Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. The Source of DOW and Sample Preparation
2.3. Animals Diet
2.4. Animals Grouping and Experiment Schedule
2.5. Serum Biochemistry Parameters
2.6. Hepatic Lipid, TBARS and Anti-Oxidation Enzyme
2.7. Determination of Protein Expression
2.8. Statistical Analysis
3. Results
3.1. The Contents of Magnesium, Calcium and Potassium Minerals Intake from the Sample and Diets
3.2. Body Weight and the Ratio of Liver Weight to Body Weight
3.3. Aspartate Aminotransferase and Alanine Aminotransferase Activity Levels and Blood Urea Nitrogen Concentration Level in Serum
3.4. Influence of Lipid Concentration in Serum
3.5. Lipid Concentration in the Liver
3.6. Lipid Peroxidation in the Liver
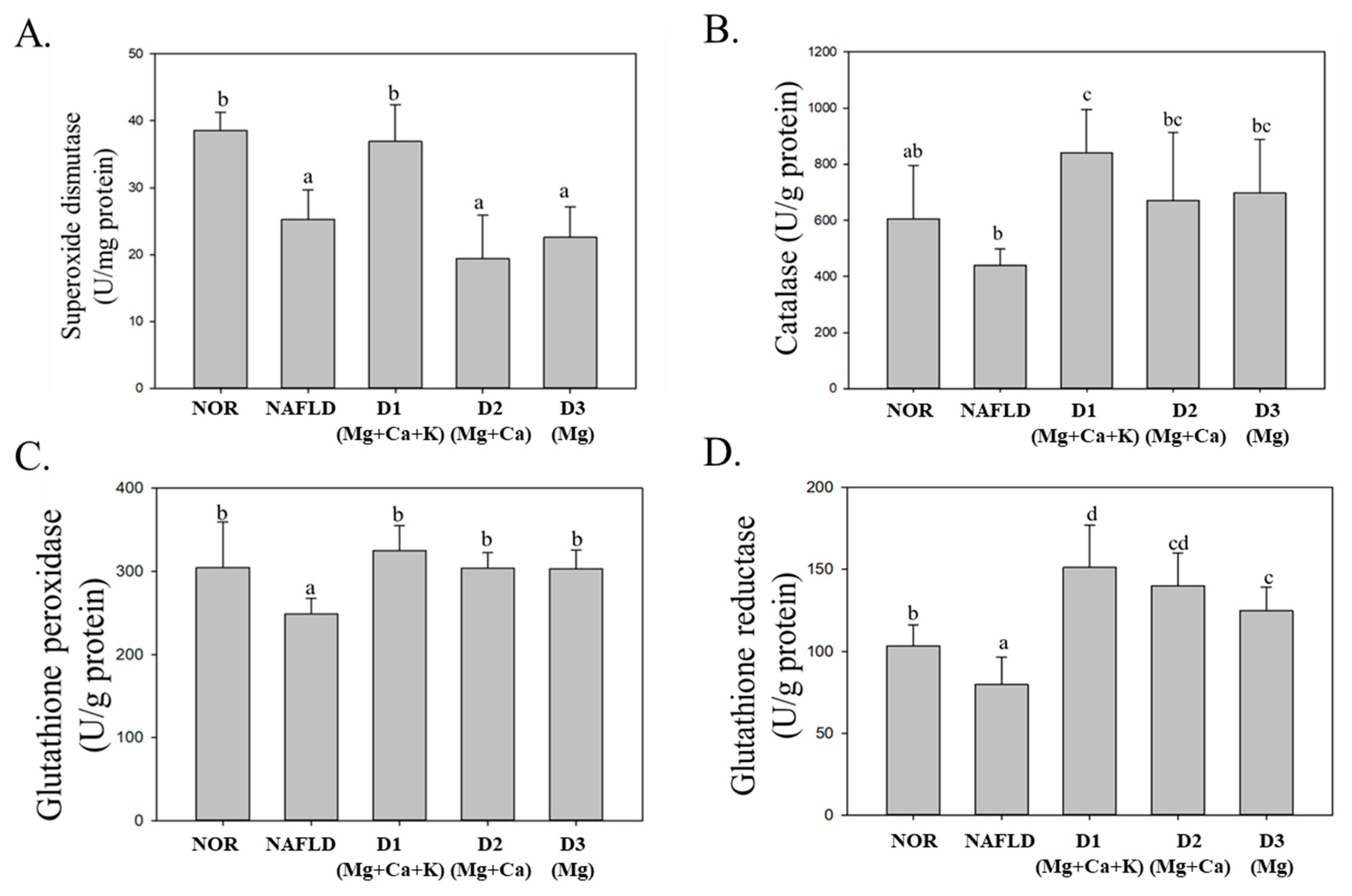
3.7. Activity of Antioxidative Enzyme in the Liver
3.8. Biopsy of Liver Tissues
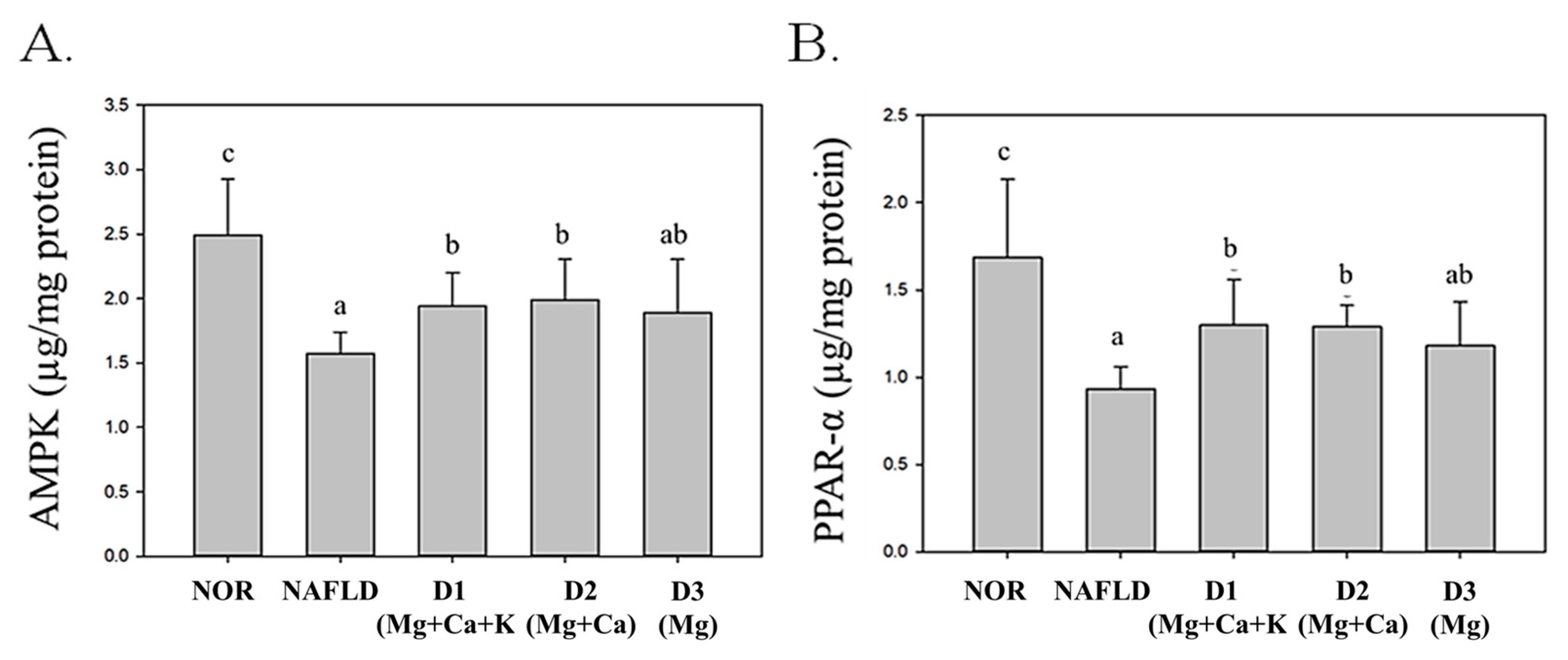
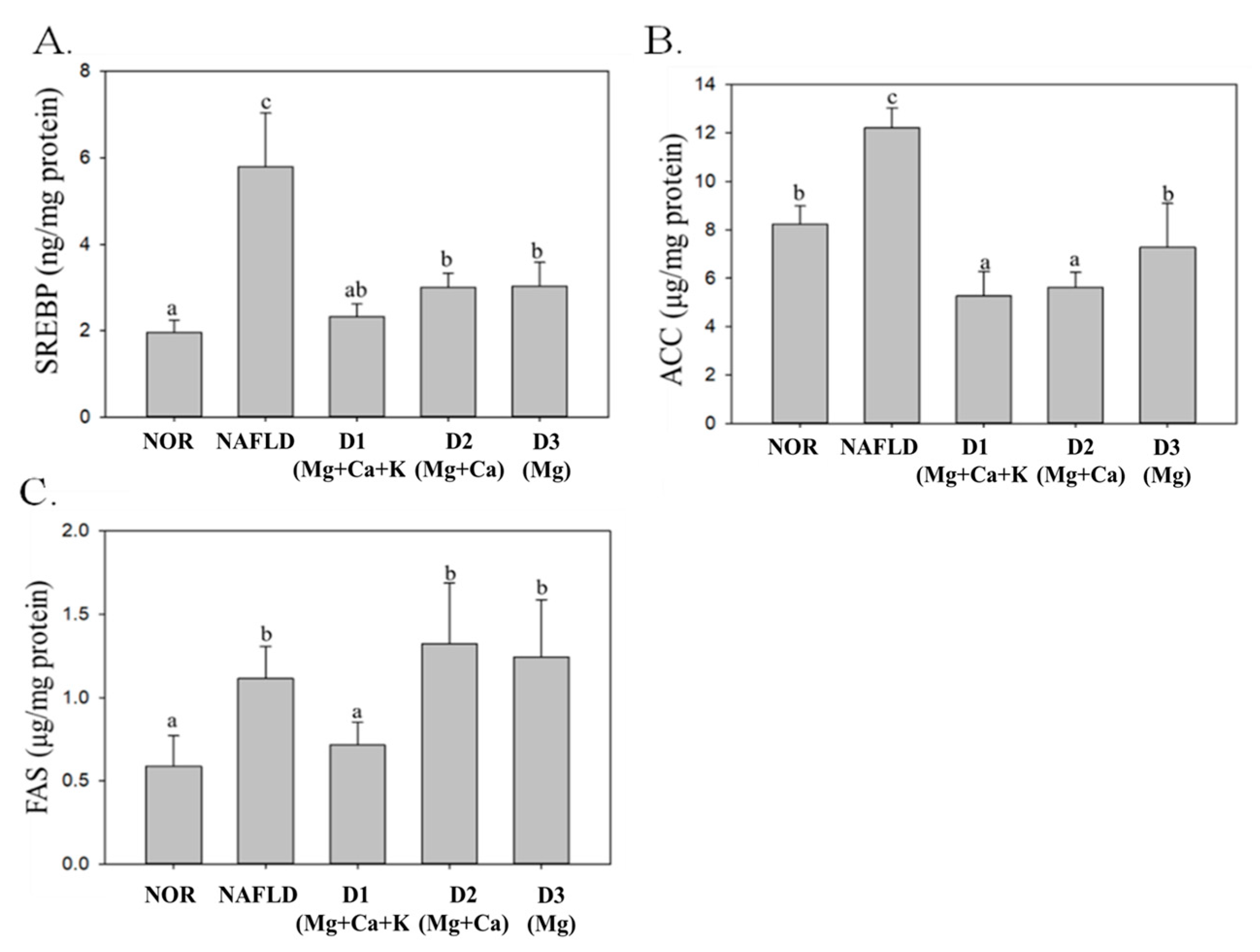
3.9. Lipid Metabolism and Biosynthesis-Related Protein Expression
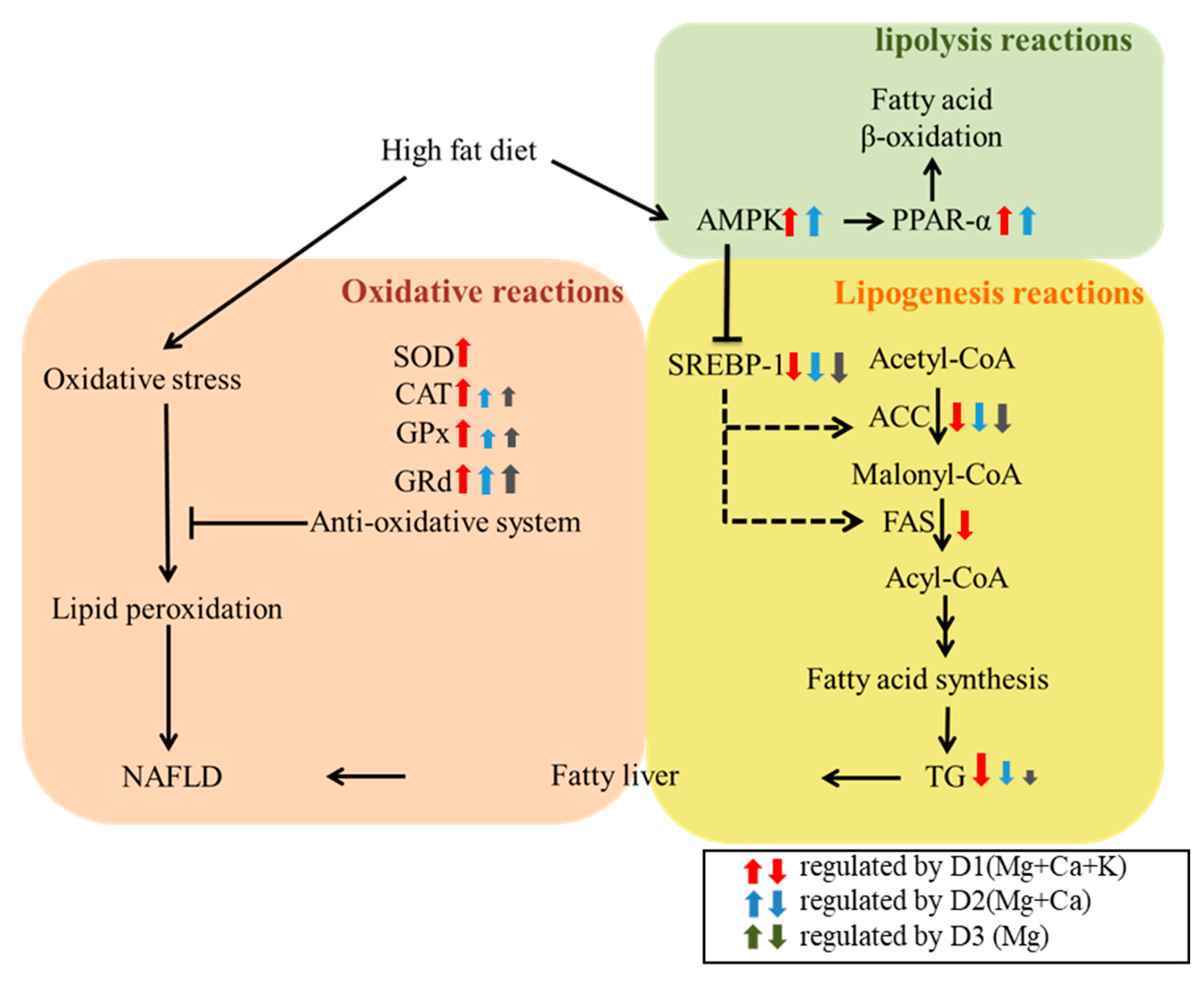
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, C.P. Natural history of NAFLD: Remarkably benign in the absence of cirrhosis. Gastroenterology 2005, 129, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; DesMeules, M.; Morrison, H.; Wen, S.W. Obesity, high energy intake, lack of physical activity, and the risk of kidney cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2006, 15, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Kessoku, T.; Imajo, K.; Honda, Y.; Kato, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci. Rep. 2016, 6, 22251. [Google Scholar] [CrossRef]
- Xu, L.; Kitade, H.; Ni, Y.; Ota, T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 2015, 5, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Fujita, D. Deep ocean water. Shokuhin Eiseigaku Zasshi 2001, 42, J340–J342. [Google Scholar] [PubMed]
- Othmer, D.F.; Roels, O.A. Power, fresh water, and food from cold, deep sea water. Science 1973, 182, 121–125. [Google Scholar] [CrossRef]
- Mohd Nani, S.Z.; Majid, F.A.; Jaafar, A.B.; Mahdzir, A.; Musa, M.N. Potential health benefits of deep sea water: A review. Evid Based Complement. Alternat Med. 2016, 2016, 6520475. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Yamamoto, M.; Maeda, H.; Nakagawa, A.; KatareGopalrao, R.; Okada, H.; Nishimori, H.; Wariishi, S.; Toda, E.; Ogawa, H.; et al. Intake of dissolved organic matter from deep seawater inhibits atherosclerosis progression. Biochem. Biophys. Res. Commun. 2009, 387, 25–30. [Google Scholar] [CrossRef]
- Bak, J.P.; Kim, Y.M.; Son, J.; Kim, C.J.; Kim, E.H. Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement. Altern. Med. 2012, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Park, J.E.; Shin, E.J.; Shon, Y.H. Effects of balanced deep-sea water on adipocyte hypertrophy and liver steatosis in high-fat, diet-induced obese mice. Obesity 2014, 22, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Park, J.E.; Shin, E.J.; Shon, Y.H. Modulation of glucose metabolism by balanced deep-sea water ameliorates hyperglycemia and pancreatic function in streptozotocin-induced diabetic mice. PLoS ONE 2014, 9, e102095. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, C.; Wu, H.; Sheng, L.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Tian, Y.; et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, W.; Zhang, J.; Lu, Y.; Wu, W.; Yan, H.; Wang, Y. Hyperlipidemia and atherosclerotic lesion development in Ldlr-deficient mice on a long-term high-fat diet. PLoS ONE 2012, 7, e35835. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chang, Y.Y.; Hsu, C.L.; Lin, H.W.; Chang, M.H.; Chen, J.W.; Chen, S.S.; Chen, Y.C. Alleviative effects of deep-seawater drinking water on hepatic lipid accumulation and oxidation induced by a high-fat diet. J. Chin. Med. Assoc. 2013, 76, 95–101. [Google Scholar] [CrossRef]
- Vijgen, G.H.; Bouvy, N.D.; Hoeks, J.; Wijers, S.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Impaired skeletal muscle mitochondrial function in morbidly obese patients is normalized one year after bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 936–941. [Google Scholar] [CrossRef]
- Ha, B.G.; Park, J.E.; Cho, H.J.; Shon, Y.H. Stimulatory effects of balanced deep sea water on mitochondrial biogenesis and function. PLoS ONE 2015, 10, e0129972. [Google Scholar] [CrossRef]
- Vaskonen, T.; Mervaala, E.; Seppanen-Laakso, T.; Karppanen, H. Diet enrichment with calcium and magnesium enhances the cholesterol-lowering effect of plant sterols in obese Zucker rats. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 158–167. [Google Scholar]
- Lee, K.S.; Kwon, Y.S.; Kim, S.; Moon, D.S.; Kim, H.J.; Nam, K.S. Regulatory mechanism of mineral-balanced deep sea water on hypocholesterolemic effects in HepG2 hepatic cells. Biomed. Pharmacother. 2017, 86, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Y.; Yang, F.L.; Hsu, H.W.; Lu, Y.F. Drinking deep seawater decreases serum total and low-density lipoprotein-cholesterol in hypercholesterolemic subjects. J. Med. Food 2012, 15, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Abdella, N.; al Awadi, F.; Salman, A.; Armstrong, D. Thiobarbituric acid test as a measure of lipid peroxidation in Arab patients with NIDDM. Diabetes Res. 1990, 15, 173–177. [Google Scholar]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, N.; Qi, K.; Cheng, H.; Sun, Z.; Gao, B.; Zhang, Y.; Pang, W.; Huangfu, C.; Ji, S.; et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas. Res. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Son, H.; Hwang, C.E.; Cho, K.M.; Park, S.W.; Kim, H.J. Ganoderma lucidum Ameliorates Non-Alcoholic Steatosis by Upregulating Energy Metabolizing Enzymes in the Liver. J. Clin. Med. 2018, 7, 152. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar] [CrossRef]
- Yang, R.Z.; Park, S.; Reagan, W.J.; Goldstein, R.; Zhong, S.; Lawton, M.; Rajamohan, F.; Qian, K.; Liu, L.; Gong, D.W. Alanine aminotransferase isoenzymes: Molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009, 49, 598–607. [Google Scholar] [CrossRef]
- Chang, W.T.; Lu, T.Y.; Cheng, M.C.; Lu, H.C.; Wu, M.F.; Hsu, C.L. Deep sea water improves abnormalities in lipid metabolism through lipolysis and fatty acid oxidation in high-fat diet-induced obese rats. Mar. Drugs 2017, 15, 386. [Google Scholar] [CrossRef]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Santos, J.C.; Valentim, I.B.; de Araujo, O.R.; Ataide Tda, R.; Goulart, M.O. Development of nonalcoholic hepatopathy: Contributions of oxidative stress and advanced glycation end products. Int. J. Mol. Sci. 2013, 14, 19846–19866. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Enjoji, M.; Yasutake, K.; Kohjima, M.; Nakamuta, M. Nutrition and nonalcoholic Fatty liver disease: The significance of cholesterol. Int. J. Hepatol. 2012, 2012, 925807. [Google Scholar] [CrossRef] [PubMed]
- Sozio, M.; Crabb, D.W. Alcohol and lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E10–E16. [Google Scholar] [CrossRef]
- Pan, X.; Wang, P.; Luo, J.; Wang, Z.; Song, Y.; Ye, J.; Hou, X. Adipogenic changes of hepatocytes in a high-fat diet-induced fatty liver mice model and non-alcoholic fatty liver disease patients. Endocrine 2015, 48, 834–847. [Google Scholar] [CrossRef]
- Im, A.R.; Kim, Y.H.; Kim, Y.H.; Yang, W.K.; Kim, S.H.; Song, K.H. Dolichos lablab Protects Against Nonalcoholic Fatty Liver Disease in Mice Fed High-Fat Diets. J. Med. Food 2017, 20, 1222–1232. [Google Scholar] [CrossRef]
- Yan, Z.; Fan, R.; Yin, S.; Zhao, X.; Liu, J.; Li, L.; Zhang, W.; Ge, L. Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int. J. Biol. Macromol. 2015, 80, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhao, L.; Meng, C.; Zhao, X.; Liu, Y.; Shi, R.; Han, X.; Wang, T.; Li, J. Prophylactic and therapeutic effects of different doses of vitamin C on high-fat-diet-induced non-alcoholic fatty liver disease in mice. Biomed. Pharmacother. 2020, 131, 110792. [Google Scholar] [CrossRef]
- Cho, J.; Lee, I.; Kim, D.; Koh, Y.; Kong, J.; Lee, S.; Kang, H. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J. Exerc. Nutr. Biochem. 2014, 18, 339–346. [Google Scholar] [CrossRef]
- Liou, C.J.; Lee, Y.K.; Ting, N.C.; Chen, Y.L.; Shen, S.C.; Wu, S.J.; Huang, W.C. Protective Effects of Licochalcone a Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cells 2019, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Ige, A.O.; Adewoye, E.O.; Okwundu, N.C.; Alade, O.E.; Onuobia, P.C. Oral magnesium reduces gastric mucosa susceptibility to injury in experimental diabetes mellitus. Pathophysiology 2016, 23, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sato, W.; Glushakova, O.; Heinig, M.; Clarke, T.; Campbell-Thompson, M.; Yuzawa, Y.; Atkinson, M.A.; Johnson, R.J.; Croker, B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 2007, 18, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Baydas, B.; Karagoz, S.; Meral, I. Effects of oral zinc and magnesium supplementation on serum thyroid hormone and lipid levels in experimentally induced diabetic rats. Biol. Trace Elem. Res. 2002, 88, 247–253. [Google Scholar] [CrossRef]
- Shi, H.; Dirienzo, D.; Zemel, M.B. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001, 15, 291–293. [Google Scholar] [CrossRef]


| Groups | Food Intake (g/Mouse) | Minerals Intake from the Sample (mg/Mouse) | Minerals Intake from the Diet (mg/Mouse) | Total Minerals Intake (mg/Mouse) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg2+ | Ca2+ | K+ | Na+ | Mg2+ | Ca2+ | K+ | Na+ | Mg2+ | Ca2+ | K+ | Na+ | ||
| NOR | 363 | - | - | - | - | 49.2 | 492 | 354 | 98.4 | 49.2 | 492 | 354 | 98.4 |
| NAFLD | 269 | - | - | - | - | 44.8 | 448 | 322 | 89.6 | 44.8 | 448 | 323 | 89.6 |
| D1(Mg + Ca + K) | 268 | 103 | 27.0 | 38.8 | 17.9 | 44.6 | 446 | 321 | 89.3 | 148 | 473 | 360 | 107 |
| D2(Mg + Ca) | 266 | 96 | 36.5 | 0.49 | 3.22 | 44.3 | 443 | 319 | 88.6 | 140 | 479 | 319 | 91.8 |
| D3(Mg) | 273 | 118 | 0.10 | 0.63 | 1.42 | 45.5 | 454 | 327 | 90.9 | 164 | 455 | 328 | 92.3 |
| Groups | Initial Body Weight (g) | 15 Week Body Weight (g) | Liver Weight (g) | Liver Weight/Body Weight (%) |
|---|---|---|---|---|
| NOR | 23.4 ± 0.7 a | 24.6 ± 1.7 a | 0.83 ± 0.08 a | 3.37 ± 0.14 b |
| NAFLD | 23.0 ± 0.8 a | 29.9 ± 1.7 b | 0.97 ± 0.05 c | 3.26 ± 0.17 b |
| D1(Mg + Ca + K) | 23.6 ± 0.5 a | 29.9 ± 1.7 b | 0.88 ± 0.02 ab | 2.95 ± 0.11 a |
| D2(Mg + Ca) | 23.3 ± 0.9 a | 30.6 ± 2.4 b | 0.90 ± 0.05 b | 2.96 ± 0.07 a |
| D3(Mg) | 23.4 ± 1.3 a | 30.4 ± 2.3 b | 0.91 ± 0.08 b | 3.00 ± 0.14 a |
| Groups | AST Activity (U/L) | ALT Activity (U/L) |
|---|---|---|
| NOR | 105 ± 25 a | 47.9 ± 21.6 a |
| NAFLD | 198 ± 66 b | 88.2 ± 38.3 b |
| D1(Mg + Ca + K) | 105± 17 a | 34.5 ± 4.1 a |
| D2(Mg + Ca) | 128 ± 31 a | 46.0 ± 4.4 a |
| D3(Mg) | 106 ± 25 a | 35.1 ± 6.0 a |
| Groups | TG (mg/dL) | TC (mg/dL) | BUN (mg/dL) |
|---|---|---|---|
| NOR | 31.5 ± 10.7 a | 85.1 ± 3.0 a | 24.0 ± 4.4 a |
| NAFLD | 76.0 ± 18.1 c | 121.2 ± 7.2 c | 24.9 ± 1.4 a |
| D1(Mg + Ca + K) | 55.1 ± 9.1 b | 101.2 ± 11.3 b | 22.9 ± 1.8 a |
| D2(Mg + Ca) | 68.5 ± 11 bc | 112.4 ± 11.0 c | 23.0 ± 2.8 a |
| D3(Mg) | 67.5 ± 16.3 bc | 101.4 ± 15.1 b | 23.9 ± 3.4 a |
| Groups | Liver TG (mg/g) | Liver TC (mg/g) |
|---|---|---|
| NOR | 4.5 ± 0.6 ab | 1.9 ± 0.2 b |
| NAFLD | 5.6 ± 0.8 c | 2.4 ± 0.4 c |
| D1(Mg + Ca + K) | 3.9 ± 0.7 a | 1.6 ± 0.2 a |
| D2(Mg + Ca) | 4.2 ± 0.4 ab | 1.6 ± 0.3 ab |
| D3(Mg) | 4.9 ± 1.1 bc | 1.5 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Lee, C.-L. Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients 2021, 13, 1732. https://doi.org/10.3390/nu13051732
Lee C-Y, Lee C-L. Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients. 2021; 13(5):1732. https://doi.org/10.3390/nu13051732
Chicago/Turabian StyleLee, Chung-Yu, and Chun-Lin Lee. 2021. "Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model" Nutrients 13, no. 5: 1732. https://doi.org/10.3390/nu13051732
APA StyleLee, C.-Y., & Lee, C.-L. (2021). Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients, 13(5), 1732. https://doi.org/10.3390/nu13051732







