Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial
Abstract
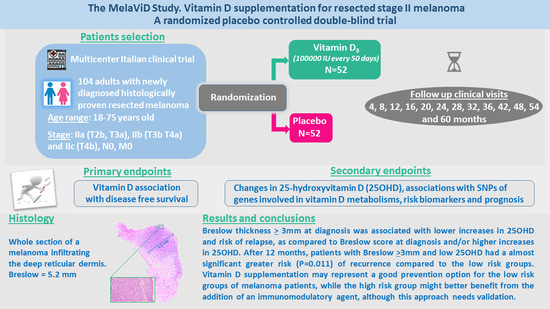
:1. Introduction
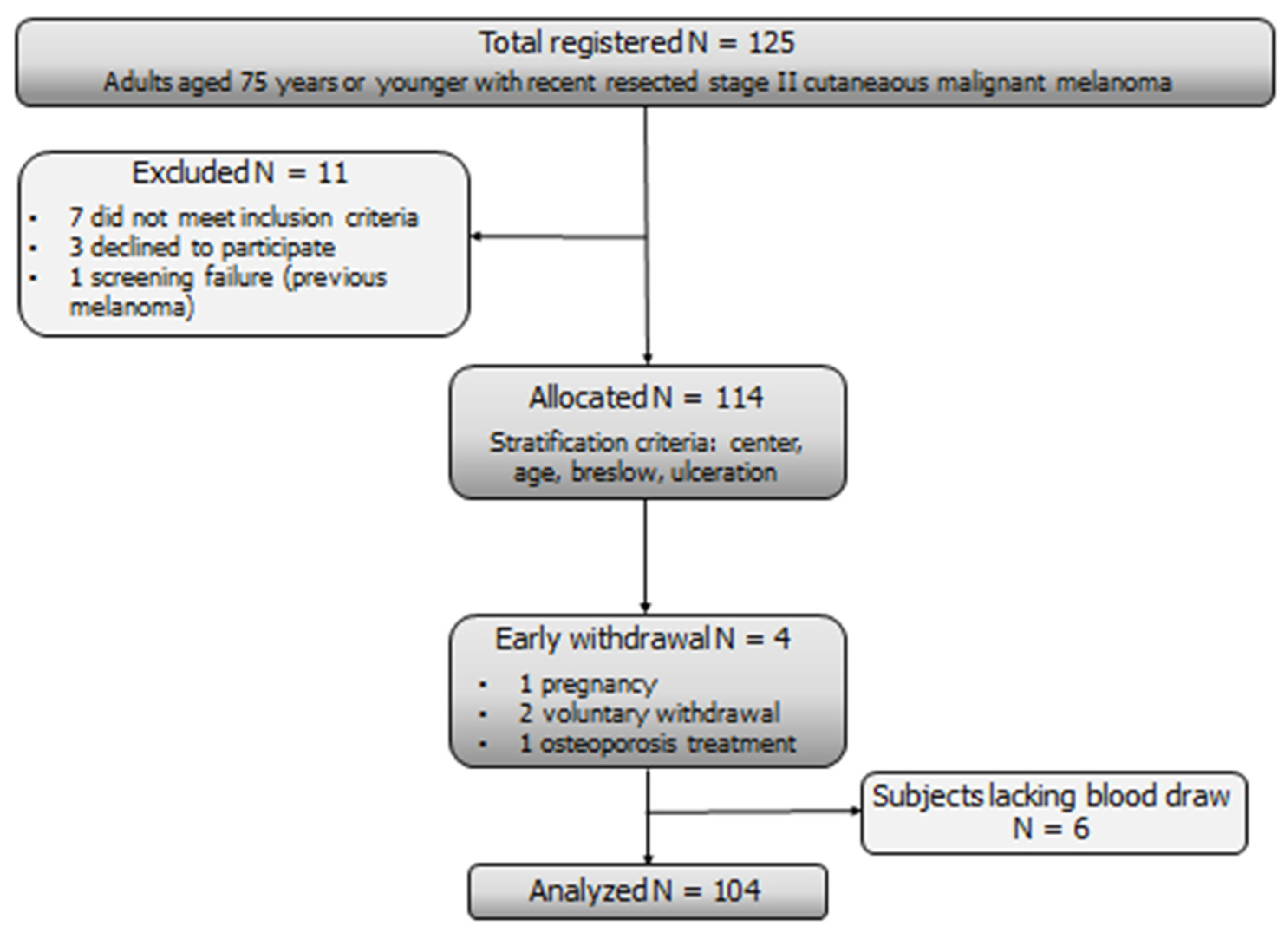
2. Materials and Methods
2.1. Study Participants
2.2. Intervention and Study Procedures
2.3. Objectives
2.4. Laboratory Methods
2.5. Statistical Methods
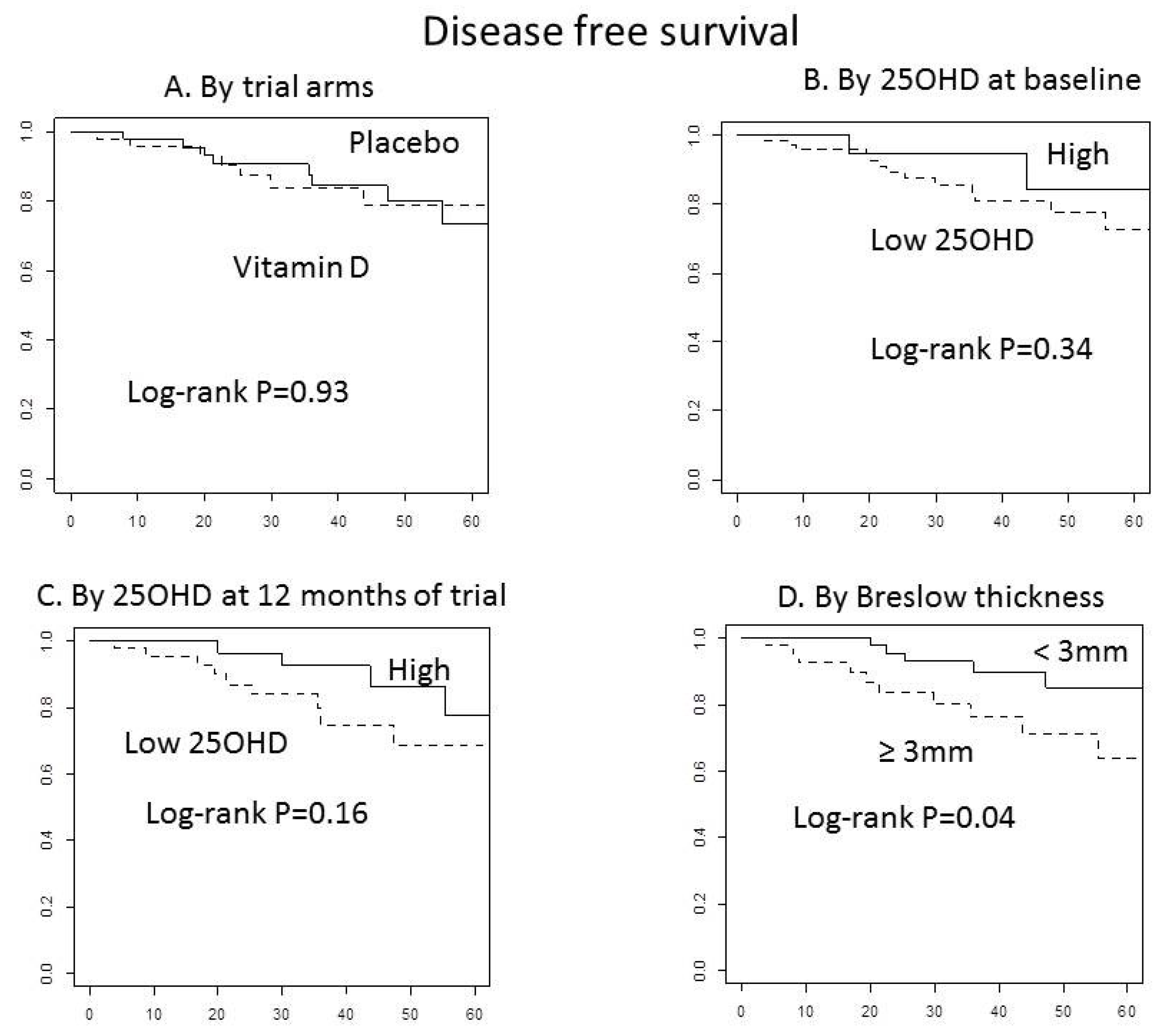
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vries, E.; Bray, F.I.; Coebergh, J.W.; Parkin, D.M. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: Rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int. J. Cancer 2003, 107, 119–126. [Google Scholar] [CrossRef]
- Egger, M.E.; Bhutiani, N.; Farmer, R.W.; Stromberg, A.J.; Martin, R.C.; Quillo, A.R.; McMasters, K.M.; Scoggins, C.R. Prognostic factors in melanoma patients with tumor-negative sentinel lymph nodes. Surgery 2016, 159, 1412–1421. [Google Scholar] [CrossRef]
- Caini, S.; Gandini, S.; Sera, F.; Raimondi, S.; Fargnoli, M.C.; Boniol, M.; Armstrong, B.K. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur. J. Cancer 2009, 45, 3054–3063. [Google Scholar] [CrossRef]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef] [Green Version]
- Rosen, C.J. Vitamin D Insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Hoffman, R.M.; Slominski, A.T. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer Res. 2020, 40, 473–489. [Google Scholar] [CrossRef] [Green Version]
- Cattaruzza, M.S.; Pisani, D.; Fidanza, L.; Gandini, S.; Marmo, G.; Narcisi, A.; Bartolazzi, A.; Carlesimo, M. 25-Hydroxyvitamin D se-rum levels and melanoma risk: A case-control study and evidence synthesis of clinical epidemiological studies. Eur. J. Cancer Prev. 2019, 28, 203–211. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.; Greenwood, D.; Manson, J.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Newton-Bishop, J.A.; Beswick, S.; Randerson-Moor, J.; Chang, Y.M.; Affleck, P.; Elliott, F.; Chan, M.; Leake, S.; Karpavicius, B.; Haynes, S.; et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J. Clin. Oncol. 2009, 27, 5439–5444. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Sui, D.; Wang, Y.; Liu, H.; Chiang, Y.-J.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; et al. Association of Vitamin D Levels With Outcome in Patients With Melanoma After Adjustment For C-Reactive Protein. J. Clin. Oncol. 2016, 34, 1741–1747. [Google Scholar] [CrossRef] [Green Version]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-reactive protein in the predic-tion of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Zanetti, R.; Masini, C.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cuta-neous melanoma: III. Family history, actinic damage and phenotypic factors. Eur. J. Cancer 2005, 41, 2040–2059. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- De Smedt, J.; Van Kelst, S.; Boecxstaens, V.; Stas, M.; Bogaerts, K.; Vanderschueren, D.; Aura, C.; Vandenberghe, K.; Lambrechts, D.; Wolter, P.; et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): A randomized controlled trial. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, P.; Zapatka, M.; Peccerella, T.; Matzk, H.; Neumaier, M.; Schadendorf, D.; Ugurel, S. Serum Amyloid A As a Prognostic Marker in Melanoma Identified by Proteomic Profiling. J. Clin. Oncol. 2009, 27, 2199–2208. [Google Scholar] [CrossRef]
- Fang, S.; Wang, Y.; Sui, D.; Liu, H.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; Schacherer, C.W.; et al. C-Reactive Protein As a Marker of Melanoma Progression. J. Clin. Oncol. 2015, 33, 1389–1396. [Google Scholar] [CrossRef]
- Mirili, C.; Yılmaz, A.; Demirkan, S.; Bilici, M.; Tekin, S.B. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int. J. Clin. Oncol. 2019, 24, 1301–1310. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Qiao, J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: Evidence from a PRIS-MA-compliant meta-analysis. Medicine 2018, 97, e11446. [Google Scholar] [CrossRef]
- Robinson, A.V.; Keeble, C.; Lo, M.C.I.; Thornton, O.; Peach, H.; Moncrieff, M.D.S.; Dewar, D.J.; Wade, R.G. The neutrophil–lymphocyte ratio and locoregional melanoma: A multicentre cohort study. Cancer Immunol. Immunother. 2020, 69, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brozyna, A.A.; Józwicki, W.; Slominski, A.T. Decreased VDR expression in cutaneous melanomas as marker of tumor progres-sion: New data and analyses. Anticancer Res. 2014, 34, 2735–2743. [Google Scholar] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.; Pols, H.A.; van Leeuwen, J.P. Genetics and biology of vitamin D receptor polymor-phisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingles, S.A.; Haile, R.W.; Henderson, B.E.; Kolonel, L.N.; Nakaichi, G.; Shi, C.Y.; Yu, M.C.; Ross, R.K.; Coetzee, G.A. Strength of linkage disequilibrium between two vitamin D receptor markers in five ethnic groups: Implications for association studies. Cancer Epidemiol. Biomark. Prev. 1997, 6, 93–98. [Google Scholar]
- Raimondi, S.; Pasquali, E.; Gnagnarella, P.; Serrano, D.; Disalvatore, D.; Johansson, H.A.; Gandini, S. BsmI polymorphism of vitamin D receptor gene and cancer risk: A comprehensive meta-analysis. Mutat. Res. Mol. Mech. Mutagen. 2014, 769, 17–34. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.; Bellerba, F.; Corso, F.; De Angelis, S.P.; Belloni, P.; Caini, S.; Gandini, S. Eth-nicity as modifier of risk for Vitamin D receptors polymorphisms: Comprehensive meta-analysis of all cancer sites. Crit. Rev. Oncol. Hematol. 2021, 158, 103202. [Google Scholar] [CrossRef]
- Orlow, I.; Reiner, A.; Thomas, N.E.; Roy, P.; Kanetsky, P.A.; Luo, L.; Paine, S.; Armstrong, B.K.; Kricker, A.; Marrett, L.D.; et al. Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: A population-based study. Carcinogenesis 2015, 37, 30–38. [Google Scholar] [CrossRef] [PubMed]


| Groups | All | Placebo (n = 52) | Vitamin D (n = 52) | p-Value | |
|---|---|---|---|---|---|
| Gender, n (%) | Males | 59 (56.7) | 27 (51.9) | 32 (61.5) | 0.322 |
| Female | 45 (43.3) | 25 (48.1) | 20 (38.5) | ||
| Staging, n (%) | stage IIa T2b | 19 (18.3) | 9 (17.3) | 10 (19.2) | 0.988 |
| stage IIa T3a | 41 (39.4) | 20 (38.5) | 21 (40.4) | ||
| stage IIb T3b | 28 (26.9) | 14 (26.9) | 14 (26.9) | ||
| stage IIb T4a | 9 (8.7) | 5 (9.6) | 4 (7.7) | ||
| stage IIc T4b | 7 (6.7) | 4 (7.7) | 3 (5.8) | ||
| Ulceration, n (%) | No | 46 (44.2) | 23 (44.2) | 23 (44.2) | 0.839 |
| Yes | 55 (52.9) | 28 (53.8) | 27 (51.9) | ||
| Missing | 3 (2.9) | 1 (1.9) | 2 (3.8) | ||
| Mitotic rates, n (%) | <2 | 26 (25) | 13 (25) | 13 (25) | 0.494 |
| ≥2 | 71 (68.3) | 34 (65.4) | 37 (71.2) | ||
| Missing | 7 (6.7) | 5 (9.6) | 2 (3.8) | ||
| 25OHD | Medium/high | 21 (20.2) | 9 (17.3) | 12 (23.1) | |
| n (%) | Low * | 83 (79.8) | 43 (82.7) | 40 (76.9) | |
| n. | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age, years | 104 | 50.5 (41.5, 59) | 50 (42, 56.5) | 52 (40.5, 62.5) | 0.688 |
| Breslow, mm. | 104 | 2.62 (2.13, 3.8) | 2.62 (2.17, 3.8) | 2.65 (2.1, 3.7) | 0.861 |
| 25OHD, ng/ml | 104 | 18.1 (12.7, 24.3) | 18.2 (13.4, 24.3) | 18.1 (12, 23.9) | 0.768 |
| LDH | 85 | 283 (201, 356) | 280 (186, 350) | 285 (201, 367) | 0.389 |
| NLR | 87 | 1.31 (1.00, 1.8) | 1.54 (1.07, 1.83) | 1.22 (0.93, 1.69) | 0.138 |
| Stage IIa | Stage IIb/IIc | |||
|---|---|---|---|---|
| 25OHD (ng/mL) | T2 or T3a n = 60 | T4 or T3b n = 44 | Total | p-Values * |
| Medium/high | 14 (23%) | 7 (16%) | 21 | 0.35 |
| Low | 46 (77%) | 37 (84%) | 83 | |
| BsmI | n = 58 | n = 43 | ||
| bb/bB | 40 (69%) | 39 (91%) | 79 | 0.009 |
| BB | 18 (31%) | 4 (9%) | 22 |
| Number of Subjects | 25 OHD (ng/mL) | PTH (pg/mL) | hs-CRP (mg/dL) | |||||
|---|---|---|---|---|---|---|---|---|
| Time-Point | Placebo | Vitamin D | Placebo | Vitamin D | Placebo | Vitamin D | Placebo | Vitamin D |
| Baseline | 52 | 52 | 18.15 (13.4–24.2) | 18.0 (12.0–23.9) | 19.8 (15.2–28.9) | 21.0 (15.0–25.4) | 0.12 (0.06–0.20) | 0.14 (0.06–0.25) |
| 4 months | 50 | 42 | 19.05 (13.0–25.9) | 32.9 (25.9–38.4) | 22.1 (15.9–30.3) | 19.3 (13.8–26.0) | 0.12 (0.07–0.26) | 0.12 (0.05–0.22) |
| 8 months | 50 | 42 | 18.7 (13.0–22.9) | 33.4 (25.1–37.1) | 20.2 (16.2–28.1) | 18.6 (14.0–24.6) | 0.10 (0.05–0.19) | 0.14 (0.05–0.34) |
| 12 months | 44 | 44 | 19.4 (14.3–25.0) | 33.8 (28.0–40.0) | 22.2 (17.2–29.6) | 16.5 (13.7–24.4) | 0.12 (0.07–0.20) | 0.12 (0.06–0.25) |
| 24 months | 37 | 32 | 21.1 (15.0–26.0) | 39.2 (31.6–46.5) | 24.0 (18.0–26.9) | 19.2 (14.6–25.8) | 0.12 (0.08–0.25) | 0.10 (0.05–0.20) |
| 36 months | 25 | 22 | 22.5 (15.9–28.8) | 41.9 (31.8–47.3) | 26.0 (19.0–31.2) | 19.7 (17.8–26.2) | 0.10 (0.05–0.15) | 0.06 (0.05–0.25) |
| 48 months | 9 | 9 | 21.4 (14.3–25.7) | 31.2 (24.3–35.5) | 22.9 (17.8–27.2) | 23.4 (22.5–30.4) | 0.10 (0.04–0.12) | 0.10 (0.03–0.23) |
| 60 months | 5 | 6 | 17.8 (14.3–24.1) | 22.4 (20.3–27.1) | 32.0 (30.7–32.4) | 25.6 (25.5–26.0) | 0.12 (0.03–0.13) | 0.12 (0.05–0.58) |
| Change in 25OHD | Std Error | p-Values | ||
|---|---|---|---|---|
| Intercept | 20.91 | 2.69 | <0.0001 | |
| Baseline 25OHD | −0.51 | 0.09 | <0.0001 | |
| Season | Late summer/autumn | 3.96 | 1.36 | 0.004 |
| Winter | −3.75 | 1.31 | 0.005 | |
| Spring/early summer | 0.00 | |||
| Arms | Placebo | 0.00 | ||
| Vitamin D | 12.01 | 2.39 | <0.0001 | |
| Placebo by time | 4 | 0.00 | ||
| 8 | −1.16 | 2.05 | 0.572 | |
| 12 | −1.69 | 2.15 | 0.431 | |
| 24 | −0.39 | 2.22 | 0.859 | |
| 36 | 4.06 | 2.51 | 0.107 | |
| Vitamin D by time | 4 | 0.00 | ||
| 8 | 3.30 | 2.27 | 0.147 | |
| 12 | 2.74 | 2.25 | 0.223 | |
| 24 | 6.43 | 2.43 | 0.009 | |
| 36 | 9.61 | 2.71 | 0.001 | |
| Breslow | <3 mm | 2.83 | 1.48 | 0.058 |
| ≥3 mm | 0.00 |
| Vitamin D | Placebo | ||||||
|---|---|---|---|---|---|---|---|
| Breslow | Months | LSM 25OHD (ng/mL) | Standard Error | p-Values | LSM 25OHD (ng/mL) | Standard Error | p-Values |
| <3 mm | 4 | 14.23 | 2.65 | <0.0001 | −0.77 | 1.62 | 0.64 |
| 8 | 18.79 | 2.68 | <0.0001 | −0.29 | 1.62 | 0.86 | |
| 12 | 16.76 | 2.69 | <0.0001 | 0.21 | 1.66 | 0.90 | |
| 24 | 20.28 | 2.91 | <0.0001 | 1.57 | 1.70 | 0.36 | |
| 36 | 30.50 | 3.78 | <0.0001 | 8.86 | 2.12 | <0.0001 | |
| ≥3 mm | 4 | 10.75 | 3.54 | 0.003 | 2.71 | 1.82 | 0.14 |
| 8 | 11.31 | 3.34 | 0.001 | −0.08 | 1.98 | 0.97 | |
| 12 | 12.75 | 3.08 | <0.0001 | −1.92 | 2.19 | 0.38 | |
| 24 | 18.33 | 3.70 | <0.0001 | −0.67 | 2.37 | 0.78 | |
| 36 | 14.51 | 3.79 | 0.0002 | −1.51 | 2.70 | 0.58 | |
| At Baseline | At 12 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | Low 95% CI | High 95% CI | p-Value | HR | Low 95% CI | High 95% CI | p-Value | ||
| Age | 1.03 | 0.99 | 1.07 | 0.21 | 1.04 | 0.99 | 1.09 | 0.104 | |
| Arm | Placebo vs. Vitamin D | 0.98 | 0.34 | 2.84 | 0.97 | 0.82 | 0.22 | 3.14 | 0.774 |
| 25OHD | High vs low * | 0.58 | 0.12 | 2.68 | 0.48 | 3.15 | 0.74 | 13.43 | 0.121 |
| Breslow | <3 mm vs. ≥3 mm | 0.35 | 0.12 | 1.03 | 0.06 | 0.26 | 0.08 | 0.90 | 0.033 |
| Age | 1.03 | 0.99 | 1.07 | 0.21 | 1.04 | 0.99 | 1.09 | 0.094 | |
| Arm | Placebo vs. Vitamin D | 0.99 | 0.36 | 2.76 | 0.98 | 0.81 | 0.26 | 2.57 | 0.723 |
| 25OHD Breslow combined score | 25OHD low and Breslow ≥3 vs. (25OHD high or Breslow <3) ** | 2.36 | 0.85 | 6.51 | 0.10 | 4.81 | 1.44 | 16.09 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, H.; Spadola, G.; Tosti, G.; Mandalà, M.; Minisini, A.M.; Queirolo, P.; Aristarco, V.; Baldini, F.; Cocorocchio, E.; Albertazzi, E.; et al. Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients 2021, 13, 1931. https://doi.org/10.3390/nu13061931
Johansson H, Spadola G, Tosti G, Mandalà M, Minisini AM, Queirolo P, Aristarco V, Baldini F, Cocorocchio E, Albertazzi E, et al. Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients. 2021; 13(6):1931. https://doi.org/10.3390/nu13061931
Chicago/Turabian StyleJohansson, Harriet, Giuseppe Spadola, Giulio Tosti, Mario Mandalà, Alessandro M. Minisini, Paola Queirolo, Valentina Aristarco, Federica Baldini, Emilia Cocorocchio, Elena Albertazzi, and et al. 2021. "Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial" Nutrients 13, no. 6: 1931. https://doi.org/10.3390/nu13061931
APA StyleJohansson, H., Spadola, G., Tosti, G., Mandalà, M., Minisini, A. M., Queirolo, P., Aristarco, V., Baldini, F., Cocorocchio, E., Albertazzi, E., Zichichi, L., Cinieri, S., Jemos, C., Mazzarol, G., Gnagnarella, P., Macis, D., Tedeschi, I., Salè, E. O., Stucci, L. S., ... on behalf of the Italian Melanoma Intergroup (IMI). (2021). Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients, 13(6), 1931. https://doi.org/10.3390/nu13061931