Perioperative Nutritional Aspects in Total Pancreatectomy: A Comprehensive Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria, Data Extraction and Quality Assessment
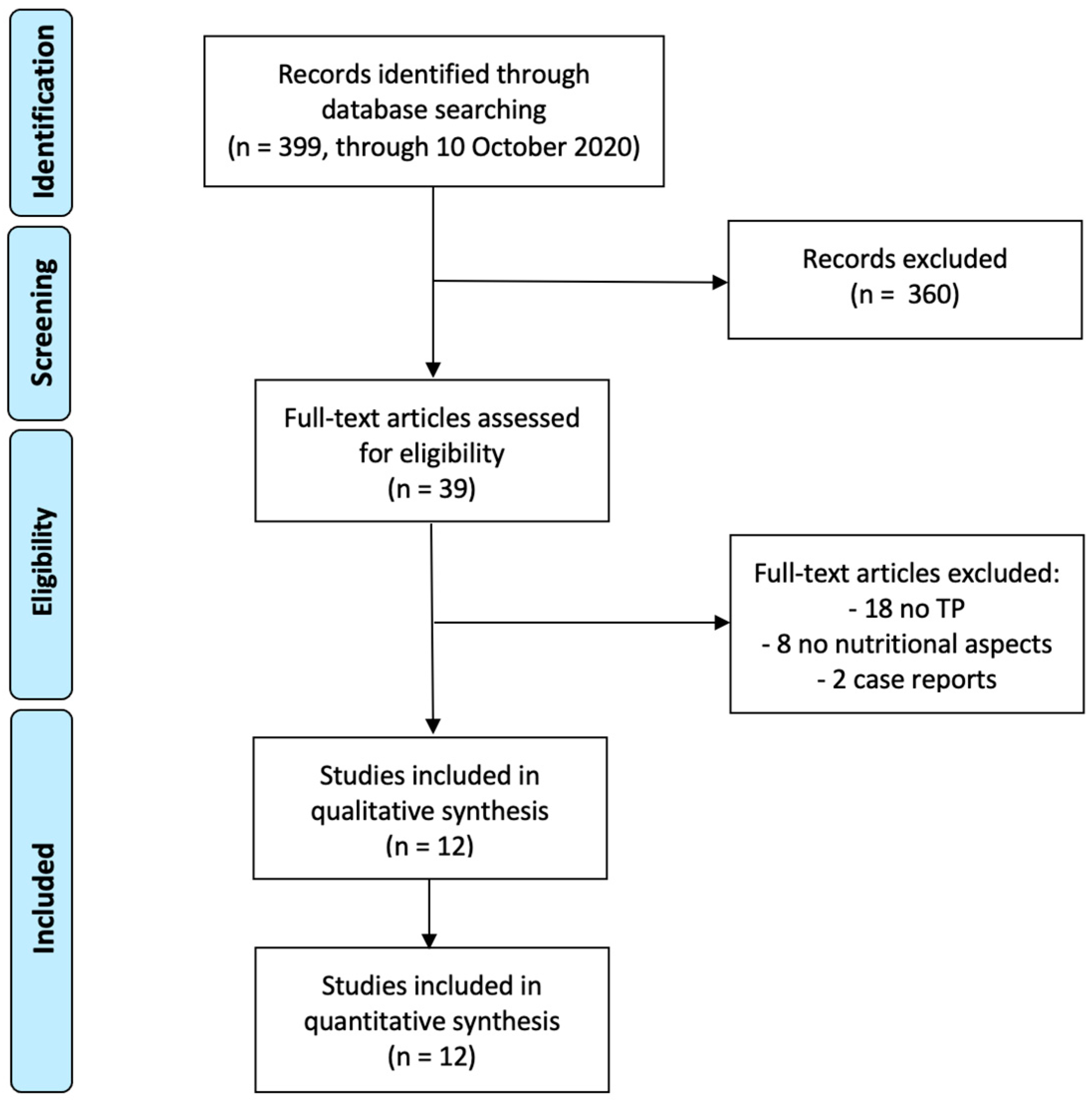
3. Results
3.1. Literature Research
3.2. Study Characteristics
4. Discussion
4.1. Pre-Operative Nutritional Status
4.2. Peri-Operative Nutritional Support
4.3. Nutritional Consequences of TP
4.3.1. Diabetes and Glycemic Control
4.3.2. Glucagon
4.3.3. Fatty Liver and Hepatic Steatosis
4.3.4. Fatty Pancreas and Diabetes after Tpiat
4.3.5. Enteroendocrine Hormones after Tpiat
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casadei, R.; Monari, F.; Buscemi, S.; Laterza, M.; Ricci, C.; Rega, D.; D’Ambra, M.; Pezzilli, R.; Calculli, L.; Santini, D.; et al. Total pancreatectomy: Indications, operative technique, and results: A single centre experience and review of literature. Updates Surg. 2010, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Rangelova, E.; Segersvärd, R.; Arnelo, U. Are there still indications for total pancreatectomy? Updates Surg. 2016, 68, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, G.; Gianardi, D.; Palmeri, M.; Furbetta, N.; Guadagni, S.; Bianchini, M.; Bonari, F.; Sbrana, A.; Vasile, E.; Pollina, L.E.; et al. Pancreatic resections for metastases: A twenty-year experience from a tertiary care center. Eur. J. Surg. Oncol. 2020, 46, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, G.; Palmeri, M.; Sbrana, A.; Gianardi, D.; Furbetta, N.; Guadagni, S.; Bianchini, M.; Stefanini, G.; Adamo, G.; Pollina, L.E.; et al. Renal cell carcinoma: The role of radical surgery on different patterns of local or distant recurrence. Surg. Oncol. 2020, 35, 106–113. [Google Scholar] [CrossRef]
- Bozzetti, F. Screening the nutritional status in oncology: A preliminary report on 1000 outpatients. Support. Care Cancer 2009, 17, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ockenga, J.; Valentini, L. Review article: Anorexia and cachexia in gastrointestinal cancer. Aliment. Pharmacol. Ther. 2005, 22, 583–594. [Google Scholar] [CrossRef]
- Di Luzio, R.; Moscatiello, S.; Marchesini, G. Role of nutrition in gastrointestinal oncological patients. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 277–284. [Google Scholar]
- Min, M.; Patel, B.; Han, S.; Bocelli, L.; Kheder, J.; Vaze, A.; Wassef, W. Exocrine pancreatic insufficiency and malnutrition in chronic pancreatitis: Identification, treatment, and consequences. Pancreas 2018, 47, 1015–1018. [Google Scholar] [CrossRef]
- Schnelldorfer, T.; Adams, D.B. The effect of malnutrition on morbidity after Surgery for chronic pancreatitis. Am. Surg. 2005, 71, 466–473. [Google Scholar] [CrossRef]
- Arvanitakis, M.; Ockenga, J.; Bezmarevic, M.; Gianotti, L.; Krznarić, Ž.; Lobo, D.N.; Löser, C.; Madl, C.; Meier, R.; Phillips, M.; et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin. Nutr. 2020, 39, 612–631. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, M.; Qin, Y.; Jiang, R.; Chen, H.; Xu, X.; Yang, T.; Jiang, K.; Miao, Y. Systematic review and meta-analysis of islet autotransplantation after total pancreatectomy in chronic pancreatitis patients. Endocr. J. 2015, 62, 227–234. [Google Scholar] [CrossRef]
- Wahoff, D.C.; Papalois, B.E.; Najarian, J.S.; Kendall, D.M.; Farney, A.C.; Leone, J.P.; Jessurun, J.; Dunn, D.L.; Robertson, R.P.; Sutherland, D.E.R.; et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. In Proceedings of the Annals of Surgery; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1995; Volume 222, pp. 562–579. [Google Scholar]
- Clayton, H.A.; Davies, J.E.; Pollard, C.A.; White, S.A.; Musto, P.P.; Dennison, A.R. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: The first 40 patients at the Leicester General Hospital. Transplantation 2003, 76, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hanazaki, K.; Maeda, H.; Okabayashi, T. Relationship between perioperative glycemic control and postoperative infections. World J. Gastroenterol. 2009, 15, 4122–4125. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Schütte, K.; Malfertheiner, P. The pancreas: Causes for malabsorption. Visz. Gastrointest. Med. Surg. 2014, 30, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Higashiguchi, T.; Kita, T.; Noguchi, T.; Kawarada, Y.; Mizumoto, R. Importance of nutritional management for the treatment of carcinoma of the pancreas. Gan To Kagaku Ryoho 1988, 14, 847–853. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Shi, H.J.; Jin, C.; Fu, D.L. Impact of postoperative glycemic control and nutritional status on clinical outcomes after total pancreatectomy. World J. Gastroenterol. 2017, 23, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Beilman, G.J.; Dunn, T.B.; Pruett, T.L.; Chinnakotla, S.C.; Radosevich, D.M.; Robertson, R.P.; Ptacek, P.; Balamurugan, A.N.; Wilhelm, J.J.; et al. Metabolic assessment prior to total pancreatectomy and islet autotransplant: Utility, limitations and potential. Am. J. Transplant. 2013, 13, 2664–2671. [Google Scholar] [CrossRef][Green Version]
- Trikudanathan, G.; Feussom, G.; Teigen, L.; Munigala, S.; Price, K.; Dirweesh, A.; Wilhelm, J.J.; Hering, B.J.; Kirchner, V.; Chinnakotla, S.; et al. Pre-operative Sarcopenia Predicts Low Islet Cell Yield Following Total Pancreatectomy with Islet Autotransplantation for Chronic Pancreatitis. J. Gastrointest. Surg. 2020, 24, 2423–2430. [Google Scholar] [CrossRef]
- Takita, M.; Naziruddin, B.; Matsumoto, S.; Noguchi, H.; Shimoda, M.; Chujo, D.; Itoh, T.; Sugimoto, K.; Tamura, Y.; Olsen, G.S.; et al. Body mass index reflects islet isolation outcome in islet autotransplantation for patients with chronic pancreatitis. Cell Transplant. 2011, 20, 313–322. [Google Scholar] [CrossRef]
- Karagianni, V.T.; Papalois, A.E.; Triantafillidis, J.K. Nutritional Status and Nutritional Support Before and After Pancreatectomy for Pancreatic Cancer and Chronic Pancreatitis. Indian J. Surg. Oncol. 2012, 3, 348–359. [Google Scholar] [CrossRef]
- Andersen, S.; Andersen, A.; Ringholm, L.; Hansen, C.P.; Storkholm, J.; Lillpers, K.; Schiøtz, C.; Mathiesen, E.R. Parenteral nutrition and insulin per protocol improve diabetes management after total pancreatectomy. Dan. Med. J. 2018, 65, 1–5. [Google Scholar]
- Wagar, M.K.; Magnuson, J.; Liu, P.T.; Kirchner, V.; Wilhelm, J.J.; Freeman, M.L.; Bellin, M.D.; Pruett, T.L.; Beilman, G.J.; Dunn, T.B. The impact of using an intraoperative goal directed fluid therapy protocol on clinical outcomes in patients undergoing total pancreatectomy and islet cell autotransplantation. Pancreatology 2017, 17, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Scholten, L.; Stoop, T.F.; Del Chiaro, M.; Busch, O.R.; van Eijck, C.; Molenaar, I.Q.; de Vries, J.H.; Besselink, M.G. Systematic review of functional outcome and quality of life after total pancreatectomy. Br. J. Surg. 2019, 106, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Bogachus, L.D.; Bellin, M.D.; Vella, A.; Paul Robertson, R. Deficient Glucagon Response to Hypoglycemia during a Mixed Meal in Total Pancreatectomy/Islet Autotransplantation Recipients. J. Clin. Endocrinol. Metab. 2018, 103, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Ishida, M.; Motoi, F.; Sakata, N.; Yoshimatsu, G.; Naitoh, T.; Katayose, Y.; Egawa, S.; Unno, M. Clinical characteristics and risk factors for the development of postoperative hepatic steatosis after total pancreatectomy. Pancreas 2016, 45, 362–369. [Google Scholar] [CrossRef]
- Kizilgul, M.; Wilhelm, J.J.; Beilman, G.J.; Chinnakotla, S.; Dunn, T.B.; Pruett, T.L.; Abdulla, M.; Heller, D.; Freeman, M.L.; Schwarzenberg, S.J.; et al. Effect of intrapancreatic fat on diabetes outcomes after total pancreatectomy with islet autotransplantation. J. Diabetes 2018, 10, 286–295. [Google Scholar] [CrossRef] [PubMed]
- McEachron, K.R.; Yang, Y.; Hodges, J.S.; Beilman, G.J.; Pruett, T.L.; Kirchner, V.A.; Dunn, T.B.; Freeman, M.L.; Trikudanathan, G.; Mulier, K.E.; et al. Alterations in Enteroendocrine Hormones after Total Pancreatectomy with Islet Autotransplantation. Pancreas 2020, 49, 806–811. [Google Scholar] [CrossRef]
- Smale, B.F.; Mullen, J.L.; Buzby, G.P.; Rosato, E.F. The efficacy of nutritional assessment and support in cancer surgery. Cancer 1981, 47, 2375–2381. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nippon Geka Gakkai Zasshi. 1984, 85, 1001–1005. (In Japanese) [Google Scholar]
- Nozoe, T.; Kohno, M.; Iguchi, T.; Mori, E.; Maeda, T.; Matsukuma, A.; Ezaki, T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg. Today 2012, 42, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.; Popiel, B.; Lammersfeld, C.; Gupta, D. Outcomes of Systematic Nutritional Assessment and Medical Nutrition Therapy in Pancreatic Cancer. Pancreas 2015, 44, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Qi, Q.; Sun, M.; Chen, H.; Wang, P.; Chen, Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur. J. Surg. Oncol. 2015, 41, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Fujii, T.; Kodera, Y.; Nagai, S.; Takeda, S.; Nakao, A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2011, 98, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Schiesser, M.; Kirchhoff, P.; Müller, M.K.; Schäfer, M.; Clavien, P.A. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery 2009, 145, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M.; Goto, K.; Watanabe, J.; Urushibara, S.; Osaki, T.; Endo, K.; Tatebe, S.; Nakamura, S. Clinical importance of preoperative and postoperative prognostic nutritional index in patients with pancreatic ductal adenocarcinoma. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 372. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Hiyoshi, M.; Imamura, N.; Yano, K.; Hamada, T.; Hamada, R.; Nagatomo, K.; Ikenoue, M.; Tobinaga, S.; Nagayasu, T. Clinical significance of preoperative nutritional parameter and patient outcomes after pancreatectomy: A retrospective study at two academic institute. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 168. [Google Scholar] [CrossRef][Green Version]
- Chinnakotla, S.; Beilman, G.J.; Dunn, T.B.; Bellin, M.D.; Freeman, M.L.; Radosevich, D.M.; Arain, M.; Amateau, S.K.; Mallery, J.S.; Schwarzenberg, S.J.; et al. Factors predicting outcomes after a total pancreatectomy and islet autotransplantation lessons learned from over 500 cases. Ann. Surg. 2015, 262, 610–622. [Google Scholar] [CrossRef]
- Sutherland, D.E.R.; Radosevich, D.M.; Bellin, M.D.; Hering, B.J.; Beilman, G.J.; Dunn, T.B.; Chinnakotla, S.; Vickers, S.M.; Bland, B.; Balamurugan, A.N.; et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J. Am. Coll. Surg. 2012, 214, 409–424. [Google Scholar] [CrossRef]
- Khan, K.M.; Desai, C.S.; Kalb, B.; Patel, C.; Grigsby, B.M.; Jie, T.; Gruessner, R.W.G.; Rodriguez-Rilo, H. MRI prediction of islet yield for autologous transplantation after total pancreatectomy for chronic pancreatitis. Dig. Dis. Sci. 2013, 58, 1116–1124. [Google Scholar] [CrossRef]
- Bellin, M.D.; Freeman, M.L.; Schwarzenberg, S.J.; Dunn, T.B.; Beilman, G.J.; Vickers, S.M.; Chinnakotla, S.; Balamurugan, A.N.; Hering, B.J.; Radosevich, D.M.; et al. Quality of Life Improves for Pediatric Patients After Total Pancreatectomy and Islet Autotransplant for Chronic Pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N. Negotiating the complexities of exocrine and endocrine dysfunction in chronic pancreatitis. Proc. Nutr. Soc. 2017, 76, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Kuan, L.L.; Dennison, A.R.; Garcea, G. Prevalence and Impact of Sarcopenia in Chronic Pancreatitis: A Review of the Literature. World J. Surg. 2021, 45, 590–597. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Olesen, S.S.; Büyükuslu, A.; Køhler, M.; Rasmussen, H.H.; Drewes, A.M. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology 2019, 19, 245–251. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.; Ziparo, V.; Nigri, G.; Cavallini, M.; Balducci, G.; Ramacciato, G. Malnutrition and pancreatic surgery: Prevalence and outcomes. J. Surg. Oncol. 2013, 107, 702–708. [Google Scholar] [CrossRef]
- Wehler, M.; Nichterlein, R.; Fischer, B.; Farnbacher, M.; Reulbach, U.; Hahn, E.G.; Schneider, T. Factors associated with health-related quality of life in chronic pancreatitis. Am. J. Gastroenterol. 2004, 99, 138–146. [Google Scholar] [CrossRef]
- Gruppo, M.; Angriman, I.; Martella, B.; Spolverato, Y.C.; Zingales, F.; Bardini, R. Perioperative albumin ratio is associated with post-operative pancreatic fistula. ANZ J. Surg. 2018, 88, E602–E605. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Hearty, A.; Prichard, R.S.; Cunningham, A.; Rowley, S.P.; Reynolds, J.V. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J. Gastrointest. Surg. 2007, 11, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V.; Lohsiriwat, D.; Boonnuch, W.; Chinswangwatanakul, V.; Akaraviputh, T.; Lert-Akayamanee, N. Pre-operative hypoalbuminemia is a major risk factor for postoperative complications following rectal cancer surgery. World J. Gastroenterol. 2008, 14, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Cull, W.; Henderson, W.; Daley, J.; Hur, K.; Khuri, S.F. Preoperative serum albumin level as a predictor of operative mortality and morbidity: Results from the National VA Surgical Risk Study. Arch. Surg. 1999, 134, 36–42. [Google Scholar] [CrossRef]
- Billingsley, K.G.; Hur, K.; Henderson, W.G.; Daley, J.; Khuri, S.F.; Bell, R.H. Outcome after pancreaticoduodenectomy for periampullary cancer: An analysis from the Veterans Affairs National Surgical Quality Improvement Program. In Proceedings of the Journal of Gastrointestinal Surgery; Elsevier Inc.: Amsterdam, The Netherlands, 2003; Volume 7, pp. 484–491. [Google Scholar]
- Nakano, Y.; Kitago, M.; Shinoda, M.; Yagi, H.; Abe, Y.; Takano, K.; Oshima, G.; Takeuch, A.; Endo, Y.; Kitagawa, Y. Prognostic significance of the postoperative level and recovery rate of serum albumin in patients with curatively resected pancreatic ductal adenocarcinoma. Mol. Clin. Oncol. 2019, 11, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Cancer anorexia-cachexia syndrome: Are neuropeptides the key? Cancer Res. 1999, 59, 4493–4501. [Google Scholar] [PubMed]
- Ramos, E.J.B.; Suzuki, S.; Marks, D.; Inui, A.; Asakawa, A.; Meguid, M.M. Cancer anorexia-cachexia syndrome: Cytokines and neuropeptides. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.N.; Trebble, T.M.; Ellis, R.D.; Duncan, H.D.; Johns, T.; Goggin, P.M. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut 2005, 54, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.F.; Pisters, P.W.T.; Posner, M.; Quesada, O.; Shike, M. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann. Surg. 1994, 220, 436–444. [Google Scholar] [CrossRef]
- Ward, N. Nutrition support to patients undergoing gastrointestinal surgery. Nutr. J. 2003, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Z.; Chen, H.; Ma, L.; Liu, L.; Zhang, J.; He, Y.; Chen, J.; Qian, Q. Treatment with growth hormone, somatostatin, and insulin in combination with hypocaloric parenteral nutrition in gastrointestinal cancer patients after surgery. Nutrition 2011, 27, 633–640. [Google Scholar] [CrossRef]
- Baradi, H.; Walsh, R.M.; Henderson, J.M.; Vogt, D.; Popovich, M. Postoperative jejunal feeding and outcome of pancreaticoduodenectomy. In Proceedings of the Journal of Gastrointestinal Surgery. J. Gastrointest. Surg. 2004, 8, 428–433. [Google Scholar] [CrossRef]
- Paccagnella, A.; Morassutti, I.; Rosti, G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr. Opin. Oncol. 2011, 23, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.D.; Ross, J.A.; Voss, A.C.; Tisdale, M.J.; Fearon, K.C.H. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br. J. Cancer 1999, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, Z.; Lou, C.; Wu, C.; Yuan, Q.; Wang, J.; Shu, G.; Wang, Y. Enteral nutrition is superior to total parenteral nutrition for pancreatic cancer patients who underwent pancreaticoduodenectomy. Asia Pac. J. Clin. Nutr. 2011, 20, 154–160. [Google Scholar] [PubMed]
- Braga, M.; Gianotti, L.; Gentilini, O.; Parisi, V.; Salis, C.; Di Carlo, V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit. Care Med. 2001, 29, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Maskell, C.; Daniels, P.; Johnson, C.D. Dietary intake after pancreatectomy. Br. J. Surg. 1999, 86, 323–326. [Google Scholar] [CrossRef]
- Johnson, P.M.; Chen, S.S.; Santomango, T.S.; Williams, P.E.; Lacy, D.B.; McGuinness, O.P. Continuous low-dose fructose infusion does not reverse glucagon-mediated decrease in hepatic glucose utilization. Metabolism 2011, 60, 867–873. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, X.Y.; Zhang, C.H.; He, Y.L.; Yuan, Y.X.; Cai, S.R.; Luo, N.X.; Zhan, W.H.; Cui, J. Is albumin administration beneficial in early stage of postoperative hypoalbuminemia following gastrointestinal surgery?: A prospective randomized controlled trial. Am. J. Surg. 2008, 196, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Jamal, W.; Dokmak, S.; Aussilhou, B.; Corcos, O.; Ruszniewski, P.; Belghiti, J.; Sauvanet, A. Impact of total pancreatectomy: Short- and long-term assessment. HPB 2013, 15, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.J.; Blanco, G.; Webber, J.; Marudanayagam, R.; Sutcliffe, R.P.; Muiesan, P.; Bramhall, S.R.; Isaac, J.; Mirza, D.F. How severe is diabetes after total pancreatectomy? A case-matched analysis. HPB 2014, 16, 814–821. [Google Scholar] [CrossRef]
- Tattersall, R.B. Brittle diabetes revisited: The third Arnold Bloom memorial lecture. Diabet. Med. 1997, 14, 99–110. [Google Scholar] [CrossRef]
- Butler, P.C.; Rizza, R.A. Regulation of carbohydrate metabolism and response to hypoglycemia. Endocrinol. Metab. Clin. N. Am. 1989, 18, 1–25. [Google Scholar] [CrossRef]
- Dresler, C.M.; Fortner, J.G.; McDermott, K.; Bajorunas, D.R. Metabolic consequences of (regional) total pancreatectomy. Ann. Surg. 1991, 214, 131–140. [Google Scholar] [CrossRef]
- Jamil, L.H.; Chindris, A.M.; Gill, K.R.S.; Scimeca, D.; Stauffer, J.A.; Heckman, M.G.; Meek, S.E.; Nguyen, J.H.; Asbun, H.J.; Raimondo, M.; et al. Glycemic control after total pancreatectomy for intraductal papillary mucinous neoplasm: An exploratory study. HPB Surg. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jethwa, P.; Sodergren, M.; Lala, A.; Webber, J.; Buckels, J.A.C.; Bramhall, S.R.; Mirza, D.F. Diabetic control after total pancreatectomy. Dig. Liver Dis. 2006, 38, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dodson, R.; Makary, M.A.; Weiss, M.J.; Hirose, K.; Cameron, J.L.; Ahuja, N.; Pawlik, T.M.; Wolfgang, C.L.; He, J. A contemporary evaluation of the cause of death and long-term quality of life after total pancreatectomy. World J. Surg. 2016, 40, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Parazzoli, S.; Oseid, E.; Bogachus, L.D.; Schuetz, C.; Patti, M.E.; Dunn, T.; Pruett, T.; Balamurugan, A.N.; Hering, B.; et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am. J. Transplant. 2014, 14, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Holst Pedersen, J.; Baldissera, F.; Stadil, F. Circulating glucagon after total pancreatectomy in man. Diabetologia 1983, 25, 396–399. [Google Scholar] [CrossRef]
- Yasui, K. Effects of total pancreatectomy on the secretion of gut glucagon in humans. Jpn. J. Surg. 1983, 13, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A.; Brennan, M.F.; Tan, M.H.; Aoki, T.T. Studies of glucagon secretion in pancreatectomized patients. Diabetes 1974, 23, 512–516. [Google Scholar] [CrossRef]
- Tanjoh, K.; Tomita, R.; Mera, K.; Hayashi, N. Metabolic modulation by concomitant administration of insulin and glucagon in pancreatectomy patients. Hepatogastroenterology 2002, 49, 538–543. [Google Scholar] [PubMed]
- Rodriguez-Diaz, R.; Caicedo, A. Novel approaches to studying the role of innervation in the biology of pancreatic islets. Endocrinol. Metab. Clin. N. Am. 2013, 42, 39–56. [Google Scholar] [CrossRef]
- Taborsky, G.J.; Mundinger, T.O. Minireview: The role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology 2012, 153, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Price, T.B.; Bergeron, R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: Impact of type 1 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4656–4664. [Google Scholar] [CrossRef] [PubMed]
- Bogachus, L.D.; Oseid, E.; Bellin, M.; Vella, A.; Robertson, R.P. Deficient endogenous glucose production during exercise after total pancreatectomy/islet autotransplantation. J. Clin. Endocrinol. Metab. 2017, 102, 3288–3295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bajorunas, D.R.; Fortner, J.G.; Jaspan, J.; Sherwin, R.S. Total pancreatectomy increases the metabolic response to glucagon in humans. J. Clin. Endocrinol. Metab. 1986, 63, 439–446. [Google Scholar] [CrossRef]
- Heptulla, R.A.; Rodriguez, L.M.; Bomgaars, L.; Raymond, M.W. The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes 2005, 54, 1100–1107. [Google Scholar] [CrossRef]
- Heidt, D.G.; Burant, C.; Simeone, D.M. Total pancreatectomy: Indications, operative technique, and postoperative sequelae. J. Gastrointest. Surg. 2007, 11, 209–216. [Google Scholar] [CrossRef]
- Suzuki, S.; Miura, J.; Shimizu, K.; Tokushige, K.; Uchigata, Y.; Yamamoto, M. Clinicophysiological outcomes after total pancreatectomy. Scand. J. Gastroenterol. 2016, 51, 1526–1531. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.O.T.; Poitout, V. β-Cell Glucose Toxicity, Lipotoxicity, and Chronic Oxidative Stress in Type 2 Diabetes. In Proceedings of the Diabetes; American Diabetes Association Inc.: Arlington County, VA, USA, 2004; Volume 53. [Google Scholar]
- Pitt, H.A. Hepato-pancreato-biliary fat: The good, the bad and the ugly. HPB 2007, 9, 92–97. [Google Scholar] [CrossRef]
- Cusi, K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 2010, 10, 306–315. [Google Scholar] [CrossRef]
- Newsholme, P.; Keane, D.; Welters, H.J.; Morgan, N.G. Life and death decisions of the pancreatic β-cell: The role of fatty acids. Clin. Sci. 2007, 112, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, M.; Khashab, M.; Zyromski, N.; Pungpapong, S.; Wallace, M.B.; Scolapio, J.; Woodward, T.; Noh, K.; Raimondo, M. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: A case-control study. Pancreas 2009, 38, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Wang, C.Y. Association between non-alcoholic fatty pancreatic disease (nafpd) and the metabolic syndrome: Case-control retrospective study. Cardiovasc. Diabetol. 2013, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.; Lal, S.; McLaughlin, J.T. Enteroendocrine cells in gastrointestinal pathophysiology. Curr. Opin. Pharmacol. 2013, 13, 941–945. [Google Scholar] [CrossRef]
- Spreckley, E.; Murphy, K.G. The L-Cell in nutritional sensing and the regulation of appetite. Front. Nutr. 2015, 2, 23. [Google Scholar] [CrossRef]
- Beglinger, C.; Degen, L. Gastrointestinal satiety signals in humans—Physiologic roles for GLP-1 and PYY ? Physiol. Behav. 2006, 89, 460–464. [Google Scholar] [CrossRef]
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Ørskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273. [Google Scholar] [CrossRef]
- Stanley, S.; Wynne, K.; Bloom, S. Gastrointestinal Satiety Signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, 693–697. [Google Scholar] [CrossRef]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.A.; Kramer, M.H.H.; Cahen, D.L.; van Raalte, D.H. Gastrointestinal actions of glucagon-like peptide-1-based therapies: Glycaemic control beyond the pancreas. Diabetes Obes. Metab. 2016, 18, 224–235. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Studt Design | Sample Size | Intervention Timing | Outcomes | Conclusion |
|---|---|---|---|---|---|---|
| Shi et al. [18] | 2017 | Single-centre | 52 TP | Postoperative | Glycemic control Nutritional status | Improvement of glycemic control and nutritional status after TP is important to prevent early complications and tumor recurrence and to improve survival |
| Lundberg et al. [19] | 2013 | Single-centre | 60 TPIAT | Preoperative chronic pancreatitis, stimulated insulin and C-peptide levels | Number of islet isolated | Normal stimulated C-peptide and fasting glucose correlate with low risk for low islet yield |
| Trikudanathan et al. [20] | 2020 | Single-centre | 138 TPIAT (46 vs. 92) | Preoperative sarcopenia | Discharging to rehabilitation Islet yield LoS30-day readmission rate | Association between sarcopenia and an increased chance of discharge to a residential rehabilitation facility and with a poor islet yield during TPIAT |
| Takita et al. [21] | 2011 | Single-centre | 12 TPIAT | BMI of pancreatic donor | Insulin independence Islet yield | Decreased C-peptide levels in the low-BMI group Better long-term graft function and islet yields in the high BMI group |
| Karagianni et al. [22] | 2012 | Review | 11 studies | EN or PN postoperative support | Cachexia Toxicity to chemotherapy Nutritional status Reduced mortality (infectious complications, LoS) Postoperative gastric stasis | EN reduces gastrointestinal toxicity derived from chemotherapy Cyclic EN reduces postoperative gastrointestinal stasis Postoperative PN in every patient is not recommended |
| Andersen et al. [23] | 2018 | Single-centre | 97 TP (57 vs. 40) | Postoperative parenteral nutrition vs. glucose infusion | Glycemic control Non-infectious post-operative complications | PN improves glycemic control and reduces non-infectious post-operative complications with respect to glucose infusion |
| Wagar et al. [24] | 2017 | Single-centre | 67 TPIAT 44 vs. 23 | Intraoperative goal-directed fluid therapy protocol vs. standard fluid therapy | Intraoperative complications (resuscitation, transfusion) Postoperative complications (graft function, 30-days complications) | Decreased intraoperative fluid resuscitation and blood transfusion using a goal-directed fluid therapy protocol vs. standard fluid Similar postoperative complications |
| Scholten et al. [25] | 2019 | Systematic review | 21 studies TP 1536 pts | Functional outcome and quality of life after total pancreatectomy | QoL Endocrine insufficiency Exocrine insufficiency | QoL is affected adversely, in particular by the consider-able impact of diarrhea improvement in the management of diabetes after TP |
| Bogachus et al. [26] | 2018 | Single-centre | 20 TPIAT (10 vs. 10) | Postoperative postprandial hypoglycemia | Decreases in postprandial glucose | Absent glucagon response contributes to postprandial hypoglycemia post-TPIAT |
| Hata et al. [27] | 2016 | Single-centre | 43 TP | Postoperative development of hepatic steatosis | Relationship between postoperative hepatic steatosis and pancreatic insufficiency | Development of hepatic steatosis after TP is related to female sex and early nutritional status and prevented with high-dose pancreatic enzyme replacement therapy |
| Kizilgul et al. [28] | 2018 | Single-centre | 79 TPIAT (53 vs. 26) | Preoperative pancreatic fat content | Insulin-dependent Postprandial glucose excursion | Intrapancreatic fat causes beta cell disfunction after TPIAT |
| McEachron et al. [29] | 2020 | Single-centre | 34 TPIAT | Enteroendocrine postoperative changes | Stimulated levels of GLP-1, PYY and PP | GLP-1 and PYY levels are higher after TPIAT PP is not significantly lower after TPIAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furbetta, N.; Comandatore, A.; Gianardi, D.; Palmeri, M.; Di Franco, G.; Guadagni, S.; Caprili, G.; Bianchini, M.; Fatucchi, L.M.; Picchi, M.; et al. Perioperative Nutritional Aspects in Total Pancreatectomy: A Comprehensive Review of the Literature. Nutrients 2021, 13, 1765. https://doi.org/10.3390/nu13061765
Furbetta N, Comandatore A, Gianardi D, Palmeri M, Di Franco G, Guadagni S, Caprili G, Bianchini M, Fatucchi LM, Picchi M, et al. Perioperative Nutritional Aspects in Total Pancreatectomy: A Comprehensive Review of the Literature. Nutrients. 2021; 13(6):1765. https://doi.org/10.3390/nu13061765
Chicago/Turabian StyleFurbetta, Niccolò, Annalisa Comandatore, Desirée Gianardi, Matteo Palmeri, Gregorio Di Franco, Simone Guadagni, Giovanni Caprili, Matteo Bianchini, Lorenzo Maria Fatucchi, Martina Picchi, and et al. 2021. "Perioperative Nutritional Aspects in Total Pancreatectomy: A Comprehensive Review of the Literature" Nutrients 13, no. 6: 1765. https://doi.org/10.3390/nu13061765
APA StyleFurbetta, N., Comandatore, A., Gianardi, D., Palmeri, M., Di Franco, G., Guadagni, S., Caprili, G., Bianchini, M., Fatucchi, L. M., Picchi, M., Bastiani, L., Biancofiore, G., Di Candio, G., & Morelli, L. (2021). Perioperative Nutritional Aspects in Total Pancreatectomy: A Comprehensive Review of the Literature. Nutrients, 13(6), 1765. https://doi.org/10.3390/nu13061765







