Zinc Pharmacotherapy for Elderly Osteoporotic Patients with Zinc Deficiency in a Clinical Setting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Statistical Analysis
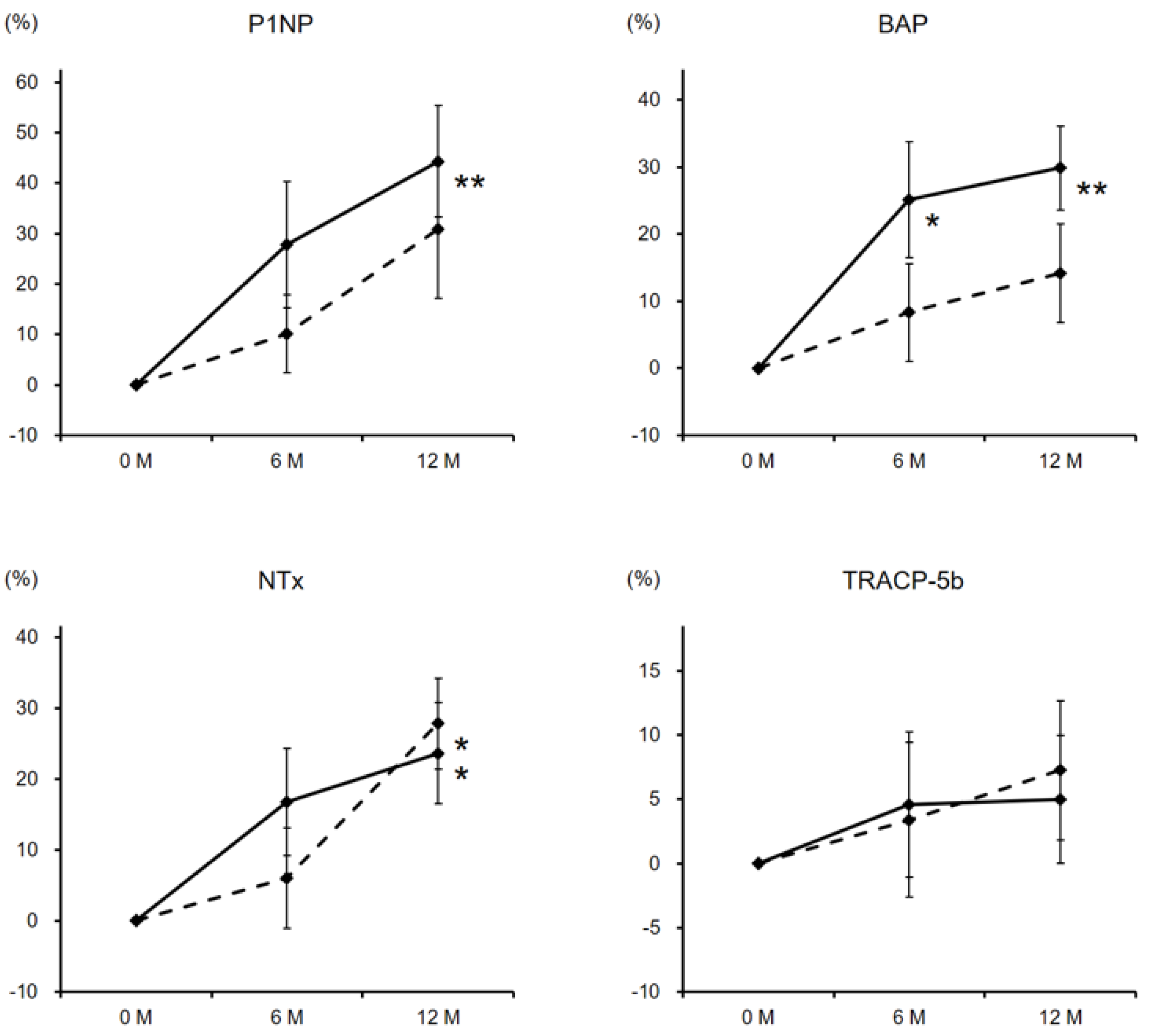
3. Results
3.1. Patient Characteristics
3.2. Laboratory Data and BMD Changes
3.3. Safety Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, A.F.; Vallee, B.L. Zinc metalloenzymes: Characteristics and significance in biology and medicine. Am. J. Clin. Nutr. 1969, 22, 1222–1239. [Google Scholar] [CrossRef]
- Lim, K.H.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef]
- Zofková, I.; Nemcikova, P.; Matucha, P. Trace elements and bone health. Clin. Chem. Lab. Med. 2013, 51, 1555–1561. [Google Scholar] [CrossRef]
- Oner, G.; Bhaumick, B.; Bala, R.M. Effect of zinc deficiency on serum somatomedin levels and skeletal growth in young rats. Endocrinology 1984, 114, 1860–1863. [Google Scholar] [CrossRef]
- da Cunha Ferreira, R.M.; Marquiegui, I.M.; Elizaga, I.V. Teratogenicity of zinc deficiency in the rat: Study of the fetal skeleton. Teratology 1989, 39, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Herzberg, M.; Foldes, J.; Steinberg, R.; Menczel, J. Zinc excretion in osteoporotic women. J. Bone Miner. Res. 1990, 5, 251–257. [Google Scholar] [CrossRef]
- Pemmer, B.; Roschger, A.; Wastl, A.; Hofstaetter, J.G.; Wobrauschek, P.; Simon, R.; Thaler, H.W.; Roschger, P.; Klaushofer, K.; Streli, C. Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone 2013, 57, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Relea, P.; Revilla, M.; Ripoll, E.; Arribas, I.; Villa, L.F.; Rico, H. Zinc, biochemical markers of nutrition, and type I osteoporosis. Age Ageing 1995, 24, 303–307. [Google Scholar] [CrossRef]
- Okyay, E.; Ertugrul, C.; Acar, B.; Sisman, A.R.; Onvural, B.; Ozaksoy, D. Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas 2013, 76, 320–325. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J. Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: A meta-analysis. Biol. Trace Elem. Res. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Ebrahimi, M.; Ebrahimi, A. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin. Cases Miner. Bone Metab. 2015, 12, 18–21. [Google Scholar] [CrossRef]
- Igarashi, A.; Yamaguchi, M. Increase in bone growth factors with healing rat fractures: The enhancing effect of zinc. Int. J. Mol. Med. 2001, 8, 433–438. [Google Scholar] [CrossRef]
- Sadighi, A.; Roshan, M.M.; Moradi, A.; Ostadrahimi, A. The effects of zinc supplementation on serum zinc, alkaline phosphatase activity and fracture healing of bones. Saudi Med. J. 2008, 29, 1276–1279. [Google Scholar]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s Practical Guidelines for Zinc Deficiency with a particular focus on taste disorders, inflammatory bowel disease, and liver cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef]
- Soen, S.; Fukunaga, M.; Sugimoto, T.; Sone, T.; Fujiwara, S.; Endo, N.; Gorai, I.; Shiraki, M.; Hagino, H.; Hosoi, T.; et al. Japanese Society for Bone and Mineral Research and Japan Osteoporosis Society Joint Review Committee for the Revision of the Diagnostic Criteria for Primary Osteoporosis Diagnostic criteria for primary osteoporosis: Year 2012 revision. J. Bone Miner. Metab. 2013, 31, 247–257. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 2 July 2019).
- Hashizume, M.; Yamaguchi, M. Stimulatory effect of beta-alanyl-L-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol. Cell. Biochem. 1993, 122, 59–64. [Google Scholar] [CrossRef]
- Hashizume, M.; Yamaguchi, M. Effect of beta-alanyl-L-histidinato zinc on differentiation of osteoblastic MC3T3-E1 cells: Increases in alkaline phosphatase activity and protein concentration. Mol. Cell. Biochem. 1994, 131, 19–24. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Goto, M.; Uchiyama, S.; Nakagawa, T. Effect of zinc on gene expression in osteoblastic MC3T3-E1 cells: Enhancement of Runx2, OPG, and regucalcin mRNA expressions. Mol. Cell. Biochem. 2008, 312, 157–166. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Hashizume, M. Effect of beta-alanyl-L-histidinato zinc on protein components in osteoblastic MC3T3-El cells: Increase in osteocalcin, insulin-like growth factor-I and transforming growth factor-beta. Mol. Cell. Biochem. 1994, 136, 163–169. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Segawa, Y.; Shimokawa, N.; Tsuzuike, N.; Tagashira, E. Inhibitory effect of beta-alanyl-L-histidinato zinc on bone resorption in tissue culture. Pharmacology 1992, 45, 292–300. [Google Scholar] [CrossRef]
- Kishi, S.; Yamaguchi, M. Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochem. Pharmacol. 1994, 48, 1225–1230. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Uchiyama, S. Receptor activator of NF-kappaB ligand-stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int. J. Mol. Med. 2004, 14, 81–85. [Google Scholar]
- Hie, M.; Tsukamoto, I. Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur. J. Pharmacol. 2011, 668, 140–146. [Google Scholar] [CrossRef]
- Liang, D.; Yang, M.; Guo, B.; Cao, J.; Yang, L.; Guo, X. Zinc upregulates the expression of osteoprotegerin in mouse osteoblasts MC3T3-E1 through PKC/MAPK pathways. Biol. Trace Elem. Res. 2012, 146, 340–348. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Díez-Pérez, A.; Boonen, S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: A systematic review. Bone 2014, 58, 126–135. [Google Scholar] [CrossRef]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Wang, H.; Liu, Y.; San Martin, J. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 2008, 93, 2149–2157. [Google Scholar] [CrossRef] [Green Version]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]


| Mean ± SD | Median (Minimum–Maximum) | Reference Value | |
|---|---|---|---|
| Age, years | 74.5 ± 7.1 | 75 (65–91) | |
| BMI, kg/m2 | 21.4 ± 3.3 | 21.8 (13.7–28.3) | 18.5–25 |
| Albumin, g/dL | 4.1 ± 0.2 | 4.1 (3.6–4.4) | 3.8–5.2 |
| HbA1c, % | 5.7 ± 0.4 | 5.8 (4.9–6.2) | 4.6–6.2 |
| eGFR, mL/min/1.73 m2 | 62.7 ± 10.8 | 63 (44.1–79.3) | ≥60 |
| Zinc, µg/dL | 65.2 ± 9 | 63 (52–79) | 80–130 |
| Copper, µg/dL | 114 ± 21 | 119 (73–143) | 68–128 |
| Iron, µg/dL | 84.7 ± 19.2 | 82 (63–129) | 48–200 |
| Magnesium, mg/dL | 2.1 ± 0.2 | 2.2 (1.7–2.4) | 1.8–2.6 |
| Calcium, mg/dL | 9.3 ± 0.5 | 9.2 (8.7–10.7) | 8.5–10.2 |
| Phosphorus, mg/dL | 3.6 ± 0.6 | 3.6 (2.1–4.7) | 2.4–4.3 |
| 25(OH)D, ng/mL | 19.2 ± 5 | 18 (12.8–29.6) | ≥30 |
| 1,25(OH)2D3, pg/mL | 56.5 ± 14.9 | 55 (32–85.7) | 20–60 |
| P1NP, ng/mL | 27.7 ± 10.2 | 23.7 (18–55.9) | 18.1–98.2 |
| BAP, µg/L | 9.8 ± 2.9 | 9.6 (5.8–16.1) | 3.7–22.6 |
| NTx, nmolBCE/mmolCre | 37.1 ± 14.8 | 36.4 (14.4–57.5) | 13–89 |
| TRACP-5b, mU/dL | 239 ± 83 | 233 (150–403) | 120–590 |
| Whole PTH, pg/mL | 24.3 ± 8.2 | 23.3 (14.6–42.5) | 8.3–38.7 |
| Lumbar BMD, g/cm2 | 0.92 ± 0.21 | 0.84 (0.68–1.38) | 1.15 ± 0.14 * |
| Total hip BMD, g/cm2 | 0.72 ± 0.12 | 0.72 (0.56–1.07) | 0.96 ± 0.13 * |
| Femoral neck BMD, g/cm2 | 0.69 ± 0.12 | 0.66 (0.54–1.02) | 0.94 ± 0.11 * |
| Osteoporosis treatment duration, years | 4.7 ± 3.3 | 3.8 (1–11.5) | |
| Diabetes mellitus, yes | 9 (7.4%) | ||
| Dyslipidemia, yes | 15 (12.3%) | ||
| Hypertension, yes | 34 (27.9%) | ||
| Rheumatoid arthritis, yes | 33 (27%) | ||
| Prevalent osteoporotic fracture, yes | 43 (35.2%) |
| r-Value from Pearson’s Product-Moment Correlation | P-Value | ρ-Value from Spearman’s Rank Correlation | P-Value | |
|---|---|---|---|---|
| vs. Lumbar BMD | 0.477 | <0.001 | 0.442 | <0.001 |
| vs. Total hip BMD | 0.395 | <0.001 | 0.321 | <0.001 |
| vs. Femoral neck BMD | 0.452 | <0.001 | 0.407 | <0.001 |
| Odds Ratio | 95% CI | P-Value | |
|---|---|---|---|
| for Lumbar BMD (+1SD) | 1.81 | 1.39–2.37 | <0.001 |
| for Total hip BMD (+1SD) | 1.77 | 1.37–2.23 | <0.001 |
| for Femoral neck BMD (+1SD) | 1.86 | 1.42–2.42 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, M.; Nakamura, Y.; Miyazaki, A.; Takahashi, J. Zinc Pharmacotherapy for Elderly Osteoporotic Patients with Zinc Deficiency in a Clinical Setting. Nutrients 2021, 13, 1814. https://doi.org/10.3390/nu13061814
Nakano M, Nakamura Y, Miyazaki A, Takahashi J. Zinc Pharmacotherapy for Elderly Osteoporotic Patients with Zinc Deficiency in a Clinical Setting. Nutrients. 2021; 13(6):1814. https://doi.org/10.3390/nu13061814
Chicago/Turabian StyleNakano, Masaki, Yukio Nakamura, Akiko Miyazaki, and Jun Takahashi. 2021. "Zinc Pharmacotherapy for Elderly Osteoporotic Patients with Zinc Deficiency in a Clinical Setting" Nutrients 13, no. 6: 1814. https://doi.org/10.3390/nu13061814
APA StyleNakano, M., Nakamura, Y., Miyazaki, A., & Takahashi, J. (2021). Zinc Pharmacotherapy for Elderly Osteoporotic Patients with Zinc Deficiency in a Clinical Setting. Nutrients, 13(6), 1814. https://doi.org/10.3390/nu13061814







