Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of the Matcha Sample
2.2. Animals and Diet
2.3. Collection of Serum and Tissue Samples
2.4. H&E Staining
2.5. Oil Red O Staining
2.6. Enzyme-Linked Immunosorbent Assays (ELISA)
2.7. Hepatic Transcriptome Sequencing and Annotation
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.9. Statistical Analysis
3. Results
3.1. Bioactive Compounds of the Matcha Sample
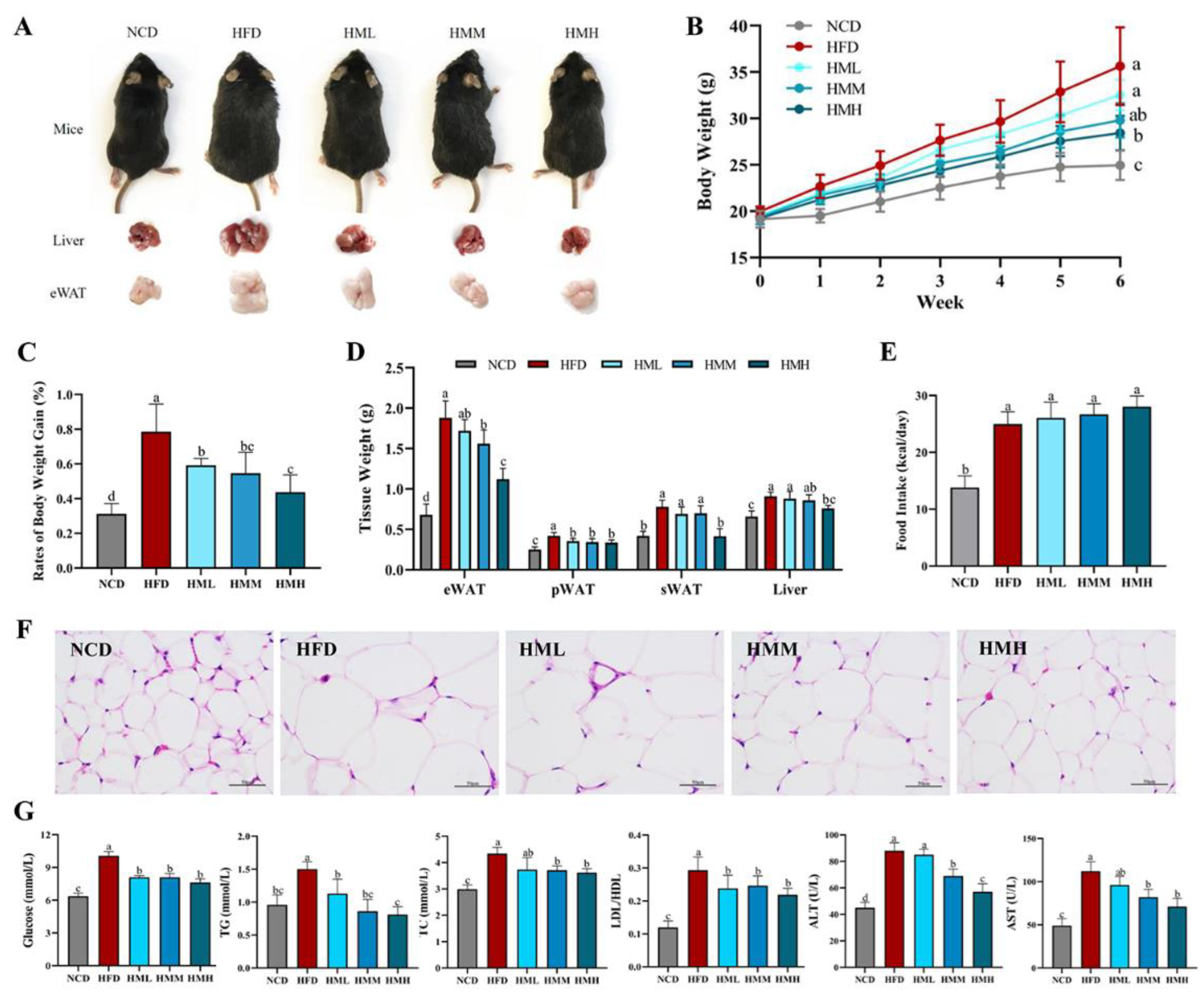
3.2. Matcha Ameliorated HFD-Induced Obesity
3.3. Matcha Improved Hepatic Lipid Accumulation and Inflammation
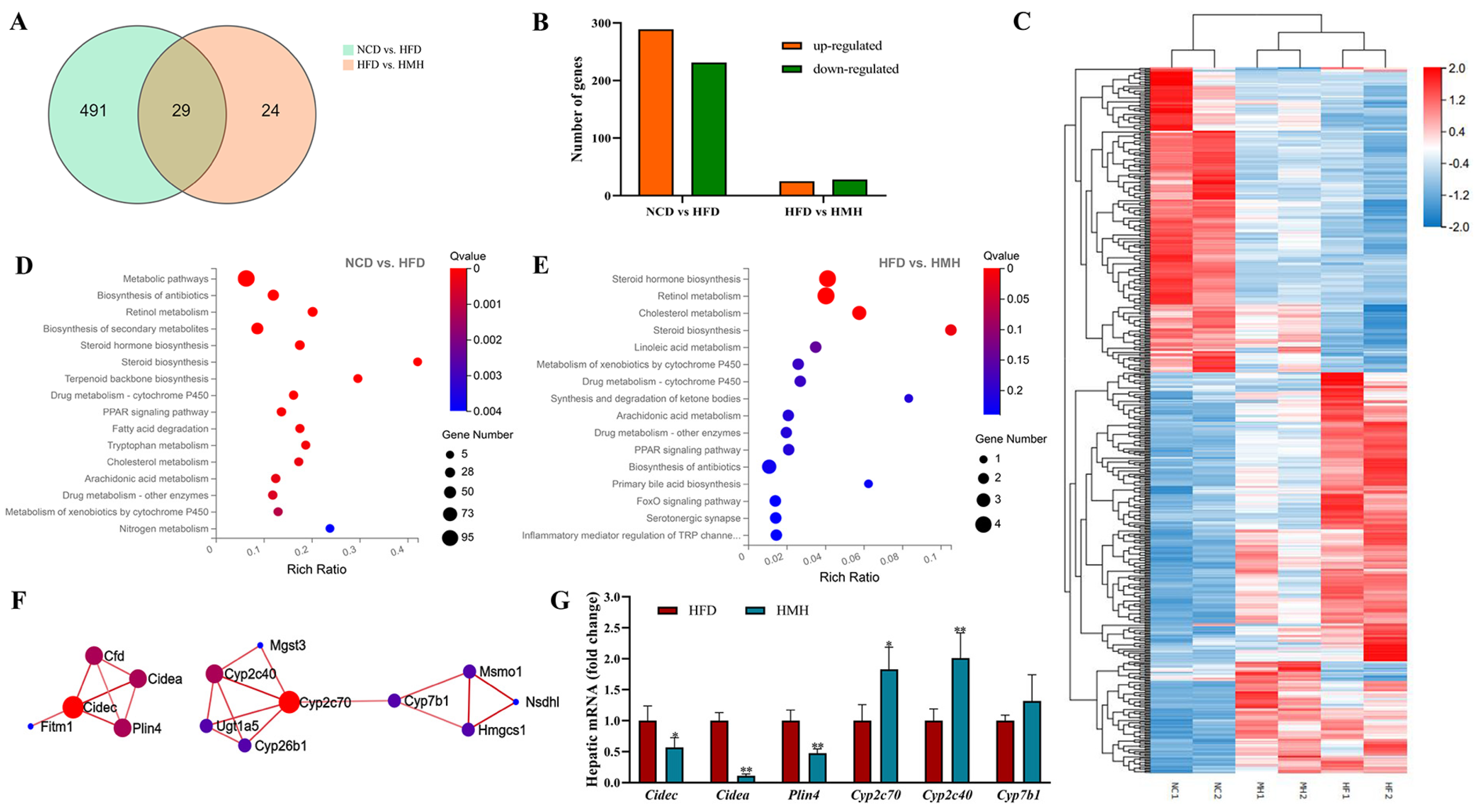
3.4. Matcha Induced Transcriptome Alterations in the Liver
4. Discussion
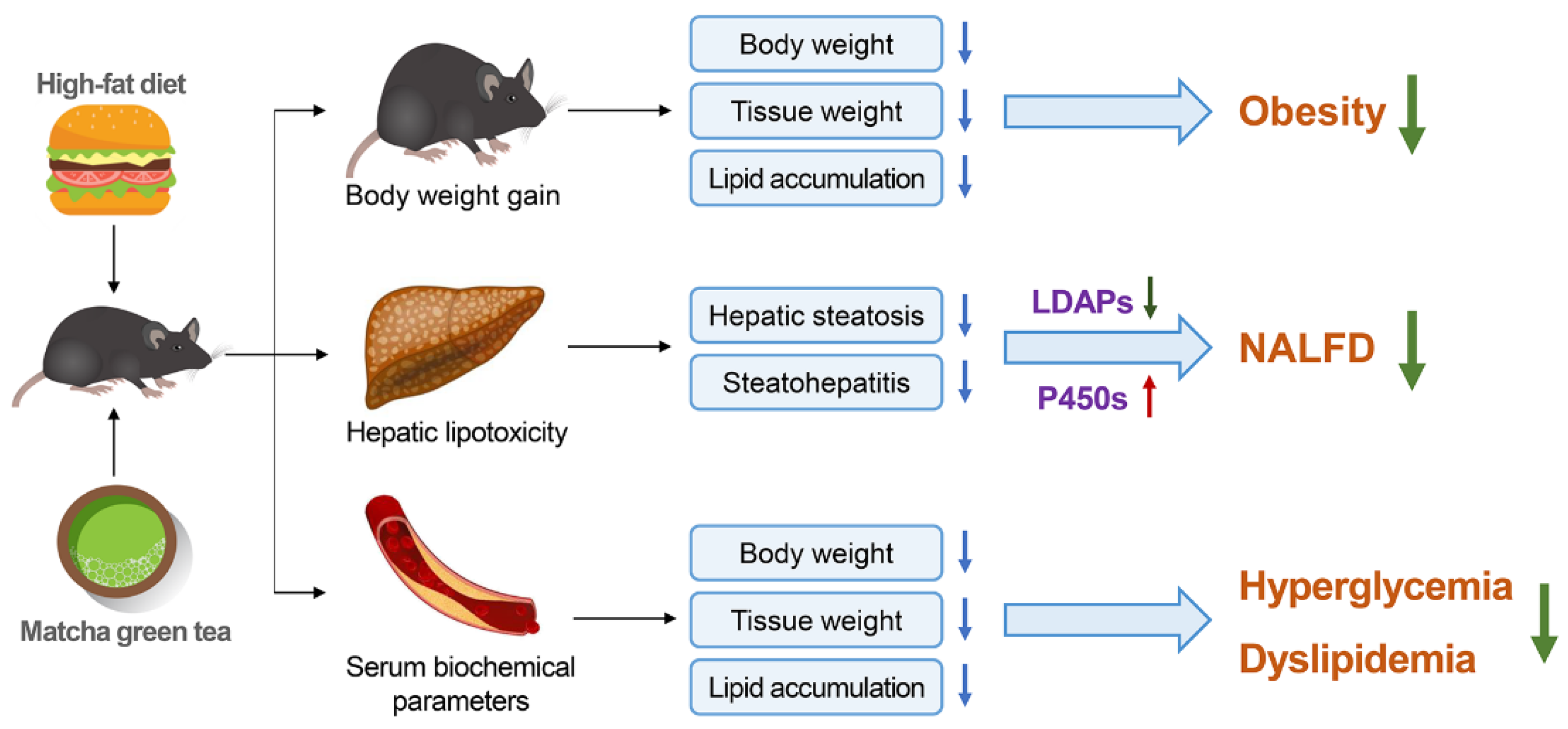
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henao-Mejia, J.; Elinav, E.; Jin, C.C.; Hao, L.M.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.R.; Chen, Y.C.; Zhou, K.; Zheng, Y.J.; Chen, Y.; Li, Q.; Zhu, C.F.; Xia, F.Z.; Gu, T.; Guo, Y.Y.; et al. Comparison of Abdominal Obesity and Fatty Liver and Their Association with Insulin Resistance and Metabolic Syndrome in Chinese Adults. Obesity 2019, 27, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Huang, C.C.; Tung, Y.T.; Huang, W.C.; Chen, Y.M.; Hsu, Y.J.; Hsu, M.C. Beneficial effects of cocoa, coffee, green tea, and garcinia complex supplement on diet induced obesity in rats. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.S.; Lee, W.K.; Yoon, W.K.; Kim, N.; Park, S.K.; Park, H.K.; Ly, S.Y.; Han, S.B.; Yun, J.; Lee, C.W.; et al. A Combination of Grape Extract, Green Tea Extract and L-Carnitine Improves High-fat Diet-induced Obesity, Hyperlipidemia and Non-alcoholic Fatty Liver Disease in Mice. Phytother. Res. 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. 2020. [Google Scholar] [CrossRef]
- Tenore, G.C.; Campiglia, P.; Giannetti, D.; Novellino, E. Simulated gastrointestinal digestion, intestinal permeation and plasma protein interaction of white, green, and black tea polyphenols. Food Chem. 2015, 169, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.J.; Zhang, H.; Qi, R.L.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Ramachandran, B.; Jayavelu, S.; Murhekar, K.; Rajkumar, T. Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia. Toxicol. Rep. 2016, 3, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults-Results of a systematic review. Regul. Toxicol. Pharm. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamabe, N.; Kang, K.S.; Hur, J.M.; Yokozawa, T. Matcha, a Powdered Green Tea, Ameliorates the Progression of Renal and Hepatic Damage in Type 2 Diabetic OLETF Rats. J. Med. Food 2009, 12, 714–721. [Google Scholar] [CrossRef]
- Kolackova, T.; Kolofikova, K.; Sytarova, I.; Snopek, L.; Sumczynski, D.; Orsavova, J. Matcha Tea: Analysis of Nutritional Composition, Phenolics and Antioxidant Activity. Plant Food Hum. Nutr. 2020, 75, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J. New tastes in green tea: A novel flavor for familiar drinks, dishes, and desserts. Libr. J. 2004, 129, 110. [Google Scholar]
- Yamamoto, N. Antihypertensive peptides derived from food proteins. Biopolymers 1997, 43, 129–134. [Google Scholar] [CrossRef]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. 2002, 26, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- Jeszka-Skowron, M.; Krawczyk, M.; Zgola-Grzeskowiak, A. Determination of antioxidant activity, rutin, quercetin, phenolic acids and trace elements in tea infusions: Influence of citric acid addition on extraction of metals. J. Food Compos. Anal. 2015, 40, 70–77. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Matcha green tea (MGT) inhibits the propagation of cancer stem cells (CSCs), by targeting mitochondrial metabolism, glycolysis and multiple cell signalling pathways. Aging 2018, 10, 1867–1883. [Google Scholar] [CrossRef]
- Das, P.R.; Kim, Y.; Hong, S.J.; Eun, J.B. Profiling of volatile and non-phenolic metabolites-Amino acids, organic acids, and sugars of green tea extracts obtained by different extraction techniques. Food Chem. 2019, 296, 69–77. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, H.; Xu, P.; Yao, L.; Xie, Q.; Mao, L.; Wang, Y. Matcha green tea prevents obesity-induced hypothalamic inflammationviasuppressing the JAK2/STAT3 signaling pathway. Food Funct. 2020, 11, 8987–8995. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Sahin, M.A.; Cook, M.D. Matcha Green Tea Drinks Enhance Fat Oxidation During Brisk Walking in Females. Int. J. Sport Nutr. Exerc. 2018, 28, 536–541. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Kong, D.D.; Gao, Y.Y.; Yan, F.; Wang, Y.F.; Xu, P. In vitro antioxidant activity of phenolic-enriched extracts from Zhangping Narcissus tea cake and their inhibition on growth and metastatic capacity of 4T1 murine breast cancer cells. J Zhejiang Univ. Sci. B 2018, 19, 199–210. [Google Scholar] [CrossRef]
- Xu, P.; Chen, L.; Wang, Y. Effect of storage time on antioxidant activity and inhibition on alpha-Amylase and alpha-Glucosidase of white tea. Food Sci. Nutr. 2019, 7, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, H.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 22–25. [Google Scholar] [CrossRef]
- Xin, B.; Tao, F.; Wang, Y.; Liu, H.Y.; Ma, C.Q.; Xu, P. Coordination of metabolic pathways: Enhanced carbon conservation in 1,3-propanediol production by coupling with optically pure lactate biosynthesis. Metab. Eng. 2017, 41, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (-)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Sohn, I.; Ahn, J.I.; Lee, K.H.; Lee, Y.S.; Lee, Y.S. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 2004, 340, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahman, S.U.; Huang, Y.Y.; Zhang, Y.F.; Ming, P.F.; Zhu, L.; Chu, X.Y.; Li, J.C.; Feng, S.B.; Wang, X.C.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Low, H.B.; Png, C.W.; Torta, F.; Kumar, J.K.; Lim, H.Y.; Zhou, Y.; Yang, H.; Angeli, V.; Shabbir, A.; et al. Protective Function of Mitogen-Activated Protein Kinase Phosphatase 5 in Aging- and Diet-Induced Hepatic Steatosis and Steatohepatitis. Hepatol. Commun. 2019, 3, 748–762. [Google Scholar] [CrossRef] [Green Version]
- Griffin, J.D.; Salter, D.M.; Bowman, T.; Greenberg, A. Role of Hepatic PLIN2 and PLIN4 in The Development of Western Type Diet Induced Hepatosteatosis. Faseb J. 2017, 31, 458-3. [Google Scholar]
- Chen, F.J.; Yin, Y.S.; Chua, B.T.; Li, P. CIDE family proteins control lipid homeostasis and the development of metabolic diseases. Traffic 2020, 21, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marcos, L.V.; Sancho-Knapik, S.; Gabas-Rivera, C.; Barranquero, C.; Gascon, S.; Romanos, E.; Martinez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Arnal, C.; et al. Pgc1a is responsible for the sex differences in hepatic Cidec/Fsp27 beta mRNA expression in hepatic steatosis of mice fed a Western diet. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E249–E261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.K.; Xu, L.; Ye, J.; Li, D.; Wang, W.S.; Li, X.H.; Wu, L.Z.; Wang, H.; Guan, F.F.; Li, P. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology 2012, 56, 95–107. [Google Scholar] [CrossRef]
- Xu, M.J.; Cai, Y.; Wang, H.; Altamirano, J.; Chang, B.X.; Bertola, A.; Odena, G.; Lu, J.; Tanaka, N.; Matsusue, K.; et al. Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans. Gastroenterology 2015, 149, 1030–1041.e6. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.; Wang, H.; Chen, H.; McLenithan, J.C.; Gong, D.W.; Yang, R.Z.; Yu, D.Z.; Fried, S.K.; Quon, M.J.; Londos, C.; et al. Consequences of lipid droplet coat protein downregulation in liver cells: Abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 2008, 57, 2037–2045. [Google Scholar] [CrossRef] [Green Version]
- Nocetti, D.; Espinosa, A.; Pino-De la Fuente, F.; Sacristan, C.; Bucarey, J.L.; Ruiz, P.; Valenzuela, R.; Chouinard-Watkins, R.; Pepper, I.; Troncoso, R.; et al. Lipid droplets are both highly oxidized and Plin2-covered in hepatocytes of diet-induced obese mice. Appl. Physiol. Nutr. Metab. 2020, 45, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Chang, B.; Wu, X.Y.; Li, L.; Sleeman, M.; Chan, L. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E770–E779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, T. Role of lipid droplet proteins in liver steatosis. J. Physiol. Biochem. 2011, 67, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Cooke, A.A.; Connaughton, R.M.; Lyons, C.L.; McMorrow, A.M.; Roche, H.M. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur. J. Pharmacol. 2016, 785, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. B 2013, 368. [Google Scholar] [CrossRef]
- Otake, Y.; Walle, T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab. Dispos. 2002, 30, 103–105. [Google Scholar] [CrossRef] [Green Version]
- de Jong, L.M.; Jiskoot, W.; Swen, J.J.; Manson, M.L. Distinct Effects of Inflammation on Cytochrome P450 Regulation and Drug Metabolism: Lessons from Experimental Models and a Potential Role for Pharmacogenetics. Genes 2020, 11, 1509. [Google Scholar] [CrossRef]
- Kroetz, D.L.; Zeldin, D.C. Cytochrome P450 pathways of arachidonic acid metabolism. Curr. Opin. Lipidol. 2002, 13, 273–283. [Google Scholar] [CrossRef]
- Kim, Y.C.; Oh, E.Y.; Kim, S.H.; Lee, M.G. Pharmacokinetics of diclofenac in rat model of diabetes mellitus induced by alloxan or steptozotocin. Biopharm. Drug Dispos. 2006, 27, 85–92. [Google Scholar] [CrossRef]
- Maximos, S.; Chamoun, M.; Gravel, S.; Turgeon, J.; Michaud, V. Tissue Specific Modulation of cyp2c and cyp3a mRNA Levels and Activities by Diet-Induced Obesity in Mice: The Impact of Type 2 Diabetes on Drug Metabolizing Enzymes in Liver and Extra-Hepatic Tissues. Pharmaceutics 2017, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, A.; Miyazaki, T.; Iwamoto, J.; Hirayama, T.; Morishita, Y.; Monma, T.; Ueda, H.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 2020, 61, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Mah, E.; Masterjohn, C.; Noh, S.K.; Park, H.J.; Clark, R.M.; Park, Y.K.; Lee, J.Y.; Bruno, R.S. Green Tea Lowers Hepatic COX-2 and Prostaglandin E2 in Rats with Dietary Fat-Induced Nonalcoholic Steatohepatitis. J. Med. Food 2015, 18, 648–655. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Cyp2c70 | TATGGGCTTTTGCTCCTGCT | GGTCTCCGATGTCTACCAATCAC |
| Cyp2c40 | GCTCACCCTGTGATCCCCAATTCA | TTGAGAAAAACAGCATAGCAG |
| Cyp7b1 | AATTGGACAGCTTGGTCTGC | TTCTCGGATGATGCTGGAGT |
| Cidec | GTGTCCACTTGTGCCGTCTT | CTCGCTTGGTTGTCTTGATT |
| Cidea | TCCTCGGCTGTCTCAATG | TGGCTGCTCTTCTGTATCG |
| Plin4 | GCAGTATCTGGAGGTGTGATG | TGTGTCCTTCGTATTGGTGAG |
| Gapdh | CCTCGTCCCGTAGACAAAATG | TGAGGTCAATGAAGGGGTCGT |
| Compounds (%) | Matcha |
|---|---|
| Tea polyphenols | 19.84 ± 0.67 |
| Amino acid | 4.19 ± 0.01 |
| Total sugars | 3.14 ± 0.15 |
| Protein | 1.15 ± 0.10 |
| GC | 1.83 ± 0.18 |
| EGC | 1.15 ± 0.09 |
| C | 0.08 ± 0.11 |
| EC | 0.44 ± 0.02 |
| EGCG | 7.02 ± 0.93 |
| GCG | 1.27 ± 0.20 |
| ECG | 0.83 ± 0.13 |
| CG | 0.09 ± 0.02 |
| Caffeine | 6.58 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yu, Y.; Ding, L.; Xu, P.; Wang, Y. Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses. Nutrients 2021, 13, 1950. https://doi.org/10.3390/nu13061950
Zhou J, Yu Y, Ding L, Xu P, Wang Y. Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses. Nutrients. 2021; 13(6):1950. https://doi.org/10.3390/nu13061950
Chicago/Turabian StyleZhou, Jihong, Yueer Yu, Lejia Ding, Ping Xu, and Yuefei Wang. 2021. "Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses" Nutrients 13, no. 6: 1950. https://doi.org/10.3390/nu13061950
APA StyleZhou, J., Yu, Y., Ding, L., Xu, P., & Wang, Y. (2021). Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses. Nutrients, 13(6), 1950. https://doi.org/10.3390/nu13061950






