Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment
Abstract
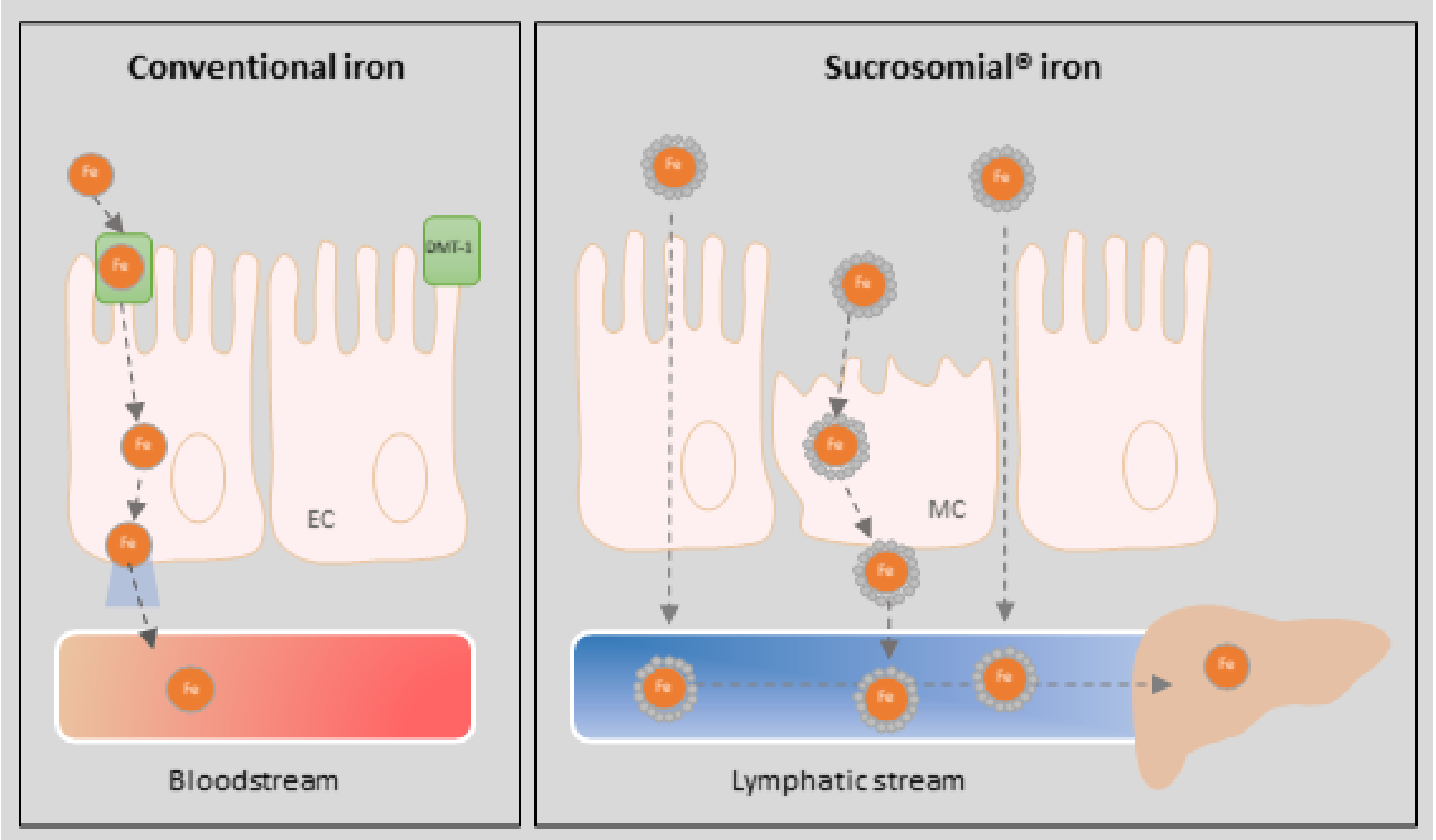
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Treatment Plan and Assessments
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Participant Flow
3.2. Outcomes
3.2.1. Tolerability of Sucrosomial® Iron
3.2.2. Adverse Events (AEs)
3.2.3. Adherence to the Study Treatment
3.2.4. Analytical Parameters
3.2.5. Signs and Symptoms of IDA
3.2.6. Quality of Life (QoL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Høivik, M.L.; Reinisch, W.; Cvancarova, M.; Moum, B. The IBSEN Study Group Anaemia in inflammatory bowel disease: A population-based 10-year follow-up. Aliment. Pharmacol. Ther. 2014, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Gisbert, J.P. Anemia and inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 4659–4665. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor- treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, C.; Henriksson, I.; Brus, O.; Zhulina, Y.; Nyhlin, N.; Tysk, C.; Montgomery, S.; Halfvarson, J. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: A population-based cohort study. Aliment. Pharmacol. Ther. 2018, 48, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Ramos-Rivers, C.; Regueiro, M.; Koutroumpakis, E.; Click, B.; Schwartz, M.; Swoger, J.; Baidoo, L.; Hashash, J.G.; Barrie, A.; et al. Five-Year Period Prevalence and Characteristics of Anemia in a Large US Inflammatory Bowel Disease Cohort. J. Clin. Gastroenterol. 2016, 50, 638–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.; Hartmann, F.; Dignass, A.U. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Hindryckx, P.; Amininejad, L.; Van De Vijver, E.; Bossuyt, P.; Belgian Group for IBD Research and Development. Belgian recommendations for the management of anemia in patients with inflammatory bowel disease. Acta Gastroenterol. Belg. 2014, 77, 333–344. [Google Scholar]
- Goldberg, N.D. Iron deficiency anemia in patients with inflammatory bowel disease. Clin. Exp. Gastroenterol. 2013, 6, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.; Dignass, A.U. Management of iron deficiency anemia in inflammatory bowel disease—A practical approach. Ann. Gastroenterol. 2013, 26, 104–113. [Google Scholar] [PubMed]
- Mücke, V.; Mücke, M.M.; Raine, T.; Bettenworth, M. Diagnosis and treatment of anemia in patients with inflammatory bowel disease. Ann. Gastroenterol. 2016, 30, 15–22. [Google Scholar] [CrossRef]
- Niepel, D.; Klag, T.; Malek, N.P.; Wehkamp, J. Practical guidance for the management of iron deficiency in patients with inflammatory bowel disease. Ther. Adv. Gastroenterol. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasche, C.; Lomer, E.M.C.; Cavill, I.; Weiss, G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.; Radeke, H.H.; Dignass, A.; Stein, J. Current evaluation and management of anaemia in patients with inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, K.; Kulnigg-Dabsch, S.; Gasche, C. Management of Iron Deficiency Anemia. Gastroenterol. Hepatol. 2015, 11, 241–250. [Google Scholar]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J. Crohns Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbati, G.; Incerti, F.; Boarini, C.; Pileri, F.; Bocchi, D.; Ventura, P.; Buzzetti, E.; Pietrangelo, A. Safety and efficacy of sucrosomial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern. Emerg. Med. 2018, 14, 423–431. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, M.; Hrelia, S.; Crespi, T.; Perazzo, P.; Malaguti, M.; De Vecchi, E.; Lombardi, G.; Banfi, G.; Riso, P.; Porrini, M.; et al. Oral Supplementation with Sucrosomial Ferric Pyrophosphate Plus L-Ascorbic Acid to Ameliorate the Martial Status: A Randomized Controlled Trial. Nutrients 2020, 12, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2014, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial® iron absorption studied by in vitro and ex-vivo models. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Sandborn, W.J.; Sutherland, L.R.; Feagan, B.G.; Geboes, K.; Hanauer, S.B.; Irvine, E.J.; Lémann, M.; Marteau, P.; Rutgeerts, P.; et al. A Review of Activity Indices and Efficacy End Points for Clinical Trials of Medical Therapy in Adults with Ulcerative Colitis. Gastroenterology 2007, 132, 763–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stange, E.; Travis, S.; Vermeire, S.; Reinisch, W.; Geboes, K.; Barakauskiene, A.; Feakins, R.; Fléjou, J.; Herfarth, H.; Hommes, D.; et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J. Crohns Colitis 2008, 2, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, R.; Bradshaw, J. A Simple Index of Crohn’s-Disease Activity. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Alcalá, M.J.; Casellas, F.; Fontanet, G.; Prieto, L.; Malagelada, J.R. Shortened questionnaire on quality of life for inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 383–391. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial Iron Supplementation in Anemic Patients with Celiac Disease Not Tolerating Oral Ferrous Sulfate: A Prospective Study. Nutrients 2018, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Barni, S. Efficacy and tolerability of Sucrosomial iron supplementation in IBD patients with iron deficiency anemia and intolerance to iron oral salts. Expert Rev. Hematol. 2016, 9 (Suppl. S1), 6–8. [Google Scholar] [CrossRef] [Green Version]
- Ciudin, A.; Simó-Servat, O.; Balibrea, J.M.; Vilallonga, R.; Hernandez, C.; Simó, R.; Mesa, J. Response to oral sucrosomial iron supplementation in patients undergoing bariatric surgery. The BARI-FER study. Endocrinol. Diabetes Nutr. 2018, 65, 17–20. [Google Scholar] [CrossRef]
- Parisi, F.; Berti, C.; Mandò, C.; Martinelli, A.; Mazzali, C.; Cetin, I. Effects of different regimens of iron prophylaxis on maternal iron status and pregnancy outcome: A randomized control trial. J. Matern. Neonatal Med. 2016, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Mafodda, A.; Giuffrida, D.; Prestifilippo, A.; Azzarello, D.; Giannicola, R.; Mare, M.; Maisano, R. Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: A pilot study. Support. Care Cancer 2017, 25, 2779–2786. [Google Scholar] [CrossRef] [Green Version]
- Bertani, L.; Tricò, D.; Zanzi, F.; Svizzero, G.B.; Coppini, F.; de Bortoli, N.; Bellini, M.; Antonioli, L.; Blandizzi, C.; Marchi, S. Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis. Nutrients 2021, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Tsantes, A.; Peyrin-Biroulet, L.; Danese, S. Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2016, 95, e2308. [Google Scholar] [CrossRef]
- Surti, B.; Spiegel, B.; Ippoliti, A.; Vasiliauskas, E.A.; Simpson, P.; Shih, D.Q.; Targan, S.R.; McGovern, D.P.; Melmed, G.Y. Assessing Health Status in Inflammatory Bowel Disease Using a Novel Single-Item Numeric Rating Scale. Dig. Dis. Sci. 2013, 58, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Gomollon, F. Common Misconceptions in the Diagnosis and Management of Anemia in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2008, 103, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Bermejo, F.; Pajares, R.; Pérez-Calle, J.-L.; Rodríguez, M.; Algaba, A.; Mancenido, N.; De La Morena, F.; Carneros, J.A.; McNicholl, A.G.; et al. Oral and intravenous iron treatment in inflammatory bowel disease: Hematological response and quality of life improvement. Inflamm. Bowel Dis. 2009, 15, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.W.; Lewis, S.; Barton, R.J.; Corbett, S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef] [Green Version]


| Characteristic | Value |
|---|---|
| Age, years [median (range)] | 41.2 (20.0–81.2) |
| Sex, F/M [n (%)] | 39 (84.8)/7 (15.2) |
| BMI, kg/m2 [median (range)] | 23.5 (16.0–45.0) |
| <18.5 | 5 (10.9) |
| 18.5–<25 | 26 (56.5) |
| 2–<30 | 10 (21.7) |
| ≥30 | 5 (10.9) |
| Diagnosis of CD/UC [n (%)] | 31 (67.4)/15 (32.6) |
| Montreal disease extent in CD at diagnosis [n (%)] | |
| ileal (L1) | 15 (32.6) |
| colonic (L2) | 3 (6.5) |
| ileo-colonic (L3) | 12 (26.1) |
| upper disease (L4) | 0(0.0) |
| ileal + upper disease (L1 + L4) | 1 (2.2) |
| Montreal disease extent in UC at diagnosis [n (%)] | |
| ulcerative proctitis (E1) | 0 (0.0) |
| left-sided UC or distal UC (E2) | 6 (13.0) |
| extensive UC or pancolitis (E3) | 9 (19.6) |
| Modified Mayo Score (MS) [mean (SD)] | 1.27 (1.75) |
| UC severity according to MS [n (%)] | |
| remission: 0 | 12 (80.0) |
| mild disease: 1 | 2 (13.3) |
| moderate disease: 2 | 1 (6.7) |
| severe disease: 3 | 0 (0.0) |
| Harvey-Bradshaw Index (HBI) score [mean (SD)] | 2.8 (2.8) |
| CD severity according to HBI [n (%)] | |
| remission: ≤4 | 23 (76.7) |
| mild disease: 5–7 | 5 (16.7) |
| moderate disease: 8–16 | 2 (6.7) |
| severe disease: ≥16 | 0 (0.0) |
| Years since diagnosis of IBD [mean (SD)] | 9.8 (7.0) |
| Patients with symptoms of IDA [n (%)] | 34 (73.9) |
| Parameters | Visit 1 (Baseline) | Telephonic Monitoring (Week 1) | Effect Size (Telephonic Monitoring) | Visit 2 (Week 5) | Effect Size (Visit 2) | Visit 3 (Week 13) | Effect Size (Visit 3) |
|---|---|---|---|---|---|---|---|
| General well-being [n (%)] | n = 49 | n = 48 | 1.44 | n = 41 | 0.93 | n = 35 | −0.18 |
| very good health | 8 (16.3) | 31 (64.6) | 28 (68.3) | 17 (48.6) | |||
| good health | 13 (26.5) | 12 (25.0) | 13 (31.7) | 16 (45.7) | |||
| fair health | 16 (32.6) | 3 (6.2) | - | 1 (2.9) | |||
| bad health | 10 (20.4) | 2 (4.2) | - | 1 (2.9) | |||
| very bad health | 2 (4.1) | - | - | - | |||
| Number of liquid depositions [n (%)] | n = 48 | n = 48 | 0.76 | n = 41 | 1.44 | n = 34 | 1.83 |
| ≤2 | 23 (46.9) | 38 (79.2) | 31 (75.6) | 28 (80.0) | |||
| 3–5 | 16 (32.7) | 5 (10.4) | 9 (22.0) | 5 (14.3) | |||
| 6–8 | 10 (20.4) | 3 (6.2) | 1 (2.4) | 1 (2.9) | |||
| ≥9 | - | 2 (4.2) | - | 1 (2.9) | |||
| Abdominal pain [n (%)] | n = 49 | n = 47 | 1.37 | n = 40 | 1.71 | n = 35 | 1.92 |
| none | 10 (20.4) | 37 (78.7) | 29 (72.5) | 26 (74.3) | |||
| mild | 13 (26.5) | 6 (12.8) | 8 (20.0) | 5 (14.3) | |||
| moderate | 20 (40.8) | 3 (6.4) | 2 (5.0) | 3 (8.6) | |||
| severe | 6 (12.2) | 1 (2.1) | 1 (2.5) | 1 (2.9) | |||
| Constipation [n (%)] | n = 48 | n = 47 | 0.84 | n = 41 | 0.64 | n = 34 | 0.74 |
| none | 37 (77.1) | 44 (93.6) | 35 (85.4) | 29 (85.3) | |||
| mild | 5 (10.4) | 2 (4.3) | 5 (12.2) | 4 (11.8) | |||
| moderate | 5 (10.4) | 1 (2.1) | - | - | |||
| severe | 1 (2.1) | - | 1 (2.5) | 1 (2.9) | |||
| Loss of appetite [n (%)] | n = 48 | n = 46 | 1.06 | n = 40 | 1.06 | n = 35 | 0.79 |
| none | 38 (79.1) | 46 (100.0) | 39 (97.5) | 31 (88.6) | |||
| mild | 6 (12.5) | - - | 1 (2.5) | 3 (8.6) | |||
| moderate | 3 (6.2) | - | - | 1 (2.9) | |||
| severe | 1 (2.1) | - | - | - | |||
| Nausea [n (%)] | n = 49 | n = 48 | 1.05 | n = 41 | 1.13 | n = 35 | 1.25 |
| none | 29 (59.2) | 44 (91.7) | 36 (87.8) | 31 (88.6) | |||
| mild | 12 (24.5) | 3 (6.2) | 4 (9.8) | 3 (8.6) | |||
| moderate | 5 (10.2) | 1 (2.1) | 1 (2.4) | 1 (2.9) | |||
| severe | 3 (6.1) | - | - | - | |||
| Vomiting [n (%)] | n = 49 | n = 48 | 0.99 | n = 41 | 0.92 | n = 35 | 0.81 |
| none | 44 (89.8) | 48 (100.0) | 40 (97.6) | 33 (94.3) | |||
| mild | 3 (6.1) | - | 1 (2.4) | 2 (5.7) | |||
| moderate | 1 (2.0) | - | - | - | |||
| severe | 1 (2.0) | - | - | - | |||
| Teeth staining [n (%)] | n = 48 | n = 45 | 0.95 | n = 41 | 0.44 | n = 35 | -0.06 |
| none | 45 (93.7) | 45 (100.0) | 39 (95.1) | 32 (91.4) | |||
| mild | 2 (4.2) | - | 2 (4.9) | 2 (5.7) | |||
| moderate | 1 (2.1) | - | - | 1 (2.9) | |||
| severe | - | - | - | - | |||
| Change in color of stool [n (%)] | n = 49 | n = 47 | 1.40 | n = 41 | 1.81 | n = 34 | 2.09 |
| none | 11 (22.4) | 35 (74.5) | 22 (53.7) | 20 (58.8) | |||
| mild | 3 (6.1) | 7 (14.9) | 14 (34.1) | 8 (23.5) | |||
| moderate | 17 (34.7) | 2 (4.2) | 4 (9.8) | 5 (14.7) | |||
| severe | 18 (36.7) | 3 (6.4) | 1 (2.4) | 1 (2.9) | |||
| Metallic taste [n (%)] | n = 48 | n = 47 | 1.07 | n = 41 | 1.09 | n = 35 | 1.21 |
| none | 27 (56.2) | 43 (91.5) | 36 (87.8) | 31 (88.6) | |||
| mild | 12 (25.0) | 4 (8.5) | 4 (9.8) | 3 (8.6) | |||
| moderate | 9 (18.8) | - | 1 (2.4) | 1 (2.9) | |||
| severe | - | - | - | - |
| Description of Adverse Events | Number of Patients (%) (n = 49) |
| Color of stool | 27 (55.1) |
| Abdominal pain | 21 (42.9) |
| Diarrhea | 20 (40.8) |
| Nausea | 10 (20.4) |
| Constipation | 10 (20.4) |
| Metallic taste | 8 (16.3) |
| Asthenia | 7 (14.3) |
| Relatedness to SI Treatment | Number of AEs (%) (n = 166) |
| Certain | 12 (7.2) |
| Probable | 56 (33.7) |
| Possible | 17 (10.2) |
| Unlikely | 38 (22.9) |
| Not related | 43 (25.9) |
| Parameter [Mean (SD)] | Visit 1 (Baseline) | Visit 2 (Week 5) | Effect Size (Visit 2) | Visit 3 (Week 13) | Effect Size (Visit 3) |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 11.2 (0.7) | 11.4 (0.8) | 0.27 | 11.7 (1.1) | 0.56 |
| Hematocrit (%) | 35.6 (2.0) | 36.5 (2.1) | 0.44 | 37.3 (3.0) | 0.69 |
| MCV (fL) | 82.5 (7.2) | 83.1 (7.8) | 0.08 | 83.4 (7.9) | 0.12 |
| MCH (pg) | 26.0 (3.0) | 26.0 (2.9) | 0.00 | 26.2 (3.2) | 0.06 |
| Erythrocytes (1012/L) | 4.3 (0.7) | 4.4 (0.4) | 0.17 | 4.5 (0.5) | 0.32 |
| Ferritin (μg/L) | 14.3 (14.0) | 15.4 (12.8) | 0.08 | 15.9 (13.0) | 0.12 |
| TSAT (%) | 8.7 (4.4) | 13.9 (11.2) | 0.64 | 16.2 (15.9) | 0.69 |
| Sideremia (μg/dL) | 31.4 (17.0) | 47.9 (37.2) | 0.59 | 55.6 (51.6) | 0.68 |
| Transferrin (g/L) | 311.8 (66.4) | 303.2 (64.0) | −0.13 | 298.7 (86.9) | −0.17 |
| C-reactive protein (mg/L) | 7.9 (9.0) | 8.1 (13.2) | 0.02 | 4.6 (7.3) | −0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastida, G.; Herrera-de Guise, C.; Algaba, A.; Ber Nieto, Y.; Soares, J.M.; Robles, V.; Bermejo, F.; Sáez-González, E.; Gomollón, F.; Nos, P. Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment. Nutrients 2021, 13, 1770. https://doi.org/10.3390/nu13061770
Bastida G, Herrera-de Guise C, Algaba A, Ber Nieto Y, Soares JM, Robles V, Bermejo F, Sáez-González E, Gomollón F, Nos P. Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment. Nutrients. 2021; 13(6):1770. https://doi.org/10.3390/nu13061770
Chicago/Turabian StyleBastida, Guillermo, Claudia Herrera-de Guise, Alicia Algaba, Yolanda Ber Nieto, Jose Manuel Soares, Virginia Robles, Fernando Bermejo, Esteban Sáez-González, Fernando Gomollón, and Pilar Nos. 2021. "Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment" Nutrients 13, no. 6: 1770. https://doi.org/10.3390/nu13061770
APA StyleBastida, G., Herrera-de Guise, C., Algaba, A., Ber Nieto, Y., Soares, J. M., Robles, V., Bermejo, F., Sáez-González, E., Gomollón, F., & Nos, P. (2021). Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment. Nutrients, 13(6), 1770. https://doi.org/10.3390/nu13061770







