Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Calcium
3.1.1. Research
3.1.2. Intake
3.1.3. Recommendation
3.1.4. Comparison with Commercial Prenatals
3.2. Chromium
3.2.1. Research
3.2.2. Intake
3.2.3. Recommendation
3.2.4. Comparison with Commercial Prenatals
3.3. Copper
3.3.1. Research
3.3.2. Intake
3.3.3. Recommendation
3.3.4. Comparison with Commercial Prenatals
3.4. Iodine
3.4.1. Research
3.4.2. Intake
3.4.3. Recommendation
3.4.4. Comparison with Commercial Prenatals
3.5. Iron
3.5.1. Research
3.5.2. Intake
3.5.3. Recommendation
3.5.4. Comparison with Commercial Prenatals
3.6. Magnesium
3.6.1. Research
3.6.2. Intake
3.6.3. Recommendation
3.6.4. Comparison with Commercial Prenatals
3.7. Manganese
3.7.1. Research
3.7.2. Intake
3.7.3. Recommendation
3.7.4. Comparison with Commercial Prenatals
3.8. Molybdenum
3.8.1. Research
3.8.2. Intake
3.8.3. Recommendation
3.8.4. Comparison with Commercial Prenatals
3.9. Selenium
3.9.1. Research
3.9.2. Intake
3.9.3. Recommendation
3.9.4. Comparison with Commercial Prenatals
3.10. Zinc
3.10.1. Research
3.10.2. Intake
3.10.3. Recommendation
3.10.4. Comparison with Commercial Prenatals
4. Discussion
4.1. Associations between Health Outcomes and Mineral Status
4.2. Limitations and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lerch, C.; Meissner, T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database Syst. Rev. 2007, Cd006164. [Google Scholar] [CrossRef]
- Pettifor, J.M. Vitamin D &/or calcium deficiency rickets in infants & children: A global perspective. Indian J. Med. Res. 2008, 127, 245–249. [Google Scholar]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Pitkin, R.M.; Gebhardt, M.P. Serum calcium concentrations in human pregnancy. Am. J. Obstet. Gynecol. 1977, 127, 775–778. [Google Scholar] [CrossRef]
- Almaghamsi, A.; Almalki, M.H.; Buhary, B.M. Hypocalcemia in Pregnancy: A Clinical Review Update. Oman Med. J. 2018, 33, 453–462. [Google Scholar] [CrossRef]
- Kolthoff, N.; Eiken, P.; Kristensen, B.; Nielsen, S.P. Bone mineral changes during pregnancy and lactation: A longitudinal cohort study. Clin. Sci. 1998, 94, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Lang, L.; Li, Y.; Liu, Q.; Yao, Y. Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy-induced hypertension and healthy pregnant women: A meta-analysis. Hypertens. Pregnancy 2016, 35, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Duley, L.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2014, Cd001059. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, H.; He, X.; Li, M.; Yan, P.; Xun, Y.; Lu, C.; Yang, K.; Zhang, X. The association between calcium supplement and preeclampsia and gestational hypertension: A systematic review and meta-analysis of randomized trials. Hypertens. Pregnancy 2019, 38, 129–139. [Google Scholar] [CrossRef]
- Herrera, J.A.; Shahabuddin, A.K.; Ersheng, G.; Wei, Y.; Garcia, R.G.; López-Jaramillo, P. Calcium plus linoleic acid therapy for pregnancy-induced hypertension. Int. J. Gynaecol. Obstet. 2005, 91, 221–227. [Google Scholar] [CrossRef]
- Herrera, J.A.; Arevalo-Herrera, M.; Herrera, S. Prevention of preeclampsia by linoleic acid and calcium supplementation: A randomized controlled trial. Obstet. Gynecol. 1998, 91, 585–590. [Google Scholar] [CrossRef]
- Mann, J.R.; McDermott, S.; Bao, H.; Hardin, J.; Gregg, A. Pre-eclampsia, birth weight, and autism spectrum disorders. J. Autism. Dev. Disord. 2010, 40, 548–554. [Google Scholar] [CrossRef]
- Li, Y.M.; Shen, Y.D.; Li, Y.J.; Xun, G.L.; Liu, H.; Wu, R.R.; Xia, K.; Zhao, J.P.; Ou, J.J. Maternal dietary patterns, supplements intake and autism spectrum disorders: A preliminary case-control study. Medicine 2018, 97, e13902. [Google Scholar] [CrossRef]
- Bassaw, B.; Roopnarinesingh, S.; Roopnarinesingh, A.; Homer, H. Prevention of hypertensive disorders of pregnancy. J. Obstet. Gynaecol. 1998, 18, 123–126. [Google Scholar] [CrossRef]
- Rogers, M.S.; Fung, H.Y.; Hung, C.Y. Calcium and low-dose aspirin prophylaxis in women at high risk of pregnancy-induced hypertension. Hypertens. Pregnancy 1999, 18, 165–172. [Google Scholar] [CrossRef]
- Cong, K.; Chi, S.; Liu, G. Calcium supplementation during pregnancy for reducing pregnancy induced hypertension. Chin. Med. J. 1995, 108, 57–59. [Google Scholar]
- World Heath Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Available online: http://apps.who.int/iris/bitstream/handle/10665/44703/9789241548335_eng.pdf (accessed on 1 January 2021).
- U.S. Department of Agriculture ARS. Total Nutrient Intakes: Percent Reporting and Mean Amounts of Selected Vitamins and Minerals from Food and Beverages and Dietary Supplements, by Gender and Age. In What We Eat in America; NHANES2017–2018; Food Surveys Research Group: Beltsville, MD, USA, 2018. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Aharoni, A.; Tesler, B.; Paltieli, Y.; Tal, J.; Dori, Z.; Sharf, M. Hair chromium content of women with gestational diabetes compared with nondiabetic pregnant women. Am. J. Clin. Nutr. 1992, 55, 104–107. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Rodgerson, D.O. Comparison of hair chromium levels of nulliparous and parous women. Am. J. Obstet. Gynecol. 1969, 103, 320–321. [Google Scholar] [CrossRef]
- Mahalko, J.R.; Bennion, M. The effect of parity and time between pregnancies on maternal hair chromium concentration. Am. J. Clin. Nutr. 1976, 29, 1069–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Baig, J.A.; Jamali, M.K.; Arain, M.B.; Sarfraz, R.A.; Sheikh, H.U.R.; Kandhro, G.A.; Shah, A.Q. Status of essential trace metals in biological samples of diabetic mother and their neonates. Arch. Gynecol. Obstet. 2009, 280, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, P.G.; Sridhar, G.R.; Sujatha, V.; Anita, V. Serum chromium levels in gestational diabetes mellitus. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 1), S70–S73. [Google Scholar] [CrossRef]
- Woods, S.E.; Ghodsi, V.; Engel, A.; Miller, J.; James, S. Serum chromium and gestational diabetes. J. Am. Board Fam. Med. 2008, 21, 153–157. [Google Scholar] [CrossRef]
- Jovanovic-Peterson, L.; Peterson, C.M. Vitamin and mineral deficiencies which may predispose to glucose intolerance of pregnancy. J. Am. Coll. Nutr. 1996, 15, 14–20. [Google Scholar] [CrossRef]
- Jamilian, M.; Bahmani, F.; Siavashani, M.A.; Mazloomi, M.; Asemi, Z.; Esmaillzadeh, A. The Effects of Chromium Supplementation on Endocrine Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2016, 172, 72–78. [Google Scholar] [CrossRef]
- Amiri Siavashani, M.; Zadeh Modarres, S.; Mirhosseini, N.; Aghadavod, E.; Salehpour, S.; Asemi, Z. The Effects of Chromium Supplementation on Gene Expression of Insulin, Lipid, and Inflammatory Markers in Infertile Women with Polycystic Ovary Syndrome Candidate for in vitro Fertilization: A Randomized, Double-Blinded, Placebo-Controlled Trial. Front. Endocrinol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suksomboon, N.; Poolsup, N.; Yuwanakorn, A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014, 39, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Iovieno, N.; Dalton, E.D.; Fava, M.; Mischoulon, D. Second-tier natural antidepressants: Review and critique. J. Affect. Disord. 2011, 130, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Alebic-Juretic, A.; Frkovic, A. Plasma copper concentrations in pathological pregnancies. J. Trace Elem. Med. Biol. 2005, 19, 191–194. [Google Scholar] [CrossRef]
- Vukelić, J.; Kapamadzija, A.; Petrović, D.; Grujić, Z.; Novakov-Mikić, A. Variations of serum copper values in pregnancy. Srp. Arh. Celok Lek. 2012, 140, 42–46. [Google Scholar] [CrossRef]
- Buamah, P.K.; Russell, M.; Milford-Ward, A.; Taylor, P.; Roberts, D.F. Serum copper concentration significantly less in abnormal pregnancies. Clin. Chem. 1984, 30, 1676–1677. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.S.; Elwood, P.C.; Abernethy, M. Trace elements in water and congenital malformations of the central nervous system in South Wales. Br. J. Prev. Soc. Med. 1976, 30, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.D.; Chen, H.C.; Shan, L.F. Study on the relationship between copper, lysyl oxidase and premature rupture of membranes. Zhonghua Fu Chan Ke Za Zhi 2006, 41, 7–11. [Google Scholar]
- Song, X.; Li, B.; Li, Z.; Wang, J.; Zhang, D. High serum copper level is associated with an increased risk of preeclampsia in Asians: A meta-analysis. Nutr. Res. 2017, 39, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Kang, Y.; Zhang, M. A meta-analysis of copper level and risk of preeclampsia: Evidence from 12 publications. Biosci. Rep. 2016, 36, e00370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thauvin, E.; Fusselier, M.; Arnaud, J.; Faure, H.; Favier, M.; Coudray, C.; Richard, M.-J.; Favier, A. Effects of a multivitamin mineral supplement on zinc and copper status during pregnancy. Biol. Trace Elem. Res. 1992, 32, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kashanian, M.; Hadizadeh, H.; Faghankhani, M.; Nazemi, M.; Sheikhansari, N. Evaluating the effects of copper supplement during pregnancy on premature rupture of membranes and pregnancy outcome. J. Matern. Fetal Neonatal Med. 2018, 31, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Borekci, B.; Gulaboglu, M.; Gul, M. Iodine and magnesium levels in maternal and umbilical cord blood of preeclamptic and normal pregnant women. Biol. Trace Elem. Res. 2009, 129, 1–8. [Google Scholar] [CrossRef]
- Adams, J.B.; Holloway, C.E.; George, F.; Quig, D. Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol. Trace Elem. Res. 2006, 110, 193–209. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- De Escobar, G.M.; Obregón, M.J.; del Rey, F.E. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007, 10, 1554–1570. [Google Scholar] [CrossRef] [Green Version]
- De Benoist, B.; McLean, E.; Andersson, M.; Rogers, L. Iodine deficiency in 2007: Global progress since 2003. Food Nutr. Bull. 2008, 29, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.M.; Braverman, L.E.; Pearce, E.N. History of U.S. iodine fortification and supplementation. Nutrients 2012, 4, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G.; Staehling, N.W.; Hannon, W.H.; Flanders, D.W.; Gunter, E.W.; Maberly, G.F.; Braverman, L.E.; Pino, S.; Miller, D.T.; Garbe, P.L.; et al. Iodine nutrition in the United States. Trends and public health implications: Iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J. Clin. Endocrinol. Metab. 1998, 83, 3401–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrick, K.A.; Perrine, C.G.; Aoki, Y.; Caldwell, K.L. Iodine Status and Consumption of Key Iodine Sources in the U.S. Population with Special Attention to Reproductive Age Women. Nutrients 2018, 10, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; WHO Library Cataloguing-in-Publication Data: Geneva, Swizterland, 2007. [Google Scholar]
- Glinoer, D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr. Rev. 1997, 18, 404–433. [Google Scholar] [CrossRef]
- Liberman, C.S.; Pino, S.C.; Fang, S.L.; Braverman, L.E.; Emerson, C.H. Circulating iodide concentrations during and after pregnancy. J. Clin. Endocrinol. Metab. 1998, 83, 3545–3549. [Google Scholar] [CrossRef]
- Brander, L.; Als, C.; Buess, H.; Haldimann, F.; Harder, M.; Hänggi, W.; Herrmann, U.; Lauber, K.; Niederer, U.; Zürcher, T.; et al. Urinary iodine concentration during pregnancy in an area of unstable dietary iodine intake in Switzerland. J. Endocrinol. Investig. 2003, 26, 389–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delange, F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 2007, 10, 1571–1580; discussion 1581–1583. [Google Scholar] [CrossRef]
- Berbel, P.; Mestre, J.L.; Santamaría, A.; Palazon, I.; Franco, A.; Graells, M.; Gonzalez-Torga, A.; de Escobar, G.M. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Rakeman, M.A.; Zhi-Hong, D.; Xue-Yi, C.; Mei, Z.Y.; DeLong, N.; Brenner, G.; Tai, M.; Dong, W.; DeLong, G.R. Effects of iodine supplementation during pregnancy on child growth and development at school age. Dev. Med. Child Neurol. 2002, 44, 76–81. [Google Scholar] [CrossRef]
- Braverman, L.E.; Uller , P.R. (Eds.) Werner and Ingbar’s the Thyroid, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Moreno-Reyes, R.; Glinoer, D.; Van Oyen, H.; Vandevijvere, S. High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: A population-based study. J. Clin. Endocrinol. Metab. 2013, 98, 3694–3701. [Google Scholar] [CrossRef] [Green Version]
- Vermiglio, F.; Lo Presti, V.P.; Moleti, M.; Sidoti, M.; Tortorella, G.; Scaffidi, G.; Castagna, M.G.; Mattina, F.; Violi, M.A.; Crisa, A.; et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: A possible novel iodine deficiency disorder in developed countries. J. Clin. Endocrinol. Metab. 2004, 89, 6054–6060. [Google Scholar] [CrossRef] [Green Version]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef] [Green Version]
- Van Mil, N.H.; Tiemeier, H.; Bongers-Schokking, J.J.; Ghassabian, A.; Hofman, A.; Hooijkaas, H.; Jaddoe, V.W.V.; de Keizer-Schrama, S.M.; Steegers, E.A.P.; Visser, T.J.; et al. Low urinary iodine excretion during early pregnancy is associated with alterations in executive functioning in children. J. Nutr. 2012, 142, 2167–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levie, D.; Korevaar, T.I.M.; Bath, S.C.; Dalmau-Bueno, A.; Murcia, M.; Espada, M.; Dineva, M.; Ibarluzea, J.M.; Sunyer, J.; Tiemeier, H.; et al. Thyroid Function in Early Pregnancy, Child IQ, and Autistic Traits: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab. 2018, 103, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, P.; Shariat, M.; Azizi, F. Effects of iodine supplementation during pregnancy on pregnant women and their offspring: A systematic review and meta-analysis of trials over the past 3 decades. Eur. J. Endocrinol. 2021, 184, 91–106. [Google Scholar] [CrossRef]
- Dineva, M.; Fishpool, H.; Rayman, M.P.; Mendis, J.; Bath, S.C. Systematic review and meta-analysis of the effects of iodine supplementation on thyroid function and child neurodevelopment in mildly-to-moderately iodine-deficient pregnant women. Am. J. Clin. Nutr. 2020, 112, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Drover, S.S.M.; Villanger, G.D.; Aase, H.; Skogheim, T.S.; Longnecker, M.P.; Zoeller, R.T.; Reichborn-Kjennerud, T.; Knudsen, G.P.; Zeiner, P.; Engel, S.M. Maternal Thyroid Function During Pregnancy or Neonatal Thyroid Function and Attention Deficit Hyperactivity Disorder: A Systematic Review. Epidemiology 2019, 30, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Watutantrige-Fernando, S.; Groccia, G.; Manso, J.; Plebani, M.; Faggian, D.; Mion, M.M.; Venturini, R.; Andrisani, A.; Casaro, A.; et al. The Effects of Iodine Supplementation in Pregnancy on Iodine Status, Thyroglobulin Levels and Thyroid Function Parameters: Results from a Randomized Controlled Clinical Trial in a Mild-to-Moderate Iodine Deficiency Area. Nutrients 2019, 11, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonangeli, L.; Maccherini, D.; Cavaliere, R.; Di Giulio, C.; Reinhardt, B.; Pinchera, A.; Aghini-Lombardi, F. Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: A longitudinal study. Eur. J. Endocrinol. 2002, 147, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Markhus, M.W.; Dahl, L.; Moe, V.; Abel, M.H.; Branstaeter, A.L.; Oyen, J.; Meltzer, H.M.; Stormark, K.M.; Graff, I.E.; Smith, L.; et al. Maternal Iodine Status is Associated with Offspring Language Skills in Infancy and Toddlerhood. Nutrients 2018, 10, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, P.H.; Cardona, G.R.; Fang, S.L.; Previtit, M.; Alex, S.; Carrasco, N.; Chin, W.W.; Braverman, L.E. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 1999, 140, 3404–3410. [Google Scholar] [CrossRef]
- Shi, X.; Han, C.; Li, C.; Mao, J.; Wang, W.; Xie, X.; Li, C.; Xu, B.; Meng, T.; Du, J.; et al. Optimal and Safe Upper Limits of Iodine Intake for Early Pregnancy in Iodine-Sufficient Regions: A Cross-Sectional Study of 7190 Pregnant Women in China. J. Clin. Endocrinol. Metab. 2015, 100, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, S.; Mikeda, T.; Okada, T.; Nakamura, K.; Kotani, T.; Hishinuma, A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid 2004, 14, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Connelly, K.J.; Boston, B.A.; Pearce, E.N.; Sesser, D.; Snyder, D.; Braverman, L.E.; Pino, S.; LaFranchi, S.H. Congenital Hypothyroidism Caused by Excess Prenatal Maternal Iodine Ingestion. J. Pediatrics 2012, 161, 760–762. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef]
- Mills, J.L.; Reische, E.C.; Kannan, K.; Gao, C.; Shaw, G.M.; Sundaram, R. Newborn Iodine Status Is Not Related to Congenital Hypothyroidism. J. Nutr. 2020, 150, 2429–2434. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogswell, M.E.; Parvanta, I.; Ickes, L.; Yip, R.; Brittenham, G.M. Iron supplementation during pregnancy, anemia, and birth weight: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Siega-Riz, A.M.; Hartzema, A.G.; Turnbull, C.; Thorp, J.; McDonald, T.; Cogswell, M.E. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: A randomized controlled trial. Am. J. Obstet. Gynecol. 2006, 194, 512–519. [Google Scholar] [CrossRef]
- Gupta, P.M.; Hamner, H.C.; Suchdev, P.S.; Flores-Ayala, R.; Mei, Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1640s–1646s. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.J.; Tancredi, D.J.; Krakowiak, P.; Hansen, R.L.; Ozonoff, S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol. 2014, 180, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Krapels, I.P.; van Rooij, I.A.; Ocké, M.C.; West, C.E.; van der Horst, C.M.; Steegers-Theunissen, R.P. Maternal nutritional status and the risk for orofacial cleft offspring in humans. J. Nutr. 2004, 134, 3106–3113. [Google Scholar] [CrossRef] [Green Version]
- Chandler, A.L.; Hobbs, C.A.; Mosley, B.S.; Berry, R.J.; Canfield, M.A.; Qi, Y.P.; Siega-Riz, A.M.; Shaw, G.M. Neural tube defects and maternal intake of micronutrients related to one-carbon metabolism or antioxidant activity. Birth Defects Res. Clin. Mol. Teratol. 2012, 94, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Makrides, M.; Crowther, C.A.; Gibson, R.A.; Gibson, R.S.; Skeaff, C.M. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.R.; Nickerson, H.J.; Olson, K.A.; Berg, R.L.; Meyer, J.A. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin. Med. Res. 2003, 1, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preziosi, P.; Prual, A.; Galan, P.; Daouda, H.; Boureima, H.; Hercberg, S. Effect of iron supplementation on the iron status of pregnant women: Consequences for newborns. Am. J. Clin. Nutr. 1997, 66, 1178–1182. [Google Scholar] [CrossRef]
- Kilbride, J.; Baker, T.G.; Parapia, L.A.; Khoury, S.A.; Shuqaidef, S.W.; Jerwood, D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: A case-control study in Jordan. Int. J. Epidemiol. 1999, 28, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Scholl, T.O. Maternal iron status: Relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr. Rev. 2011, 69 (Suppl. 1), S23–S29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Rosas, J.P.; Viteri, F.E. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2009, Cd004736. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, Cd004736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jorge, F.B.; Delascio, D.; De Ulhoa Cintra, A.B.; Antunes, M.L. Magnesium Concentration in the Blood Serum of Normal Pregnant Women. Obstet. Gynecol. 1965, 25, 253–254. [Google Scholar] [PubMed]
- Standley, C.A.; Whitty, J.E.; Mason, B.A.; Cotton, D.B. Serum ionized magnesium levels in normal and preeclamptic gestation. Obstet. Gynecol. 1997, 89, 24–27. [Google Scholar] [CrossRef]
- Hall, D.G. Serum magnesium in pregnancy. Obstet. Gynecol. 1957, 9, 158–162. [Google Scholar]
- Arikan, G.M.; Panzitt, T.; Gücer, F.; Scholz, H.S.; Reinisch, S.; Haas, J.; Weiss, P.A.M. Course of maternal serum magnesium levels in low-risk gestations and in preterm labor and delivery. Fetal. Diagn. Ther. 1999, 14, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zarcone, R.; Cardone, G.; Bellini, P. Role of magnesium in pregnancy. Panminerva Med. 1994, 36, 168–170. [Google Scholar]
- Jafrin, W.; Mia, A.R.; Chakraborty, P.K.; Hoque, M.R.; Paul, U.K.; Shaha, K.R.; Akhter, S.; Roy, A.S. An evaluation of serum magnesium status in pre-eclampsia compared to the normal pregnancy. Mymensingh Med. J. 2014, 23, 649–653. [Google Scholar]
- Tavana, Z.; Hosseinmirzaei, S. Comparison of Maternal Serum Magnesium Level in Pre-eclampsia and Normal Pregnant Women. Iran. Red Crescent Med. J. 2013, 15, e10394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enaruna, N.O.; Ande, A.; Okpere, E.E. Clinical significance of low serum magnesium in pregnant women attending the University of Benin Teaching Hospital. Niger. J. Clin. Pract. 2013, 16, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Uludağ, E.; Gözükara, I.; Kucur, S.K.; Uluğ, P.; Özdeğirmenci, Ö.; Erkaya, S. Maternal magnesium level effect on preterm labor treatment. J. Matern. Fetal Neonatal Med. 2014, 27, 1449–1453. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, P.; Chen, C.; Lu, L.; Shechter, M.; Rosanoff, A.; He, K. Magnesium levels in relation to rates of preterm birth: A systematic review and meta-analysis of ecological, observational, and interventional studies. Nutr. Rev. 2021, 79, 188–199. [Google Scholar] [CrossRef]
- Makrides, M.; Crosby, D.D.; Shepherd, E.; Crowther, C.A. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Harrison, V.; Fawcus, S.; Jordaan, E. Magnesium supplementation and perinatal hypoxia: Outcome of a parallel group randomised trial in pregnancy. BJOG: Int. J. Obstet. Gynaecol. 2007, 114, 994–1002. [Google Scholar] [CrossRef]
- D’Almeida, A.; Carter, J.P.; Anatol, A.; Prost, C. Effects of a combination of evening primrose oil (gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) versus magnesium, and versus placebo in preventing pre-eclampsia. Women Health 1992, 19, 117–131. [Google Scholar] [CrossRef]
- Li, S.; Tian, H. Oral low-dose magnesium gluconate preventing pregnancy induced hypertension. Zhonghua Fu Chan Ke Za Zhi 1997, 32, 613–615. [Google Scholar] [PubMed]
- Arikan, G.; Panzitt, T.; Gücer, F.; Boritsch, J.; Trojovski, A.; Haeusler, M.C.H. Oral magnesium supplementation and the prevention of preterm labor. Am. J. Obstet. Gynecol. 1997, 176, S45. [Google Scholar] [CrossRef]
- Spätling, L.; Spätling, G. Magnesium supplementation in pregnancy. A double-blind study. Br. J. Obstet. Gynaecol. 1988, 95, 120–125. [Google Scholar] [CrossRef]
- Kovács, L.; Molnár, B.G.; Huhn, E.; Bódis, L. Magnesium substitution in pregnancy. A prospective, randomized double-blind study. Geburtshilfe Frauenheilkd. 1988, 48, 595–600. [Google Scholar] [CrossRef]
- Sibai, B.M.; Villar, M.A.; Bray, E. Magnesium supplementation during pregnancy: A double-blind randomized controlled clinical trial. Am. J. Obstet. Gynecol. 1989, 161, 115–119. [Google Scholar] [CrossRef]
- De Araújo, C.A.L.; Ray, J.G.; Figueiroa, J.N.; Alves, J.G. BRAzil magnesium (BRAMAG) trial: A double-masked randomized clinical trial of oral magnesium supplementation in pregnancy. BMC Pregnancy Childbirth 2020, 20, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.W.; Crowther, C.A.; Middleton, P.; Marret, S.; Rouse, D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst. Rev. 2009, Cd004661. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A. Whole blood manganese levels in pregnancy and the neonate. Nutrition 1999, 15, 731–734. [Google Scholar] [CrossRef]
- Vigeh, M.; Yokoyama, K.; Ramezanzadeh, F.; Dahaghin, M.; Fakhriazad, E.; Seyedaghamiri, Z.; Araki, S. Blood manganese concentrations and intrauterine growth restriction. Reprod. Toxicol. 2008, 25, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Ettinger, A.S.; Bouchard, M.; Amarasiriwardena, C.J.; Schwartz, J.; Hu, H.; Wright, R.O. Maternal Blood Manganese Levels and Infant Birth Weight. Epidemiology 2009, 20, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Than, N.G.; Romero, R.; Tarca, A.L.; Draghici, S.; Erez, O.; Tinnakorn, C.; Kim, Y.M.; Kim, S.K.; Vaisbuch, E.; Tromp, G. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J. Matern. Fetal Neonatal Med. 2009, 22, 1000–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eum, J.-H.; Cheong, H.-K.; Ha, E.-H.; Eum, J.H.; Cheong, H.K.; Ha, E.H.; Ha, M.; Kim, Y.; Hong, Y.C.; Park, H.; et al. Maternal blood manganese level and birth weight: A MOCEH birth cohort study. Environ. Health Glob. Access Sci. Source 2014, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.M.; de Joode, B.v.W.; Mergler, D.; Córdoba, L.; Cano, C.; Quesada, R.; Smith, D.R.; Menezes-Filho, J.A.; Eskenazi, B. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Environ. Res. 2015, 136, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Wright, J.M.; Rice, G.; Buckley, B.T.; Magsumbol, M.S.; Barr, D.B.; Williams, B.L. Metal exposures in an inner-city neonatal population. Environ. Int. 2010, 36, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Cheong, H.K.; Ha, E.H.; Kim, B.N.; Ha, M.; Kim, Y.; Hong, Y.C.; Park, H.; Oh, S.Y. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ. Health Perspect. 2015, 123, 717–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Chen, Y.C.; Su, F.C.; Lin, C.M.; Liao, H.F.; Hwang, Y.H.; Hsieh, W.S.; Jeng, S.F.; Su, Y.N.; Chen, P.C. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ. Res. 2013, 123, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Klovrza, L.V. Sulphur Metabolism in Autism. J. Nutr. Environ. Med. 2000, 10, 25–32. [Google Scholar] [CrossRef]
- Vázquez-Salas, R.A.; López-Carrillo, L.; Menezes-Filho, J.A.; Vazquez-Sales, R.A.; Lopez-Carrillo, L.; Menezes-Filho, J.A.; Rothenberg, S.J.; Cebrian, M.E.; Schnass, L.; Viana, G.F.; et al. Prenatal molybdenum exposure and infant neurodevelopment in Mexican children. Nutr. Neurosci. 2014, 17, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T.; Gunderson, E.L. History of the Food and Drug Administration’s Total Diet Study-1961 to 1987. J. Assoc. Off. Anal. Chem. 1987, 70, 772–782. [Google Scholar] [CrossRef]
- Zachara, B.A.; Wardak, C.; Didkowski, W.; Maciag, A.; Marchaluk, E. Changes in blood selenium and glutathione concentrations and glutathione peroxidase activity in human pregnancy. Gynecol. Obstet. Investig. 1993, 35, 12–17. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.; Kim, H.; Kim, S.; Chung, J.; Oh, S.; Lee, S. A Prospective Study of Serum Trace Elements in Healthy Korean Pregnant Women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef] [Green Version]
- Varsi, K.; Bolann, B.; Torsvik, I.; Rosvold Eik, T.C.; Høl, P.J.; Bjørke-Monsen, A.L. Impact of Maternal Selenium Status on Infant Outcome during the First 6 Months of Life. Nutrients 2017, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.J.; Ma, L.L.; Chen, S.P.; Li, G.; Zhou, J.Q. Serum selenium level and gestational diabetes mellitus: A systematic review and meta-analysis. Nutr. J. 2016, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariath, A.B.; Bergamaschi, D.P.; Rondó, P.H.; Tanaka, A.C.; de Fragas Hinnig, P.; Abbade, J.F.; Diniz, S.G. The possible role of selenium status in adverse pregnancy outcomes. Br. J. Nutr. 2011, 105, 1418–1428. [Google Scholar] [CrossRef]
- Bro, S.; Berendtsen, H.; Nørgaard, J.; Høst, A.; Jørgensen, P.J. Serum selenium concentration in maternal and umbilical cord blood. Relation to course and outcome of pregnancy. J. Trace Elem. Electrolytes Health Dis. 1988, 2, 165–169. [Google Scholar] [PubMed]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Majdi, M.R.; Tavallaie, S.; Azimi-Nezhad, M.; et al. Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Fetal Neonatal Med. 2011, 24, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition 2015, 31, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The Influence of Selenium Supplementation on Postpartum Thyroid Status in Pregnant Women with Thyroid Peroxidase Autoantibodies. J.Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Miller, L.V.; Mazariegos, M.; Westcott, J.; Solomons, N.W.; Raboy, V.; Kemp, J.F.; Das, A.; Goco, N.; Hartwell, T.; et al. Upregulation of Zinc Absorption Matches Increases in Physiologic Requirements for Zinc in Women Consuming High- or Moderate-Phytate Diets during Late Pregnancy and Early Lactation. J. Nutr. 2017, 147, 1079–1085. [Google Scholar] [CrossRef]
- Hunt, I.F.; Murphy, N.J.; Cleaver, A.E.; Faraji, B.; Swendseid, M.E.; Browdy, B.L.; Coulson, A.H.; Clark, V.A.; Settlage, R.H.; Smith, J.C., Jr. Zinc supplementation during pregnancy: Zinc concentration of serum and hair from low-income women of Mexican descent. Am. J. Clin. Nutr. 1983, 37, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Tamura, T.; Neggers, Y.; Copper, R.L.; Johnston, K.E.; DuBard, M.B.; Hauth, J.C. The effect of zinc supplementation on pregnancy outcome. JAMA 1995, 274, 463–468. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F.; Jacobs, M.A.; Favier, A.; Guyette, L.; Ikle, D.N. Zinc nutritional status during pregnancy: A longitudinal study. Am. J. Clin. Nutr. 1983, 37, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Goldenberg, R.L.; Johnston, K.E.; DuBard, M. Maternal plasma zinc concentrations and pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.M.; Chen, X.; Pan, J. The effects of zinc supplementation to Chinese rural pregnant women and their pregnancy outcome. J. Shanghai Sec. Med. Univ. 2001, 13, 199–224. [Google Scholar]
- Huang, H.M.; Leung, P.L.; Sun, D.Z.; Zhu, M.G. Hair and serum calcium, iron, copper, and zinc levels during normal pregnancy at three trimesters. Biol. Trace Elem. Res. 1999, 69, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Simsek, M. The changes of trace elements, malondialdehyde levels and superoxide dismutase activities in pregnancy with or without preeclampsia. Clin. Biochem. 2002, 35, 393–397. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, X.; Zhang, D. The Relationship between Serum Zinc Level and Preeclampsia: A Meta-Analysis. Nutrients 2015, 7, 7806–7820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedón, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef]
- Devereux, G.; Turner, S.W.; Craig, L.C.; McNeill, G.; Martindale, S.; Harbour, P.J.; Helms, P.J.; Seaton, A. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am. J. Respir. Crit. Care Med. 2006, 174, 499–507. [Google Scholar] [CrossRef]
- Mukherjee, M.D.; Sandstead, H.H.; Ratnaparkhi, M.V.; Johnson, L.K.; Milne, D.B.; Stelling, H.P. Maternal zinc, iron, folic acid, and protein nutriture and outcome of human pregnancy. Am. J. Clin. Nutr. 1984, 40, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Lazebnik, N.; Kuhnert, B.R.; Kuhnert, P.M.; Thompson, K.L. Zinc status, pregnancy complications, and labor abnormalities. Am. J. Obstet. Gynecol. 1988, 158, 161–166. [Google Scholar] [CrossRef]
- Hunt, I.F.; Murphy, N.J.; Cleaver, A.E.; Faraji, B.; Swendseid, M.E.; Browdy, B.L.; Coulson, A.H.; Clark, V.A.; Settlage, R.H.; Smith, J.C., Jr. Zinc supplementation during pregnancy in low-income teenagers of Mexican descent: Effects on selected blood constituents and on progress and outcome of pregnancy. Am. J. Clin. Nutr. 1985, 42, 815–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015, Cd000230. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation during Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low-and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, T.; Goldenberg, R.L.; Ramey, S.L.; Nelson, K.G.; Chapman, V.R. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. Am. J. Clin. Nutr. 2003, 77, 1512–1516. [Google Scholar] [CrossRef] [Green Version]
- Darmstadt, G.L.; Osendarp, S.J.; Ahmed, S.; Feldman, C.; Van Raaij, J.M.; Baqui, A.H.; Hautvast, J.G.; Fuchs, G.J. Effect of antenatal zinc supplementation on impetigo in infants in Bangladesh. Pediatr. Infect. Dis. J. 2012, 31, 407–409. [Google Scholar] [CrossRef]
- Bowen, A.C.; Mahé, A.; Hay, R.J.; Andrews, R.M.; Steer, A.C.; Tong, S.Y.; Carapetis, J.R. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS ONE 2015, 10, e0136789. [Google Scholar] [CrossRef] [Green Version]
| Maternal Outcome | Significant Evidence | Limited Evidence |
|---|---|---|
| Anemia | Iron [77,78,83,84,85,86,87] and [89] M | |
| Anxiety | Copper [39] | |
| Blighted Ovum | Copper [31,33] | |
| Depression | Copper [39] | |
| Edema | Magnesium [102] Selenium [130] | |
| Fertility (for women with Polycystic Ovary Syndrome) | Chromium [27,28] | |
| Gestational Diabetes | Chromium [20,24,26] | Selenium [127] M, [128] R |
| Gestational Hypertension | Calcium [7] M, [8] R, [14,15,16] Magnesium [7] M, [100,103] Selenium [124,130] Zinc [7] M, [141] M | |
| Hypothyroidism | Selenium [124,130,132] Iodine [49] | Selenium [132] |
| Infection | Selenium [126] | |
| Leg Cramps | Magnesium [97] | |
| Maternal Death/Serious Morbidity | Calcium [8] R | |
| Maternal Hospitalization | Magnesium [94,104,105] | |
| Miscarriage | Copper [31,32,33] Selenium [128] R | |
| Postpartum Depression | Chromium [30] Selenium [130] | |
| Preeclampsia | Calcium [3], [8] R, [10,11,14,17] Copper [36] M, [37] M Magnesium [95,96,97] Selenium [124,128,130] Zinc [7] M, [141] M | Iodine [40] Manganese (high levels are a problem) [116] |
| Premature Rupture of Membranes (PROM) | Copper [35] |
| Infant Outcome | Significant Evidence | Limited Evidence |
|---|---|---|
| ADD | Iodine [59] | |
| ADHD | Iodine [66] R | |
| Anemia | Iron [83,84,85,86,87,88] | |
| Anencephaly | Copper [33] Iron [82] | |
| Apgar Score | Magnesium [100] R | Zinc [138] |
| Asthma | Zinc [143] | |
| Autism | Calcium [12,13] Iodine [41,42] | Iron [80] Molybdenum [120] |
| Birth Weight | Magnesium [104,105,106] Manganese [111,112,114] | Calcium [8] R Selenium [128] R Zinc [135,138] |
| Central Nervous System (CNS) Malformations | Copper [34] | |
| Cerebral Palsy | Magnesium [109] R | |
| Congenital Diaphragmatic Hernia | Selenium [128] | |
| Dental Cavities | Calcium [8] R | |
| Fetal Distress | Zinc [144] | |
| Fetal Growth Restriction | Magnesium [111] | |
| High Blood Pressure | Calcium [8] R | |
| Hypothyroidism | Iodine [43] R, [49] | |
| Impetigo | Zinc [150] | |
| Infant Mortality | Iodine [54] | |
| Intellectual Disability | Iodine [43,54,55,56,69] Manganese [117,118] | |
| Intrauterine Growth Restriction | Selenium [128] R Zinc [138] | |
| Long Delivery | Selenium [145] | |
| Neonatal Intensive Care Unit Admissions | Calcium [8] R | |
| Neural Tube Defects | Selenium [128] R | |
| Orofacial Cleft | Iron [81] Magnesium [81] | |
| Preterm Birth | Calcium [8] R Magnesium [97,98,104,105,106,107] Zinc [147] M, [138] | Manganese [113] |
| Rickets | Calcium [1,2] | |
| Wheeze | Zinc [142] M |
| Mineral | Significant Evidence | Limited Evidence |
|---|---|---|
| Calcium | Gestational Hypertension [7] M, [8] R, [14,15,16] Maternal Death [8] R Preeclampsia [8] R, [10,11,14,17] Pregnancy Induced Hypertension [7] M, [8] R, [10,11,14,15,16] | |
| Chromium | Gestational Diabetes [20,26] | Postpartum Depression [30] Fertility in Women with Polycystic Ovary Syndrome [27] |
| Copper | Miscarriages [31,32,33] Preeclampsia [36] M, [37] M | Anxiety [39] Depression [39] PROM [35] |
| Iodine | Hypothyroidism [43,49] | Preeclampsia [40] |
| Iron | Anemia [77,78,83,84,85,87,89] | |
| Magnesium | Gestational Hypertension [7] M, [42,100,103] Maternal Hospitalization [94,104,105] Preeclampsia [95,96,97] | Leg Cramps [97] |
| Manganese | Preeclampsia (high levels are a problem) [116] | |
| Molybdenum | ||
| Selenium | Miscarriage [128] R Preeclampsia [124,128,130] Gestational Hypertension [124,130] | Gestational Diabetes [127] M, [128] R Hypothyroidism [124,130,132] Postpartum Depression [130] Postpartum Thyroid Deficiency [132] |
| Zinc | Preeclampsia [7] M, [141] M Gestational Hypertension [7] M, [141] M |
| Mineral | Significant Evidence | Limited Evidence |
|---|---|---|
| Calcium | Autism [12,13] Preterm Birth [8] R Rickets [1,2] | High Blood Pressure [8] R Dental Cavities [8] R Low Birthweight [8] R |
| Chromium | ||
| Copper | Anencephaly [33] CNS Malformations [34] | |
| Iodine | ADHD [66] R Hypothyroidism [43] R, [44] Intellectual Disability [43] R, [54,55,56] | Mortality [54] IQ [63] M Stillbirth [54] |
| Iron | Anemia [85,86,87,88,89] | Anencephaly [82] Autism [80] Orofacial Cleft [81] |
| Magnesium | Apgar Score [100] R Birth Weight [104,105,106] Cerebral Palsy [109] R Preterm Birth [97,98,104,105,106,107] | Orofacial Cleft [81] |
| Manganese | Birth Weight [111,112,114] Intellectual Disability [117,118] | Preterm Birth [113] |
| Selenium | Neural Tube Defects [138] R | Birthweight [128] R Congenital Diaphragmatic Hernia [128] R Intellectual Disability [126] Intrauterine Growth Restriction [128] R |
| Zinc | Preterm Birth [138], [147] M Wheeze [142] M | Apgar Scores [138] Birth Weight [138] Duration of Gestation [138] Impetigo [150] Asthma [143] |
| Nutrient | Our Recommendation | RDA Recommendation for Total Daily Intake for Pregnant Women | Daily Intake (Women Aged 20–39) per NHANES Unless Otherwise Noted | Tolerable Upper Limit for Pregnant Women | Change during Pregnancy |
|---|---|---|---|---|---|
| Calcium | 550 mg (1000 mg for those with greater risk of preeclampsia) | 1000 mg | 872 mg | 1000 mg | Decreases |
| Chromium | 100 mg (200 mg for women with diabetes) | 30 μg | 23 to 29 μg [19] | - | Possibly decreases |
| Copper | 1.3 mg | 1 mg | 1.1 mg | 10 mg | Levels increase, but low levels associated with health complications |
| Iodine | 150 μg/day | 220 μg | 160 μg * | 1100 μg | Possibly decreases |
| Iron | 30 mg 1st trimester, 60 mg 2nd trimester and 3rd trimester; up to 60 mg 3× day in extreme cases | 27 mg | 12.2 mg | 45 mg | Decreases |
| Magnesium | 350 mg | 400 mg | 269 mg | 400 mg | Decreases |
| Manganese | 1 mg | 2.0 mg | 2.3 mg [122] | 11 mg | Increases, but low levels are associated with health complications |
| Molybdenum | 25 μg | 50 μg | 76 μg [122] | 2000 μg | Unknown |
| Selenium | 60 μg | 70 μg | 97 μg | 400 mg | Decreases |
| Zinc | 30 mg | 11 mg | 9.4 mg | 40 mg | Decreases |
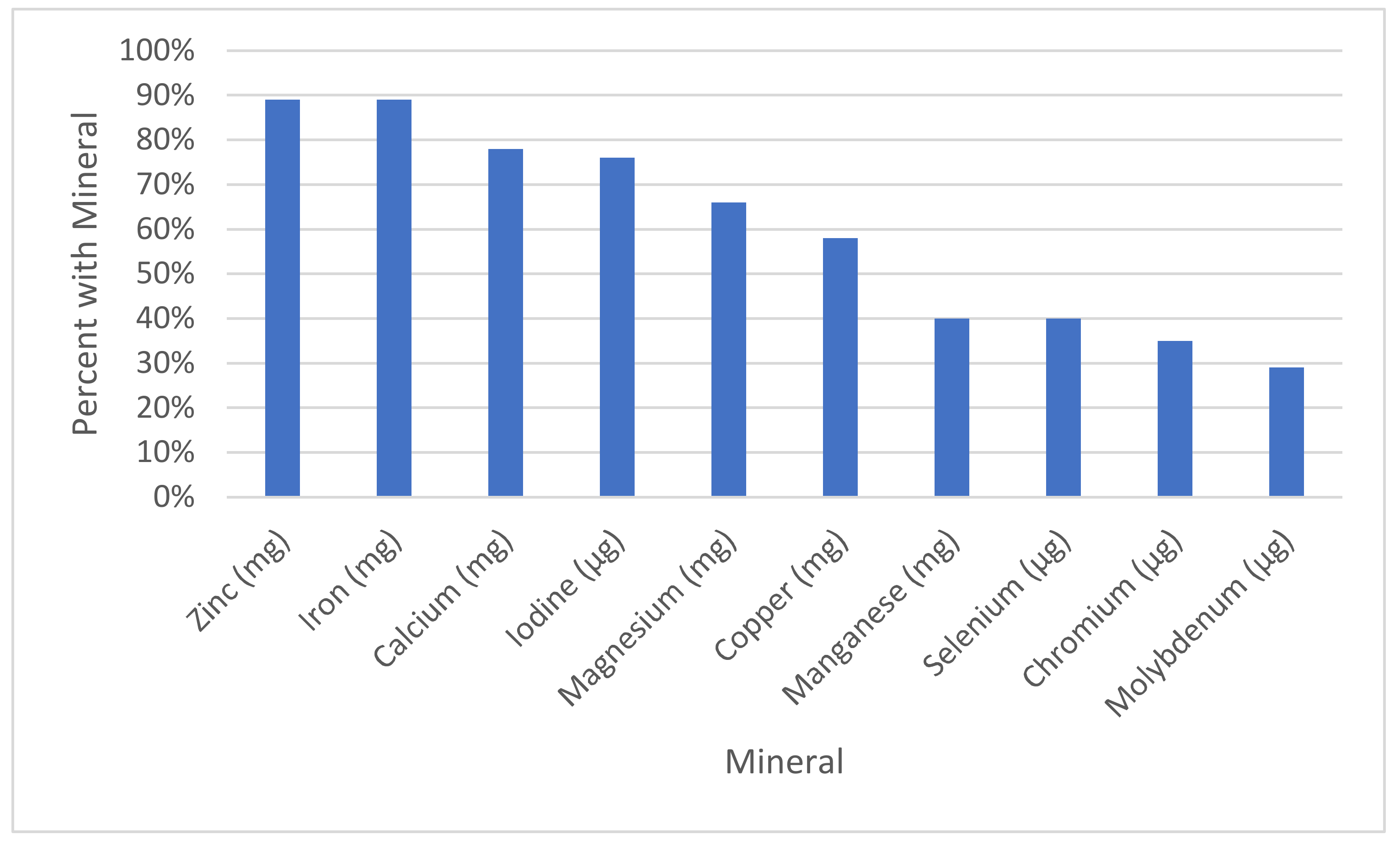
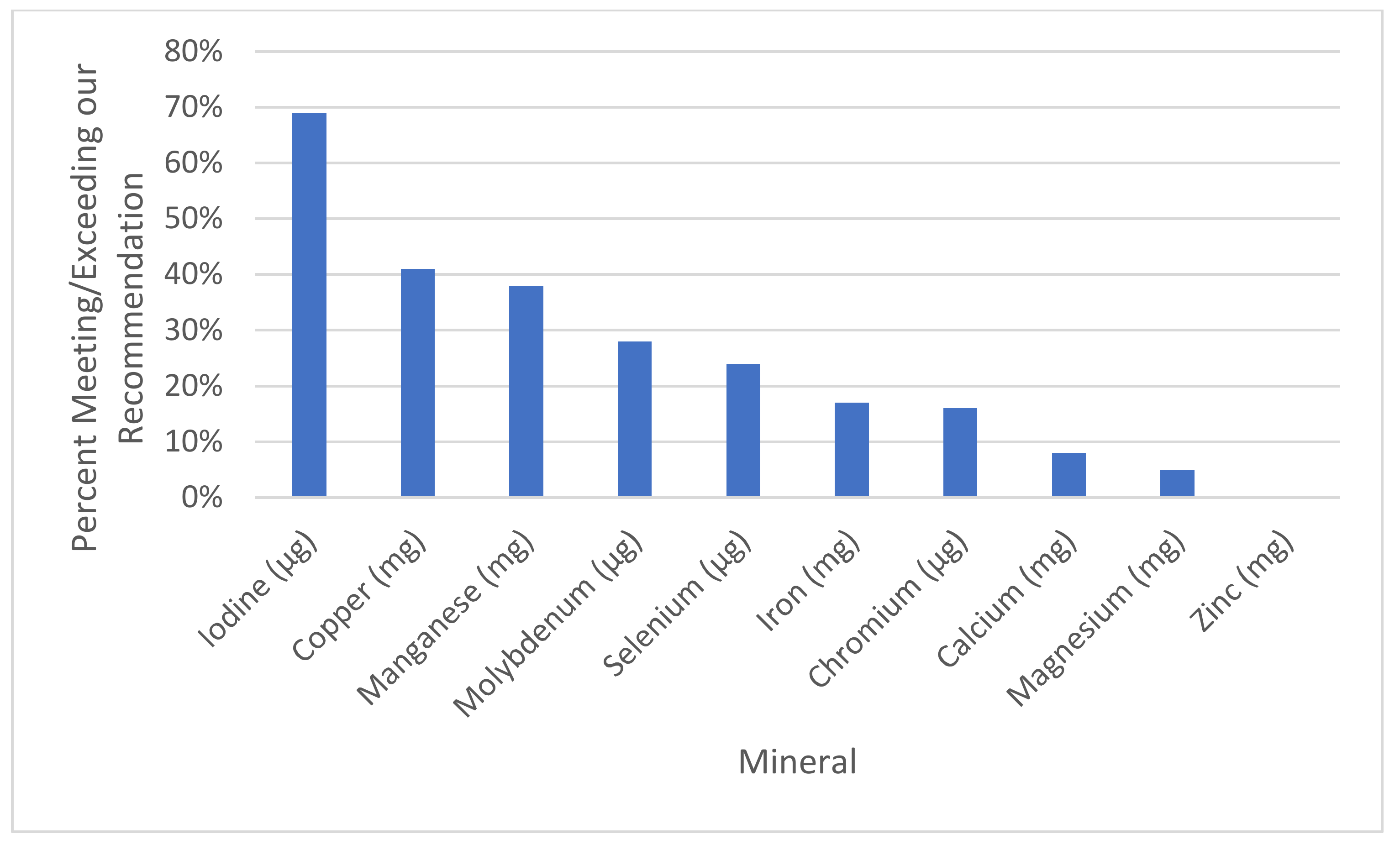
| Mineral | Our Reccomendation | % of Sups with This Mineral (Out of 188) | % Meeting or Exceeding Rec. | Average All | Average of Those with the Mineral | % of the Average of All Supplements Divided by Our Recommendation | % of the Average of Those with Supplements Divided by Our Recommendation | Range |
|---|---|---|---|---|---|---|---|---|
| Calcium (mg) | 550 | 78% (146) | 8% (15) | 204.2 ± 233.8 | 262.9 ± 233.6 | 37% | 48% | 0–1300 |
| Chromium (μg) | 100 | 35% (66) | 16% (31) | 28.9 ± 51.6 | 82.3 ± 56.2 | 29% | 82% | 0–200 |
| Copper (mg) | 1.3 | 58% (109) | 41% (77) | 0.9 ± 0.9 | 1.5 ± 0.6 | 67% | 115% | 0–2 |
| Iodine (μg) | 150 | 76% (143) | 69% (129) | 138.9 ± 98.0 | 182.6 ± 67.6 | 93% | 122% | 0–316 |
| Iron (mg) | 30 | 89% (168) | 17% (32) | 24.2 ± 15.9 | 27.1 ± 14.2 | 81% | 90% | 0–91.5 |
| Magnesium (mg) | 350 | 66% (124) | 5% (9) | 75.0 ± 110.1 | 113.7 ± 117.8 | 21% | 32% | 0–500 |
| Manganese (mg) | 1 | 40% (76) | 38% (71) | 1.1 ± 1.7 | 3.3 ± 1.6 | 100% | 273% | 0–40 |
| Molybdenum (μg) | 25 | 29% (55) | 28% (53) | 16.1 ± 28.0 | 55.1 ± 22.8 | 64% | 220% | 0–100 |
| Selenium (μg) | 60 | 40% (76) | 24% (46) | 27.3 ± 43.6 | 67.4 ± 44.3 | 45% | 112% | 0–200 |
| Zinc (mg) | 30 | 89% (167) | 0% (0) | 13.3 ± 7.7 | 15.0 ± 6.5 | 44% | 50% | 0–25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, J.B.; Sorenson, J.C.; Pollard, E.L.; Kirby, J.K.; Audhya, T. Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals. Nutrients 2021, 13, 1849. https://doi.org/10.3390/nu13061849
Adams JB, Sorenson JC, Pollard EL, Kirby JK, Audhya T. Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals. Nutrients. 2021; 13(6):1849. https://doi.org/10.3390/nu13061849
Chicago/Turabian StyleAdams, James B., Jacob C. Sorenson, Elena L. Pollard, Jasmine K. Kirby, and Tapan Audhya. 2021. "Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals" Nutrients 13, no. 6: 1849. https://doi.org/10.3390/nu13061849
APA StyleAdams, J. B., Sorenson, J. C., Pollard, E. L., Kirby, J. K., & Audhya, T. (2021). Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals. Nutrients, 13(6), 1849. https://doi.org/10.3390/nu13061849






