | 2832 | 47.1 | 9.8 | 70 | Dietary zinc intake not related to CVD mortality. |
| Eshak et al. 2018 [26] | Japan | 58,646 | 40–79 | 19.3 | 3388 | Higher intake of zinc inversely associated with mortality from coronary heart disease (n = 702) in males; 0.68 (0.58–1.03; p-trend = 0.05) but not females; 1.13 (0.71–1.49; p-trend = 0.61). |
| Bates et al. 2011 [25] | UK | 1054 | 75.8 ± 6.9 (males) 77.3 ± 7.9 (females) | n/a | 189 | Plasma zinc associated with vascular disease mortality (HR 0.73; 95% CI 0.61–0.88). |
| Lee et al. 2005 [148] | USA | 34,492 | (55–69) | >15 | 1767 | Inverse association of dietary zinc with CVD mortality. |
| Author, Year (Reference) | Country | Number of Subjects | Age (Years) † | Elemental Zinc Dose (mg/day) | Administration Duration (Days) | Outcomes |
|---|---|---|---|---|---|---|
| Escobedo-Monge et al. 2019 [153] | Peru | 48 (children) | 12.8 ± 4 | 15/30 | 365 | Increase: BMI (30 mg/day group only) |
| Kobayashi et al. 2015 [156] | Japan | 70 | 69 ± 10 | 34 | 90/180/270/360 | Increase: serum zinc Decrease: serum copper, ferritin |
| El-Shazly et al. 2015 [157] | Egypt | 30 | 13.2 ± 2.1 | 16.5 | 90 | Increase: serum zinc, BMI Decrease: serum leptin |
| Tonelli et al. 2015 [154] | Canada | 150 | 62 | 25 and 50 | 90 and 180 | None |
| Argani et al. 2014 [114] | Iran | 60 | (50,60) | 90 | 60 | Increase: serum zinc, albumin, hemoglobin, BMI Decrease: serum leptin |
| Pakfetrat et al. 2013 [158] | Iran | 97 | 51.6 ± 16.8 | 50 | 43 | Increase: serum zinc Decrease: homocysteine |
| Mazani et al. 2013 [159] | Iran | 65 | 52.7 ± 12.6 | 100 | 60 | Increase: serum zinc, GSH, MDA, SOD, TAC |
| Guo and Wang. 2013 [160] | Taiwan | 65 | 59.7 ± 9.2 | 11 | 56 | Increase: plasma zinc, albumin, hemoglobin, hematocrit, nPNA, SOD, vitamin C, vitamin E, CD4, D19 Decrease: plasma copper, CRP, MDA INF-b, TNF-𝛼, |
| Rahimi-Ardabili et al. 2012 [161] | Iran | 60 | 52.7 ± 12.7 | 100 | 60 | Increase: Apo-AI, HDL-C, PON |
| Roozbeh et al. 2009 [115] | Iran | 53 | 55.7 | 45 | 42 | Increase: serum zinc, TC, HDL-C, LDL-C, TG |
| Rashidi et al. 2009 [162] | Iran | 55 | 57.6 | 45 | 42 | Increase: serum zinc |
| Nava-Hernandez and Amato 2005 [163] | Mexico | 25 | 16.6 | 100 | 90 | n/a |
| Matson et al. 2003 [164] | UK | 15 | 60 (31–76) | 45 | 42 | Not significant |
| Chevalier et al. 2002 [165] | USA | 27 | 51.9 | 50 | 40/90/90 | Increase: serum zinc, LDL-C |
| Candan et al. 2002 [166] | Turkey | 34 | 45.6 (28,64) | 20 | 90 | Increase: serum zinc Decrease: lipid peroxidation osmotic fragility |
| Jern et al. 2000 [167] | USA | 14 | 56.5 (23,80) | 45 | 40/90 | Increase: serum zinc, nPNA |
| Brodersen et al. 1995 [168] | Germany | 40 | 60 | 60 | 112 | Increase: serum zinc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. https://doi.org/10.3390/nu13051680
Nakatani S, Mori K, Shoji T, Emoto M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients. 2021; 13(5):1680. https://doi.org/10.3390/nu13051680
Chicago/Turabian StyleNakatani, Shinya, Katsuhito Mori, Tetsuo Shoji, and Masanori Emoto. 2021. "Association of Zinc Deficiency with Development of CVD Events in Patients with CKD" Nutrients 13, no. 5: 1680. https://doi.org/10.3390/nu13051680
APA StyleNakatani, S., Mori, K., Shoji, T., & Emoto, M. (2021). Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients, 13(5), 1680. https://doi.org/10.3390/nu13051680






