Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotic Strains
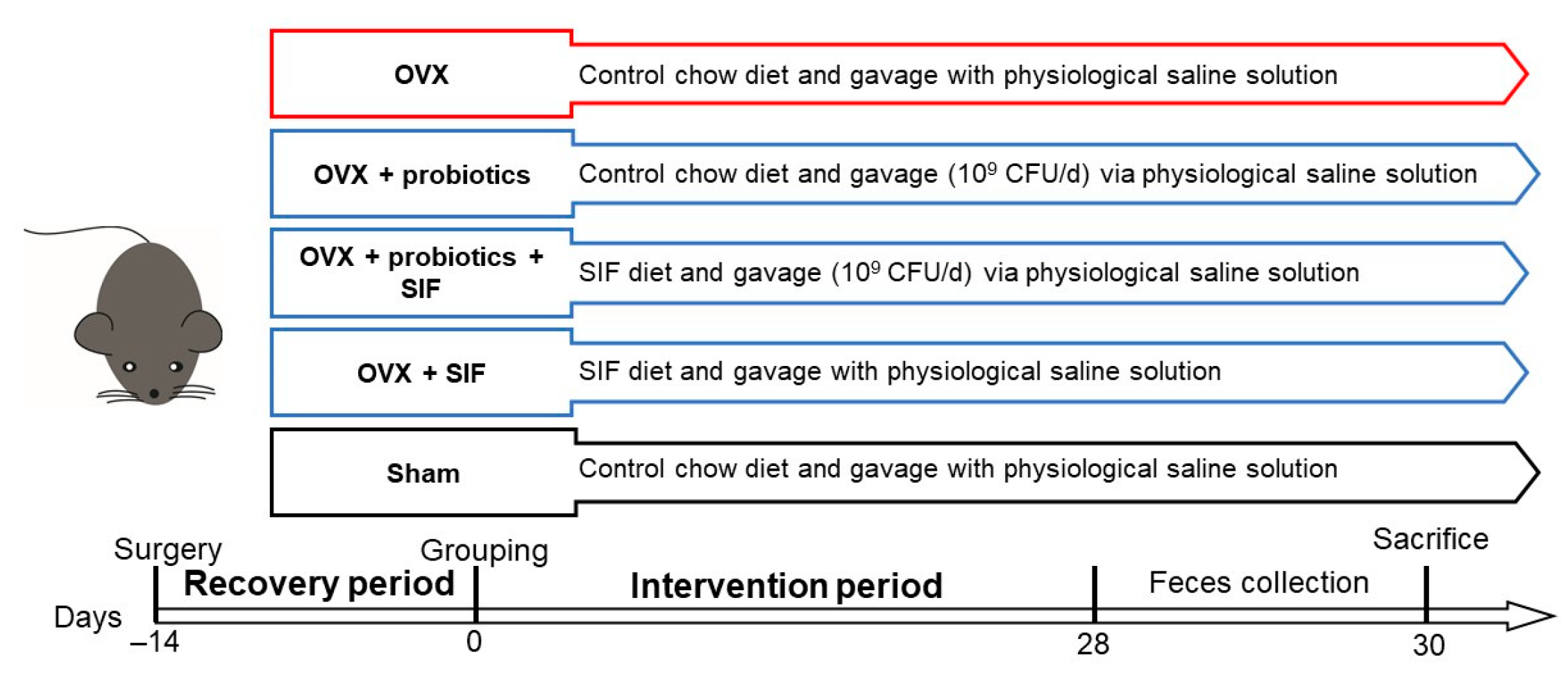
2.2. Animal and Ovariectomy
2.3. Detection of Sex Hormones Levels in Serum
2.4. Determinations of Biochemical Assays
2.5. RT-qPCR for Estrogen Receptor (ER) α in Abdominal Adipose Tissue
2.6. Feces DNA Isolation and 16S rRNA Gene Sequence Analysis
2.7. Measurement of Short-Chain Fatty Acids (SCFAs)
2.8. Statistical Analysis
3. Results
3.1. Administration of Probiotics and a SIF Diet Alleviated Metabolic Dysregulation and Inflammation Induced by OVX
3.2. L. plantarum 30M5 Elevated Serum Estradiol Concentrations and Promoted ERα Expression in Abdominal Adipose Tissue in Ovariectomized Mice
3.3. L. plantarum 30M5 and a SIF Diet Exerted Strong Influences on the Intestinal Microbiota, and Altered Microbiota Was Associated with Estradiol Production
3.4. L. plantarum 30M5 Showed a High Production Capacity for SCFAs in OVX Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yokota-Nakagi, N.; Takahashi, H.; Kawakami, M.; Takamata, A.; Uchida, Y.; Morimoto, K. Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats. Nutrients 2020, 12, 907. [Google Scholar] [CrossRef]
- Eckel, L.A. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol. Behav. 2011, 104, 517–524. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, E.J.; Choi, Y.Y.; Hong, J.; Yang, W.M. Lycium chinense Improves Post-Menopausal Obesity via Regulation of PPAR-γ and Estrogen Receptor-α/β Expressions. Am. J. Chin. Med. 2017, 45, 269–282. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Conner, E.A.; Kaunitz, A.M. Management of Menopause and the Role for Hormone Therapy. Clin. Obstet. Gynecol. 2019, 62, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Ness, R.B.; Roman, L.D.; Terry, K.L.; Schildkraut, J.M.; Chang-Claude, J.; Doherty, J.A.; Menon, U.; Cramer, D.W.; Gayther, S.A.; et al. Association Between Menopausal Estrogen-Only Therapy and Ovarian Carcinoma Risk. Obstet. Gynecol. 2016, 127, 828–836. [Google Scholar] [CrossRef]
- Fernandez, E.; Gallus, S.; Bosetti, C.; Franceschi, S.; Negri, E.; La Vecchia, C. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case-control studies. Int. J. Cancer 2003, 105, 408–412. [Google Scholar] [CrossRef]
- Sau, L.; Olmstead, C.M.; Cui, L.J.; Chen, A.; Shah, R.S.; Kelley, S.T.; Thackray, V.G. Alterations in Gut Microbiota Do Not Play a Causal Role in Diet-independent Weight Gain Caused by Ovariectomy. J. Endocr. Soc. 2021, 5, bvaa173. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Santacruz, A.; Gauffin-Cano, P. Gut microbiota in obesity and metabolic disorders. Proc. Nutr. Soc. 2010, 69, 434–441. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019, 593, 2655–2664. [Google Scholar] [CrossRef]
- Cheng, L.; Qi, C.; Zhuang, H.; Fu, T.; Zhang, X. gutMDisorder: A comprehensive database for dysbiosis of the gut microbiota in disorders and interventions. Nucleic Acids Res. 2020, 48, D554–D560. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Delzenne, N.; Neyrinck, A.M.; Bäckhed, F.; Cani, P. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-Y.; Wan, Y.-P.; Fang, Q.-Y.; Lu, W.; Cai, W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr. 2011, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Ahn, Y.T.; Park, S.H.; Huh, C.S.; Yoo, S.R.; Yu, R.; Sung, M.K.; McGregor, R.A.; Choi, M.S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Besselink, E.; Henning, S.M.; Go, V.L.W.; Heber, D. Phytoestrogens Induce Differential Estrogen Receptor Alpha- or Beta-Mediated Responses in Transfected Breast Cancer Cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Guadamuro, L.; Dohrmann, A.B.; Tebbe, C.C.; Mayo, B.; Delgado, S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Xie, C.-L.; Kang, S.S.; Cho, K.M.; Park, K.H.; Lee, D.H. Isoflavone-enriched soybean (Glycine max) leaves prevents ovariectomy-induced obesity by enhancing fatty acid oxidation. J. Funct. Foods 2018, 43, 165–172. [Google Scholar] [CrossRef]
- Tang, S.; Du, Y.; Oh, C.; No, J. Effects of Soy Foods in Postmenopausal Women: A Focus on Osteosarcopenia and Obesity. J. Obes. Metab. Syndr. 2020, 29, 180–187. [Google Scholar] [CrossRef]
- Fontaine, K.R.; Yang, D.; Gadbury, G.L.; Heshka, S.; Schwartz, L.G.; Murugesan, R.; Kraker, J.L.; Heo, M.; Heymsfield, S.B.; Allison, D.B. Results of soy-based meal replacement formula on weight, anthropometry, serum lipids & blood pressure during a 40-week clinical weight loss trial. Nutr. J. 2003, 2, 14. [Google Scholar] [CrossRef]
- Meng, Q.; Li, J.; Chao, Y.; Bi, Y.; Zhang, W.; Zhang, Y.; Ji, T.; Fu, Y.; Chen, Q.; Zhang, Q.; et al. β-estradiol adjusts intestinal function via ERβ and GPR30 mediated PI3K/AKT signaling activation to alleviate postmenopausal dyslipidemia. Biochem. Pharmacol. 2020, 180, 114134. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Q.; Tian, P.; Wang, L.; Li, X.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Gut microbiota dysbiosis might be responsible to different toxicity caused by Di-(2-ethylhexyl) phthalate exposure in murine rodents. Environ. Pollut. 2020, 261, 114164. [Google Scholar] [CrossRef]
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Medina-Contreras, J.; Villalobos-Molina, R.; Zarain-Herzberg, A.; Balderas-Villalobos, J. Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol. Cell. Biochem. 2020, 475, 261–276. [Google Scholar] [CrossRef]
- Meng, Q.; Yu, X.; Chen, Q.; Wu, X.; Kong, X.; Wang, S.; Cai, D.; Cheng, P.; Li, Y.; Bian, H. Liuwei Dihuang soft capsules inhibits the phenotypic conversion of VSMC to prevent the menopausal atherosclerosis by up-regulating the expression of myocardin. J. Ethnopharmacol. 2020, 246, 112207. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jo, K.-J.; Kim, O.-S.; Kim, B.-J.; Kang, D.-W.; Lee, K.-H.; Baik, H.-W.; Han, M.S.; Lee, S.-K. Parenteral 17beta-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci. 2010, 87, 358–366. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Ludgero-Correia, A., Jr.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 2012, 28, 316–323. [Google Scholar] [CrossRef]
- Guillaume, M.; Handgraaf, S.; Fabre, A.; Raymond-Letron, I.; Riant, E.; Montagner, A.; Vinel, A.; Buscato, M.; Smirnova, N.; Fontaine, C.; et al. Selective Activation of Estrogen Receptor α Activation Function-1 Is Sufficient to Prevent Obesity, Steatosis, and Insulin Resistance in Mouse. Am. J. Pathol. 2017, 187, 1273–1287. [Google Scholar] [CrossRef]
- Gao, Q.; Horvath, T.L. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am. J. Physiol. Metab. 2008, 294, E817–E826. [Google Scholar] [CrossRef]
- Febbraio, M.A. gp130 receptor ligands as potential therapeutic targets for obesity. J. Clin. Investig. 2007, 117, 841–849. [Google Scholar] [CrossRef]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.-M. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Jardel, C.; Bruckert, E.; Blondy, P.; Capeau, J.; Laville, M.; Vidal, H.; Hainque, B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 2000, 85, 3338–3342. [Google Scholar]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; De Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, Y.-J.; Shin, M.-J.; Yi, H. Difference in the Gut Microbiome between Ovariectomy-Induced Obesity and Diet-Induced Obesity. J. Microbiol. Biotechnol. 2017, 27, 2228–2236. [Google Scholar] [CrossRef]
- Becker, S.L.; Manson, J.E. Menopause, the gut microbiome, and weight gain: Correlation or causation? Menopause 2021, 28, 327–331. [Google Scholar] [CrossRef]
- Vieira, A.T.; Castelo, P.M.; Ribeiro, D.A.; Ferreira, C.M. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front. Microbiol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; Van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Lim, E.Y.; Lee, S.-Y.; Shin, H.S.; Lee, J.; Nam, Y.-D.; Lee, D.O.; Lee, J.Y.; Yeon, S.H.; Son, R.H.; Park, C.L.; et al. The Effect of Lactobacillus acidophilus YT1 (MENOLACTO) on Improving Menopausal Symptoms: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. J. Clin. Med. 2020, 9, 2173. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, D.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Seo, K.-H. Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity: Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; Reyes-Gavilán, C.G.D.L.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Eden, J.A. A review of the clinical effects of phytoestrogens. Obstet. Gynecol. 1996, 87, 897–904. [Google Scholar] [PubMed]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Dai, S.; Pan, M.; El-Nezami, H.S.; Wan, J.M.; Wang, M.; Habimana, O.; Lee, J.C.-Y.; Louie, J.C.; Shah, N.P. Effects of Lactic Acid Bacteria-Fermented Soymilk on Isoflavone Metabolites and Short-Chain Fatty Acids Excretion and Their Modulating Effects on Gut Microbiota. J. Food Sci. 2019, 84, 1854–1863. [Google Scholar] [CrossRef]
- Elghali, S.; Mustafa, S.; Amid, M.; Manap, M.Y.A.; Ismail, A.; Abas, F. Bioconversion of daidzein to equol by Bifidobacterium breve 15700 and Bifidobacterium longum BB536. J. Funct. Foods 2012, 4, 736–745. [Google Scholar] [CrossRef]
- Kwon, J.E.; Lim, J.; Bang, I.; Kim, I.; Kim, D.; Kang, S.C. Fermentation product with new equol-producing Lactobacillus paracasei as a probiotic-like product candidate for prevention of skin and intestinal disorder. J. Sci. Food Agric. 2019, 99, 4200–4210. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the Fecal Microbiome with Urinary Estrogens and Estrogen Metabolites in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef] [PubMed]








| Ingredient | Group | |
|---|---|---|
| Control Chow Diet (g/kg) | SIF Diet (g/kg) | |
| Cornstarch | 427.486 | 424.986 |
| Casein | 200 | 200 |
| Dextrinized Corn starch | 132 | 132 |
| Sucrose | 100 | 100 |
| Corn oil | 40 | 40 |
| Cellulose | 50 | 50 |
| Mineral mix (AIN-93M) | 35 | 35 |
| Vitamin mix (AIN-93) | 10 | 10 |
| L-Cystine | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.014 | 0.014 |
| Isoflavone-rich powder | - | 2.5 |
| Group | Food Consumption (g/day) | Body Weight Gain (%) | Abdominal Adiposity (%) |
|---|---|---|---|
| Sham | 2.709 ± 0.4212 | 7.480 ± 1.938 | 2.208 ± 0.3607 ** |
| OVX | 2.763 ± 0.2613 | 10.83 ± 2.025 | 3.510 ± 0.3814 |
| OVX + 15M1 | 2.769 ± 0.4632 | 0.420 ± 3.602 **** | 3.223 ± 0.6795 |
| OVX + 30M5 | 2.493 ± 0.2703 | 5.666 ± 1.802 ** | 2.894 ± 0.4978 |
| OVX + 15M1 + SIF | 2.642 ± 0.2762 | 5.654 ± 2.678 ** | 3.320 ± 0.6107 |
| OVX + 30M5 + SIF | 2.833 ± 0.3089 | 13.660 ± 4.703 | 3.637 ± 0.9034 |
| OVX + SIF | 2.786 ± 0.4192 | 11.380 ± 1.884 | 3.563 ± 0.8390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. https://doi.org/10.3390/nu13061793
Chen Q, Wang B, Wang S, Qian X, Li X, Zhao J, Zhang H, Chen W, Wang G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients. 2021; 13(6):1793. https://doi.org/10.3390/nu13061793
Chicago/Turabian StyleChen, Qian, Botao Wang, Shunhe Wang, Xin Qian, Xiu Li, Jianxin Zhao, Hao Zhang, Wei Chen, and Gang Wang. 2021. "Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice" Nutrients 13, no. 6: 1793. https://doi.org/10.3390/nu13061793
APA StyleChen, Q., Wang, B., Wang, S., Qian, X., Li, X., Zhao, J., Zhang, H., Chen, W., & Wang, G. (2021). Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients, 13(6), 1793. https://doi.org/10.3390/nu13061793






