Crossing the Antarctica: Exploring the Effects of Appetite-Regulating Hormones and Indicators of Nutrition Status during a 93-Day Solo-Expedition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Procedure, Equipment and Variables
3. Results
3.1. Appetite-Regulating Hormones
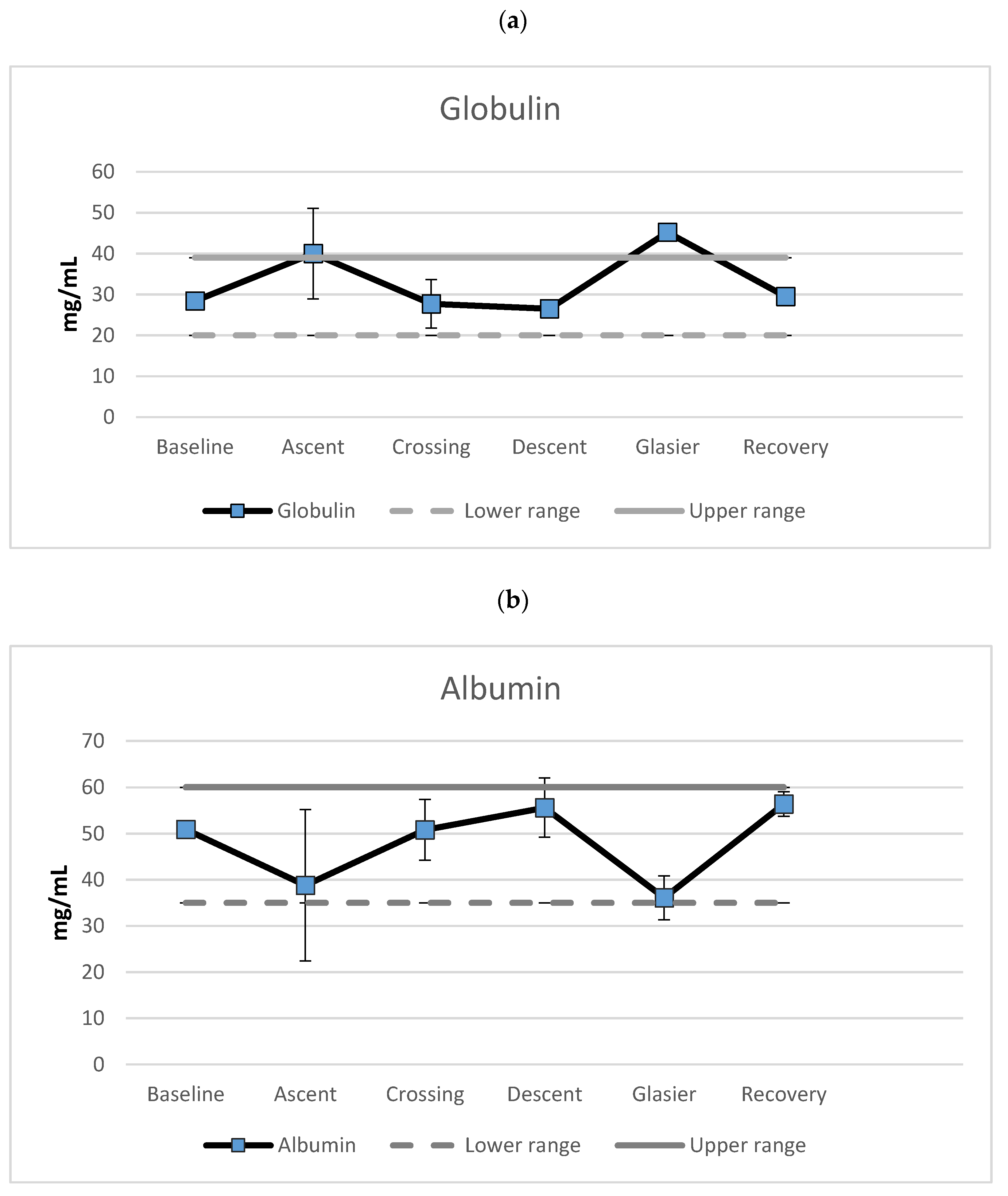
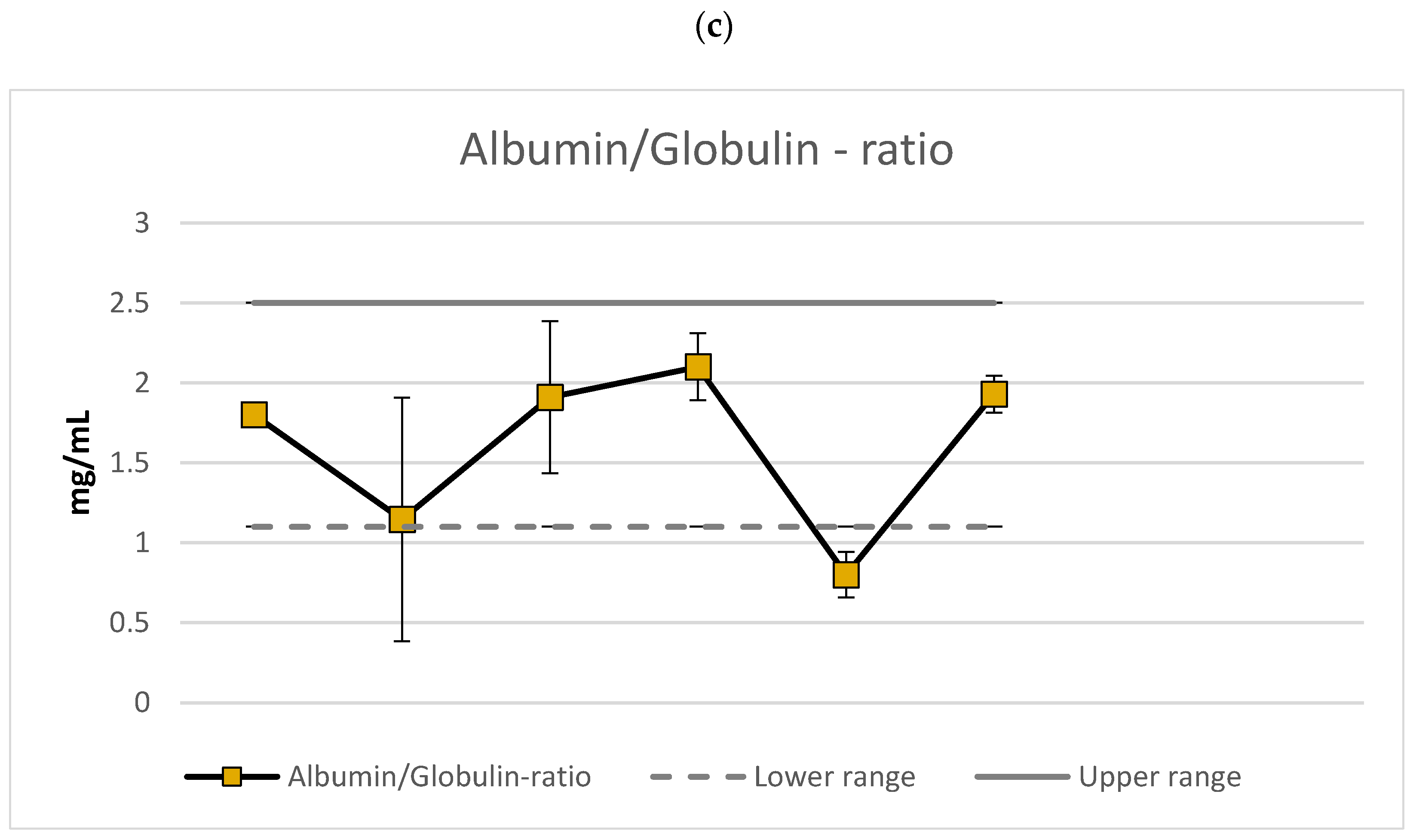
3.2. Markers of Nutrition Status
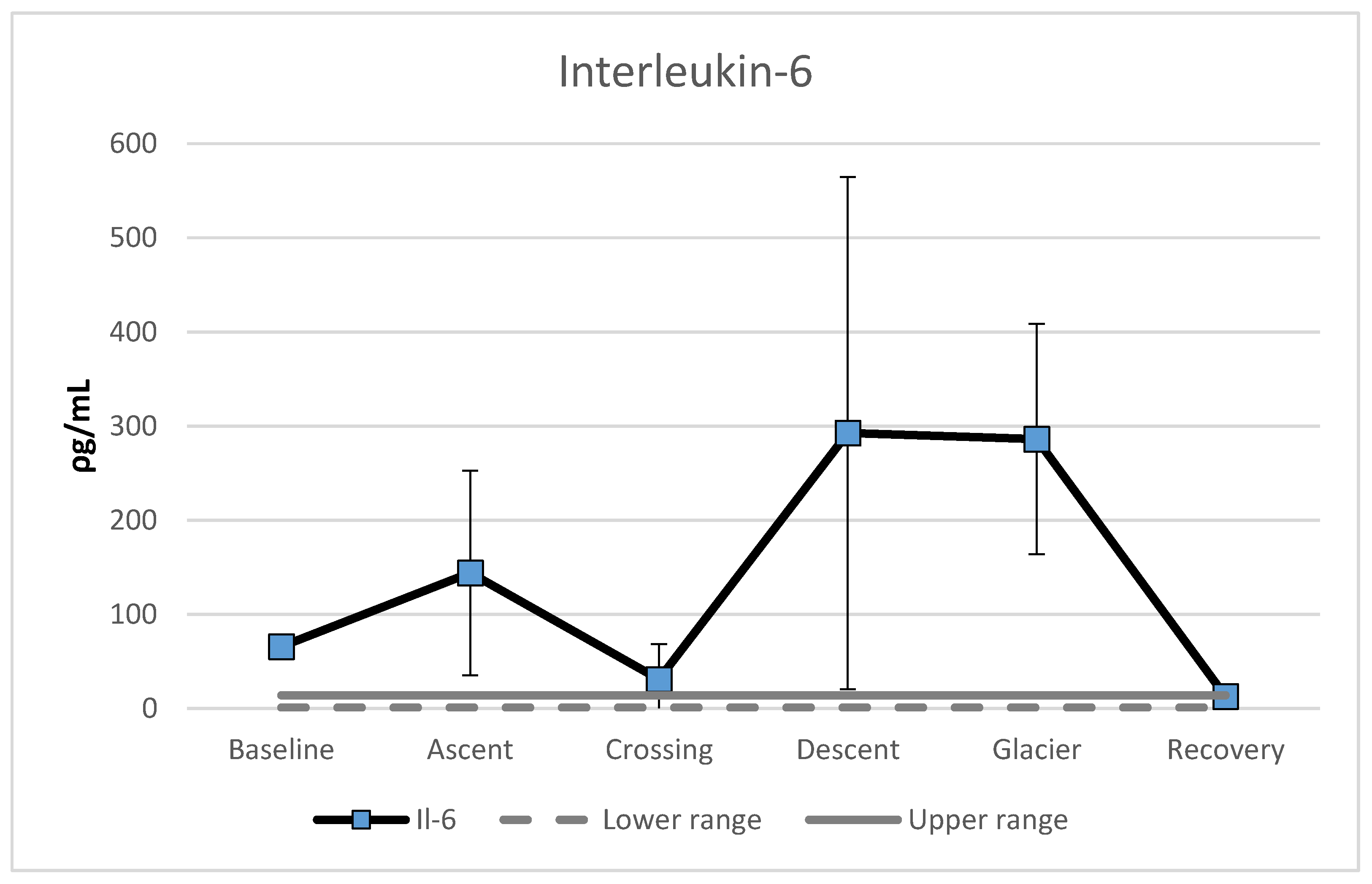
3.3. Interleukin-6
4. Discussion
4.1. Appetite-Regulating Hormones
4.2. Nutritional Status
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NASA. NASA Strategic Plan. 2014. Available online: https://www.nasa.gov/sites/default/files/files/FY2014_NASA_SP_508c.pdf (accessed on 20 May 2021).
- Bartone, P.T.; Roland, R.R.; Bartone, J.V.; Krueger, G.P.; Sciarretta, A.A.; Johnsen, B.H. Human adaptability for deep space missions: An exploratory study. J. Hum. Perform. Extrem. Environ. 2019, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskaya, I.B.; Egorov, A.D.; Sonkin, V.D. Some approaches to prophylactic measures in a martian expedition. Hum. Physiol. 2010, 36, 259–263. [Google Scholar] [CrossRef]
- Bartone, P.T.; Krueger, G.P.; Roland, R.R.; Sciarretta, A.A.; Bartone, J.V.; Johnsen, B.H. Individual Differences in Adapta-Bility for Long Duration Space Exploration Missions; Report NASA/TM-2016-219288; Johnson Space Center: Houston, TX, USA, 2017.
- Bartone, P.T.; Krueger, G.P.; Bartone, J.V. Individual Differences in Adaptability to Isolated, Confined, and Extreme Environments. Aerosp. Med. Hum. Perform. 2018, 89, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Strewe, C.; Moser, D.; Buchheim, J.-I.; Gunga, H.-C.; Stahn, A.; Crucian, B.E.; Fiedel, B.; Bauer, H.; Gössmann-Lang, P.; Thieme, D.; et al. Sex differences in stress and immune responses during confinement in Antarctica. Biol. Sex Differ. 2019, 10, 20. [Google Scholar] [CrossRef]
- Palacios-González, B.; Vadillo-Ortega, F.; Polo-Oteyza, E.; Sánchez, T.; Ancira-Moreno, M.; Romero-Hidalgo, S.; Meráz, N.; Antuna-Puente, B. Irisin levels before and after physical activity among school-age children with different BMI: A direct relation with leptin. Obesity 2015, 23, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Romanelli, M.M.C. Irisin: A Potential Link between Physical Exercise and Metabolism—An Observational Study in Differently Trained Subjects, from Elite Athletes to Sedentary People. J. Diabetes Res. 2017, 2017, 1039161. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Tournis, S.; Leontsini, D.; Jamurtas, A.Z.; Sxina, M.; Thomakos, P.M.; Manousaki, M.; Douroudos, I.I.; Taxildaris, K.; Mitrakou, A. Leptin and Adiponectin Responses in Overweight Inactive Elderly following Resistance Training and Detraining Are Intensity Related. J. Clin. Endocrinol. Metab. 2005, 90, 5970–5977. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.M. The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis. Endocrinol. Metab. 2016, 31, 1–6. [Google Scholar] [CrossRef]
- Polyzos, J.; Kountouras, K.; Shields, K.; Mantzoros, C.S. Irisin: A renaissance in metabolism? Metab.-Clin. Exp. 2013, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nat. Cell Biol. 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.-J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: A cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism 2018, 79, 24–32. [Google Scholar] [CrossRef]
- Joung, K.E.; Park, K.-H.; Zaichenko, L.; Sahin-Efe, A.; Thakkar, B.; Brinkoetter, M.; Usher, N.; Warner, D.; Davis, C.R.; Crowell, J.A.; et al. Early Life Adversity Is Associated With Elevated Levels of Circulating Leptin, Irisin, and Decreased Levels of Adiponectin in Midlife Adults. J. Clin. Endocrinol. Metab. 2014, 99, E1055–E1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dundar, A.; Kocahan, S.; Sahin, L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch. Physiol. Biochem. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.R.; Chu, H.; Castracane, V.D. Leptin and Exercise. Exp. Biol. Med. 2002, 227, 701–708. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Gabrielsen, J.S.; Simcox, J.A.; Lee, S.-H.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte iron regulates leptin and food intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, K.; Fukagawa, K.; Fukagawa, T.; Noguchi, H.; Kakuma, T.; Sakata, T.; Yoshimatsu, H. Glucagon-like peptide-1, corticotropin-releasing hormone, and hypothalamic neuronal histamine interact in the leptin-signaling pathway to regulate feeding behavior. FASEB J. 2005, 19, 1131–1133. [Google Scholar] [CrossRef] [Green Version]
- Laursen, T.L.; Zak, R.B.; Shute, R.J.; Heesch, M.W.S.; Dinan, N.E.; Bubak, M.P.; La Salle, D.T.; Slivka, D.R. Leptin, adiponectin, and ghrelin responses to endurance exercise in different ambient conditions. Temperature 2017, 4, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Leonard, W.R.; Sorensen, M.V.; Galloway, V.A.; Spencer, G.J.; Mosher, M.; Osipova, L.; Spitsyn, V.A. Climatic influences on basal metabolic rates among circumpolar populations. Am. J. Hum. Biol. 2002, 14, 609–620. [Google Scholar] [CrossRef]
- White, L.J.; Dressendorfer, R.H.; Holland, E.; McCoy, S.C.; Ferguson, M.A. Increased Caloric Intake Soon after Exercise in Cold Water. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 38–47. [Google Scholar] [CrossRef]
- Bruckmaier, M.; Tachtsidis, I.; Phan, P.; Lavie, N. Attention and Capacity Limits in Perception: A Cellular Metabolism Account. J. Neurosci. 2020, 40, 6801–6811. [Google Scholar] [CrossRef]
- Mainous, M.R.; Deitch, E.A. Nutrition and Infection. Surg. Clin. N. Am. 1994, 74, 659–676. [Google Scholar] [CrossRef]
- Gundog, M.; Basaran, H. Pretreatment low prognostic nutritional index and low albumin–globulin ratio are predictive for overall survival in nasopharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, A. Albumin usage in clinical medicine: Tradition or therapeutic? Transfus. Med. Rev. 2010, 24, 53–63. [Google Scholar] [CrossRef]
- Xu, W.Z.; Li, F.; Xu, Z.K.; Chen, X.; Sun, B.; Cao, J.W.; Liu, Y.G. Pre-operative albumin-to-globulin ratio and prognostic nutrition index predicts prognosis for glioblastoma. Oncotargets Ther. 2017, 10, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Dai, Y.; Zhou, F.; Long, Z.; Li, Y.; Liu, B.; Xie, D.; Tang, J.; Tan, J.; Yao, K.; et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 484–e1. [Google Scholar] [CrossRef]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscular Interleukin-6 and Its Role as an Energy Sensor. Med. Sci. Sports Exerc. 2012, 44, 392–396. [Google Scholar] [CrossRef]
- Smith, J.D.; Cianflone, K.; Martin, J.; Poirier, P.; Broderick, T.L.; Noël, M. Plasma Adipokine and Hormone Changes in Mountaineers on Ascent to 5300 Meters. Wilderness Environ. Med. 2011, 22, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, B.H.; Gjeldnes, R.; Neteland HO, M.; Thayer, J.F.; Phillips, T. Militærpsykologisk forskning i felt: En case-studie av biologiske markører under solokryssing av Antarktisk. Necesse 2020, 5, 181–199. [Google Scholar]
- Gjeldens, R. Beyond the Poles; Rune Gjeldnes AS: Hell, Norway, 2006; ISBN -10. [Google Scholar]
- Phillips, T.M. Rapid analysis of inflammatory cytokines in cerebrospinal fluid using chip-based immunoaffinity electrophoresis. Electrophoresis 2004, 25, 1652–1659. [Google Scholar] [CrossRef]
- Mihalopoulos, N.L.; Phillips, T.M.; Slater, H.; Thomson, J.A.; Varner, M.W.; Nanjee, M.N.; Moyer-Mileur, L.J. Validity and reliability of perinatal biomarkers of adiposity after storage as dried blood spots on paper. Am. J. Hum. Biol. 2011, 23, 717–719. [Google Scholar] [CrossRef] [Green Version]
- Phillips, T.M. Multi-analyte analysis of biological fluids with a recycling immunoaffinity column array. J. Biochem. Biophys. Methods 2001, 49, 253–262. [Google Scholar] [CrossRef]
- Larson, D. Clinical Chemistry: Fundamentals and Laboratory Technique; Saunders: Philadelphia, PA, USA, 2016; ISBN1 10 1455742147. ISBN2 13 9781455742141. [Google Scholar]
- Dutra, S.C.P.; Moura, E.G.; Rodrigues, A.L.; Lisboa, P.C.; Bonomo, I.; Toste, F.P.; Passos, M.C.F. Cold exposure restores the decrease in leptin receptors (OB-Rb) caused by neonatal leptin treatment in 30-day-old rats. J. Endocrinol. 2007, 195, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Imai, J.; Katagiri, H.; Yamada, T.; Ishigaki, Y.; Ogihara, T.; Uno, K.; Hasegawa, Y.; Gao, J.; Ishihara, H.; Sasano, H.; et al. Cold Exposure Suppresses Serum Adiponectin Levels through Sympathetic Nerve Activation in Mice. Obesity 2006, 14, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Konrad, M.; Henson, D.A.; Kennerly, K.; Shanely, R.A.; Wallner-Liebmann, S.J. Variance in the Acute Inflammatory Response to Prolonged Cycling Is Linked to Exercise Intensity. J. Interf. Cytokine Res. 2012, 32, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Yones, H.A.; Mostafa, R.; Shirin, Z.B.; Hossein, S.; Zahra, H.A. Inflammatory immune system response to short term altitude exposure and recreational physical activity. Int. J. Sport Stud. 2014, 4, 1383–1387. [Google Scholar]
- Baumert, P.; Lake, M.J.; Stewart, C.E.; Drust, B.; Erskine, R.M. Genetic variation and Exercise induced muscle damage: Im-plications for athletic performance, injury and ageing. Eur. J. Appl. Physiol. 2016, 116, 1595–1625. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Menu 1 | Carbohydrates (kcal) | Proteins (kcal) | Fat (kcal) | Total (kcal) | |
|---|---|---|---|---|---|
| Breakfast | 287.6 | 66.4 | 923.4 | 1277 | |
| Lunch | 292.8 | 68.8 | 965.3 | 1327 | |
| Dinner | 307.6 | 216.8 | 1058.4 | 1583 | |
| Dessert | 458.4 | 30 | 382.5 | 871 | |
| Total | 1346.4 | 382 | 3329.6 | 5058 | |
| Menu 2 | |||||
| breakfast | 332.4 | 74.4 | 954 | 1361 | |
| Lunch | 368 | 98.4 | 1263.1 | 1730 | |
| Dinner | 304 | 206.4 | 1562.4 | 2073 | |
| Dessert | 404.4 | 26.4 | 337.5 | 768 | |
| Total | 1408.8 | 405.6 | 4117 | 5931 |
| Breakfast | Lunch | Dinner | Dessert |
|---|---|---|---|
| Soya oil | Oatmeal | Drytech dinner | Milk chocolate |
| Solfrokost (cereal) | Hazelnuts | Soya oil | Powdered chocolate drink |
| Hazelnuts | Salted Peanuts | Potato chips/pork chips | |
| Sugar | Raisins | ||
| Cream powder | Soya oil | ||
| MCT-fat | |||
| Sugar |
| Phases | Stressors | Number of Samples | Distance Travelled (km) | Duration (days) |
|---|---|---|---|---|
| Baseline | Some psychological stress - Expectations for the coming journey | 1 | 0 | 3 |
| Ascent towards plateau | Extreme physical stress - Muscular load/strain | 6 | 485 | 20 |
| Crossing of plateau | Some physical stress - Prolonged, low-intensity muscular load/strain | 10 | 2924 | 42 |
| Descent | Some psychological stress - Occasional experiences of threats | 5 | 1246 | 22 |
| Priestly glacier | Extreme psychological stress - Continuous extreme threats | 2 | 145 | 9 |
| Recovery | Some psychological stress - Missing significant others | 3 | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnsen, B.H.; Brattebø, G.; Phillips, T.M.; Gjeldnes, R.; Bartone, P.T.; Monsen, H.-O.N.; Thayer, J.F. Crossing the Antarctica: Exploring the Effects of Appetite-Regulating Hormones and Indicators of Nutrition Status during a 93-Day Solo-Expedition. Nutrients 2021, 13, 1777. https://doi.org/10.3390/nu13061777
Johnsen BH, Brattebø G, Phillips TM, Gjeldnes R, Bartone PT, Monsen H-ON, Thayer JF. Crossing the Antarctica: Exploring the Effects of Appetite-Regulating Hormones and Indicators of Nutrition Status during a 93-Day Solo-Expedition. Nutrients. 2021; 13(6):1777. https://doi.org/10.3390/nu13061777
Chicago/Turabian StyleJohnsen, Bjørn Helge, Guttorm Brattebø, Terry M. Phillips, Rune Gjeldnes, Paul T. Bartone, Hans-Olav Neteland Monsen, and Julian F. Thayer. 2021. "Crossing the Antarctica: Exploring the Effects of Appetite-Regulating Hormones and Indicators of Nutrition Status during a 93-Day Solo-Expedition" Nutrients 13, no. 6: 1777. https://doi.org/10.3390/nu13061777






