Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Question
2.2. Search Strategy and Study Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
- -
- Study design: number of experimental groups compared with the vehicle control group.
- -
- Non-clinical models: for in vivo studies: female murine (mice or rats), adult animals (6 weeks of age) with breast cancer, such as xenograft model (e.g., MCF-7, MDA-MB-231 cells) and syngeneic model (e.g., Ehrlich and Walker-256) at any weight body; for in vitro studies: any type of breast cancer cell line.
- -
- Intervention: dose/concentration, time of treatment/incubation, and route of administration when applicable. Type, source, molecular weight, and structure of the polysaccharide.
- -
- Outcome: primary: growth of breast cancer cells; for in vitro: the amount of cell proliferation or viability or migration; for in vivo: tumor volume and/or tumor weight. Secondary: mechanism of action of polysaccharide through biochemical and molecular assay in samples (cells or tumor tissue).
2.5. Assessment of Risk of Bias
3. Results
3.1. Study Selection
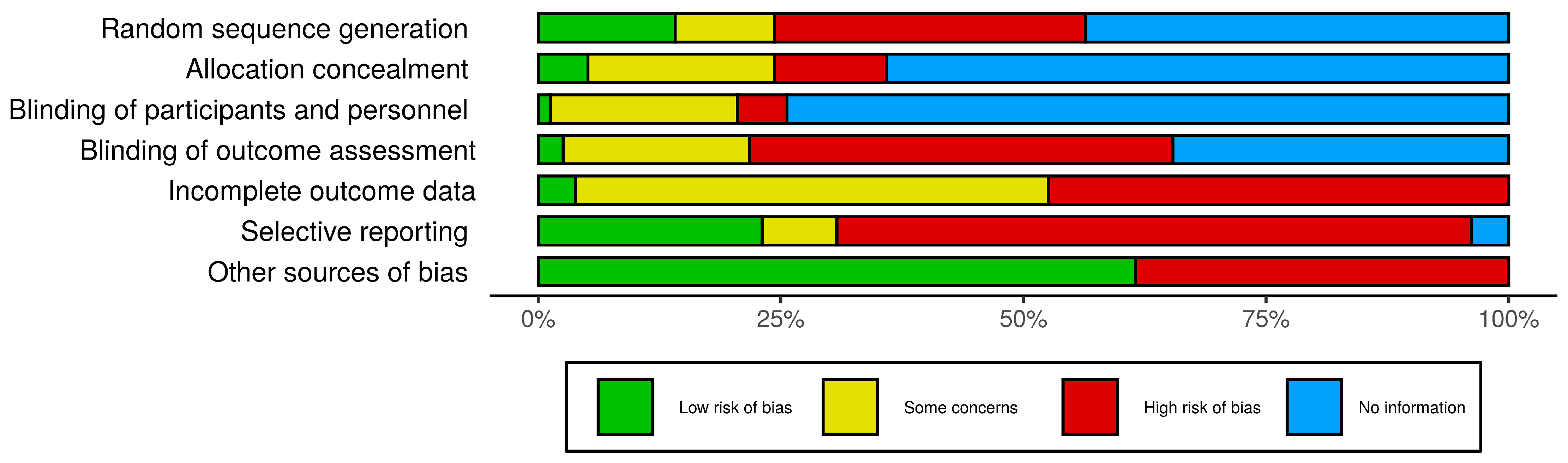
3.2. Risk of Bias Assessment
3.3. Data Extraction
3.4. Sea Animals
3.5. Algaes
3.6. Bacteria
3.7. Plants
3.8. Fruits
3.9. Fungus
3.10. Mushrooms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodai, B. Breast Cancer Survivorship: A Comprehensive Review of Long-Term Medical Issues and Lifestyle Recommendations. Perm. J. 2019, 19, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; IARC: Lyon, France, 2014. [Google Scholar]
- World Health Organization. Life Expectancy at Birth (Years); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomark. Prev. 2015, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Giordano, S.H.; Temin, S.; Kirshner, J.J.; Chandarlapaty, S.; Crews, J.R.; Davidson, N.E.; Esteva, F.J.; Gonzalez-Angulo, A.M.; Krop, I.; Levinson, J.; et al. Systemic Therapy for Patients with Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 2078–2099. [Google Scholar] [CrossRef]
- Poortmans, P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: Ending the debate. Lancet 2014, 383, 2104–2106. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–23. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- C.Ooi, V.E. Immunomodulation and Anti-Cancer Activity of Polysaccharide-Protein Complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.; Duan, J.; Fang, X.; Fang, J. Chemical modifications of the (1→3)-α-d-glucan from spores of Ganoderma lucidum and investigation of their physicochemical properties and immunological activity. Carbohydr. Res. 2001, 336, 127–140. [Google Scholar] [CrossRef]
- Smith, J.E.; Rowan, N.J.; Sullivan, R. Medicinal mushrooms: A rapidly developing area of biotechnology for cancer therapy and other bioactivities. Biotechnol. Lett. 2002, 24, 1839–1845. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L.; Cheung, P.C. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006, 341, 2261–2269. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.; Cheung, P.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, M.; Luo, A.; Chun, Z.; Luo, A. Characterization and Antitumor Activity of a Polysaccharide from Sarcodia ceylonensis. Molecules 2014, 19, 10863–10876. [Google Scholar] [CrossRef] [PubMed]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr. Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; Shin, J.-S.; Nam, K.-S. Starfish polysaccharides downregulate metastatic activity through the MAPK signaling pathway in MCF-7 human breast cancer cells. Mol. Biol. Rep. 2013, 40, 5959–5966. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer Properties and Mechanisms of Fucoidan on Mouse Breast Cancer In Vitro and In Vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Liu, Y.; Wang, Q.; Hou, L.; Zheng, Z. Fucoidan Inhibited 4T1 Mouse Breast Cancer Cell Growth In Vivo and In Vitro Via Downregulation of Wnt/β-Catenin Signaling. Nutr. Cancer 2013, 65, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGF receptor degradation in breast cancer. Carcinogenesis 2012, 34, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan Induces Cancer Cell Apoptosis by Modulating the Endoplasmic Reticulum Stress Cascades. PLoS ONE 2014, 9, e108157. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef]
- Xue, M.; Liang, H.; Tang, Q.; Xue, C.; He, X.; Zhang, L.; Zhang, Z.; Liang, Z.; Bian, K.; Zhang, L.; et al. The Protective and Immunomodulatory Effects of Fucoidan Against 7,12-Dimethyl benz[a]anthracene-Induced Experimental Mammary Carcinogenesis Through the PD1/PDL1 Signaling Pathway in Rats. Nutr. Cancer 2017, 69, 1–11. [Google Scholar] [CrossRef]
- He, X.; Xue, M.; Jiang, S.; Li, W.; Yu, J.; Xiang, S. Fucoidan Promotes Apoptosis and Inhibits EMT of Breast Cancer Cells. Biol. Pharm. Bull. 2019, 42, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-J.; Lin, M.-H.; Kuo, T.-C.; Chou, C.-M.; Mi, F.-L.; Cheng, C.-H.; Lin, C.-W. Fucoidan from Laminaria japonica exerts antitumor effects on angiogenesis and micrometastasis in triple-negative breast cancer cells. Int. J. Biol. Macromol. 2020, 149, 600–608. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan Extract Induces Apoptosis in MCF-7 Cells via a Mechanism Involving the ROS-Dependent JNK Activation and Mitochondria-Mediated Pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan Extract Enhances the Anti-Cancer Activity of Chemotherapeutic Agents in MDA-MB-231 and MCF-7 Breast Cancer Cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-Y.; Yan, M.-D.; Wu, A.T.; Yuan, K.S.-P.; Liu, S.H. Brown Seaweed Fucoidan Inhibits Cancer Progression by Dual Regulation of mir-29c/ADAM12 and miR-17-5p/PTEN Axes in Human Breast Cancer Cells. J. Cancer 2016, 7, 2408–2419. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Important Role of β1-Integrin in Fucoidan-Induced Apoptosis via Caspase-8 Activation. Biosci. Biotechnol. Biochem. 2012, 76, 1163–1168. [Google Scholar] [CrossRef] [Green Version]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal sulfated carrageenan inhibits proliferation of MDA-MB-231 cells via apoptosis regulatory genes. Mol. Med. Rep. 2014, 11, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, A.; Murad, H.; Jazzara, M.; Odeh, A.; Allaf, A.W. Isolation, Structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. Int. J. Biol. Macromol. 2018, 108, 916–926. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.; Liu, T.; Hu, Y.; Zheng, Q.; Wang, B.; Lin, H.; Li, X. Separation, characterization and anticancer activities of a sulfated polysaccharide from Undaria pinnatifida. Int. J. Biol. Macromol. 2016, 83, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, H.; Wang, X.; Zhang, X.; Liu, W.; Wang, Y.; Zhang, Y.; Pan, H.; Wang, Q.; Han, Y. Effect of polysaccharide from Undaria pinnatifida on proliferation, migration and apoptosis of breast cancer cell MCF7. Int. J. Biol. Macromol. 2019, 121, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.A.; Fortes, Z.B.; da Cunha, M.A.; Sarilmiser, H.K.; Dekker, A.M.B.; Öner, E.T.; Dekker, R.F.; Khaper, N. Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int. J. Biol. Macromol. 2017, 102, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, X.; Liu, X.; Sun, Z.; Li, J. Antitumor Effects of Ruyiping on Cell Growth and Metastasis in Breast Cancer. Cancer Biother. Radiopharm. 2019, 34, 297–305. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Wang, K.; Zhuang, J.; Chu, F.; Gao, C.; Liu, L.; Feng, F.; Zhou, C.; Zhang, W.; et al. Identifying the Antiproliferative Effect of Astragalus Polysaccharides on Breast Cancer: Coupling Network Pharmacology with Targetable Screening from the Cancer Genome Atlas. Front. Oncol. 2019, 9, 368. [Google Scholar] [CrossRef]
- Qin, N.; Lu, S.; Chen, N.; Chen, C.; Xie, Q.; Wei, X.; Ye, F.; He, J.; Li, Y.; Chen, L.; et al. Yulangsan polysaccharide inhibits 4T1 breast cancer cell proliferation and induces apoptosis in vitro and in vivo. Int. J. Biol. Macromol. 2019, 121, 971–980. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Sun, J.; Wang, Y.-B. Selective estrogen receptor modulator: A novel polysaccharide from Sparganii Rhizoma induces apoptosis in breast cancer cells. Carbohydr. Polym. 2017, 163, 199–207. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, C.; Wang, J.; Kumar, V.; Anwar, F.; Xiao, F.; Mushtaq, G.; Liu, Y.; Kamal, M.A.; Yuan, D. Inhibition on the growth of human MDA-MB-231 breast cancer cells in vitro and tumor growth in a mouse xenograft model by Se-containing polysaccharides from Pyracantha fortuneana. Nutr. Res. 2016, 36, 1243–1254. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.-Y.; Jiang, Q.-Y.; Kang, X.-M.; Zhao, L. Polysaccharides derived from Lycium barbarum suppress IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways in MCF-7 cells. Food Chem. 2012, 131, 1479–1484. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, M.S.; Kim, S.N.; Kim, H.Y.; Kim, K.H.; Shin, K.-S.; Kang, K.S. Polysaccharides from Korean Citrus hallabong peels inhibit angiogenesis and breast cancer cell migration. Int. J. Biol. Macromol. 2016, 85, 522–529. [Google Scholar] [CrossRef]
- Delphi, L.; Sepehri, H. Apple pectin: A natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed. Pharmacother. 2016, 84, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Adami, E.R.; Corso, C.R.; de Oliveira, N.M.T.; Galindo, C.M.; Milani, L.; Stipp, M.C.; Nascimento, G.E.D.; Chequin, A.; da Silva, L.C.M.; de Andrade, S.F.; et al. Antineoplastic effect of pectic polysaccharides from green sweet pepper (Capsicum annuum) on mammary tumor cells in vivo and in vitro. Carbohydr. Polym. 2018, 201, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Jafaar, Z.M.; Litchfield, L.M.; Ivanova, M.M.; Radde, B.N.; Al-Rayyan, N.; Klinge, C.M. β-D-glucan inhibits endocrine-resistant breast cancer cell proliferation and alters gene expression. Int. J. Oncol. 2014, 44, 1365–1375. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, E.A.; Fortes, Z.B.; da Cunha, M.A.; Barbosa, A.M.; Khaper, N.; Dekker, R.F. Antiproliferative and pro-apoptotic effects of three fungal exocellular β-glucans in MCF-7 breast cancer cells is mediated by oxidative stress, AMP-activated protein kinase (AMPK) and the Forkhead transcription factor, FOXO3a. Int. J. Biochem. Cell Biol. 2015, 67, 14–24. [Google Scholar] [CrossRef]
- Wang, G.; Liu, C.; Liu, J.; Liu, B.; Li, P.; Qin, G.; Xu, Y.; Chen, K.; Liu, H.; Chen, K. Exopolysaccharide from Trichoderma pseudokoningii induces the apoptosis of MCF-7 cells through an intrinsic mitochondrial pathway. Carbohydr. Polym. 2016, 136, 1065–1073. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Y.; Meng, J.; Wen, T.; Liu, J.; Xu, H. Cationic polysaccharide spermine-pullulan drives tumor associated macrophage towards M1 phenotype to inhibit tumor progression. Int. J. Biol. Macromol. 2019, 123, 1012–1019. [Google Scholar] [CrossRef]
- Gan, D.; Zeng, X.; Liu, R.H.; Ye, H. Potential mechanism of mycelium polysaccharide from Pholiota dinghuensis Bi in regulating the proliferation and apoptosis of human breast cancer MCF-7 cells through p38/MAPK pathway. J. Funct. Foods 2015, 12, 375–388. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, X.; Xiong, H.; Qiu, H.; Yuan, X.; Zhu, F.; Wang, Y.; Zou, Y. A polysaccharide from Huaier induced apoptosis in MCF-7 breast cancer cells via down-regulation of MTDH protein. Carbohydr. Polym. 2016, 151, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Lyu, X.; Yu, J.; Sun, L.; Du, D.; Lai, Y.; Li, H.; Wang, Y.; Zhang, L.; Yin, H.; et al. MHP-1 inhibits cancer metastasis and restores topotecan sensitivity via regulating epithelial-mesenchymal transition and TGF-β signaling in human breast cancer cells. Phytomedicine 2016, 23, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zou, S.; Xu, X. The β-glucan from Lentinus edodes suppresses cell proliferation and promotes apoptosis in estrogen receptor positive breast cancers. Oncotarget 2017, 8, 86693–86709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, J.; Hu, H.; Li, Q.; Liu, Y.; Wang, K. Functional polysaccharide Lentinan suppresses human breast cancer growth via inducing autophagy and caspase-7-mediated apoptosis. J. Funct. Foods 2018, 45, 75–85. [Google Scholar] [CrossRef]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 Breast Tumor Model. Curr. Protoc. Immunol. 2000, 39, 20.2.1–20.2.16. [Google Scholar] [CrossRef]
- Hammadi, M.; Oulidi, A.; Gackière, F.; Katsogiannou, M.; Slomianny, C.; Roudbaraki, M.; Dewailly, E.; Delcourt, P.; Lepage, G.; Lotteau, S.; et al. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: Involvement of GRP. FASEB J. 2012, 27, 1600–1609. [Google Scholar] [CrossRef]
- Sriburi, R.; Jackowski, S.; Mori, K.; Brewer, J.W. XBP1 a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004, 167, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Murad, H.; Hawat, M.; Ekhtiar, A.; Aljapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, S.; Shirataki, Y. Inhibitory effects of Astragali Radix, a crude drug in Oriental medicines, on lipid peroxidation and protein oxidative modification by copper. J. Ethnopharmacol. 1999, 68, 331–333. [Google Scholar] [CrossRef]
- Toda, S.; Yase, Y.; Shirataki, Y. Inhibitory effects of Astragali Radix, crude drug in oriental medicines on lipid peroxida-tion and protein oxidative modification of mouse brain homogenate by copper. Phyther. Res. 2000, 14, 294–296. [Google Scholar] [CrossRef]
- Yin, G.; Jeney, G.; Racz, T.; Xu, P.; Jun, X.; Jeney, Z. Effect of two Chinese herbs (Astragalus radix and Scutellaria radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture 2006, 253, 39–47. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, O.K.; Park, C.W.; Yang, C.H.; Jeon, T.W.; Yoo, W.K.; Kim, S.H.; Kim, H.J. Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali radix in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2005, 100, 289–294. [Google Scholar] [CrossRef]
- Huang, G.-C.; Wu, L.-S.; Chen, L.-G.; Yang, L.-L.; Wang, C.-C. Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine model of cyclophosphamide-induced leucopenia. J. Ethnopharmacol. 2007, 109, 229–235. [Google Scholar] [CrossRef]
- Cho, W.C.; Leung, K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [CrossRef]
- Doan, V.M.; Chen, C.; Lin, X.; Nguyen, V.P.; Nong, Z.; Li, W.; Chen, Q.; Ming, J.; Xie, Q.; Huang, R. Yulangsan polysaccharide improves redox homeostasis and immune impairment in d-galactose-induced mimetic aging. Food Funct. 2015, 6, 1712–1718. [Google Scholar] [CrossRef]
- Huang, J.; Nguyen, V.; Tang, X.; Wei, J.; Lin, X.; Lai, Z.; Doan, V.; Xie, Q.; Huang, R.; Van Nguyen, P.; et al. Protection from diclofenac-induced liver injury by Yulangsan polysaccharide in a mouse model. J. Ethnopharmacol. 2016, 193, 207–213. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, X.; Lin, X.; Zhang, S.; Huang, Z.; Chen, C.; Guo, Y.; Xuan, F.; Xu, X.; Huang, R. Mechanism of the Protective Effect of Yulangsan Flavonoid on Myocardial Ischemia/Reperfusion Injury in Rats. Cell. Physiol. Biochem. 2014, 34, 1050–1062. [Google Scholar] [CrossRef]
- Lin, X.; Huang, Z.; Chen, X.; Rong, Y.; Zhang, S.; Jiao, Y.; Huang, Q.; Huang, R. Protective effect of Millettia pulchra polysaccharide on cognitive impairment induced by d-galactose in mice. Carbohydr. Polym. 2014, 101, 533–543. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, N.; Sun, P.; Bucheli, P.; Li, L.; Hou, Y.; Wang, J. Effects of Soluble Tea Polysaccharides on Hyperglycemia in Alloxan-Diabetic Mice. J. Agric. Food Chem. 2007, 55, 5523–5528. [Google Scholar] [CrossRef]
- Monobe, M.; Ema, K.; Kato, F.; Maeda-Yamamoto, M. Immunostimulating Activity of a Crude Polysaccharide Derived from Green Tea (Camellia sinensis) Extract. J. Agric. Food Chem. 2008, 56, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, Z.; Yi, M.; Wang, X.; Peng, F.; Xiao, F.; Chen, T.; Wang, C.; Mushtaq, G.; Kamal, M. Effects of Polysaccharides from Selenium-enriched Pyracantha fortuneana on Mice Liver Injury. Med. Chem. 2015, 11, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, C.; Bu, Y.; Xiang, T.; Huang, X.; Wang, Z.; Yi, F.; Ren, G.; Liu, G.; Song, F. Antioxidative and immunoprotective effects of Pyracantha fortuneana (Maxim.) Li polysaccharides in mice. Immunol. Lett. 2010, 133, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan Induces Apoptosis through Activation of Caspase-8 on Human Breast Cancer MCF-7 Cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Del Cornò, M.; Gessani, S.; Conti, L. Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers 2020, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.; Hwang, P. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Demir, G.; Klein, H.; Mandel-Molinas, N.; Tuzuner, N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int. Immunopharmacol. 2007, 7, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Esfahani, A.; Jafarabadi, M.A.; Ziaei, J.E.; Movassaghpourakbari, A.; Farrin, N. Effect of Beta Glucan on Quality of Life in Women with Breast Cancer Undergoing Chemotherapy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Adv. Pharm. Bull. 2014, 4, 471–477. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Ziaei, J.E.; Esfahani, A.; Jafarabadi, M.A.; Movassaghpourakbari, A.; Farrin, N. Effect of Beta Glucan on White Blood Cell Counts and Serum Levels of IL-4 and IL-12 in Women with Breast Cancer Undergoing Chemotherapy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Asian Pac. J. Cancer Prev. 2014, 15, 5733–5739. [Google Scholar] [CrossRef]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Mol. Med. 2005, 15, 695–699. [Google Scholar] [CrossRef] [PubMed]



| CRITERIA | DESCRIPTION |
|---|---|
| PARTICIPANTS | Non-clinical studies referring to the biological activities of extracts or isolated polysaccharides in breast cancer models (e.g., female murine models of breast cancer and breast cancer cells) |
| INTERVENTION | Extracts or isolated polysaccharides treatment |
| COMPARISON(S) | Comparison control groups (which were not treated with polysaccharide) |
| OUTCOMES (S) | Changes in parameters related to the model of breast cancer studied |
| SOURCE | Species | Polysaccharide | In Vitro Model | In Vivo Model | Concentration/ Dose | Mechanism of Action/ Antitumor Effect | Reference |
|---|---|---|---|---|---|---|---|
| SEA ANIMALS | Asterina pectinifera | Ps | MCF-7 | - | 10, 20, 40 *, 80 *, 120 * µg/mL | Inhibit metastasis through COX-2 and MMP-9 via downregulation of MAPK pathway | Lin, Shi, and Nam (2013) [23] |
| ALGAES | Fucus vesiculosus | Fucoidan | 4T1 | 4T1 (mice) | 50 *, 100 *, and 200 * µg/mL 5 * and 10 * mg/kg | Apoptosis through mitochondrial pathway, inhibit angiogenesis through downregulation of VEGF and ERK signaling and inhibit metastasis | Xue et al. (2012) [24] |
| 4T1 | 4T1 (mice) | 25 *, 50 *, 100 *, and 200 * µg/mL 5 * and 10 * mg/kg | Inhibit proliferation through downregulation of cyclin d1 and wnt/β-catenin signaling | Xue et al. (2013) [25] | |||
| 4T1 MDA-MB-231 | 4T1 (mice) | 60, 90 *, 100 *, and 120 * µg/mL 0.25 * mg/kg | Inhibit metastasis through tgfr/smad/snail, slug, twist and emt axes | Hsu et al. (2013) [26] | |||
| MDA-MB-231 | - | 10, 50 *, and 100 * µg/mL | Apoptosis through er stress | Chen et al. (2014) [27] | |||
| MDA-MB-231 | DMBA (rat) | 6.25 *, 12.5 *, and 25 * µg/mL 200 * and 400 * mg/kg | Inhibit proliferation and induce apoptosis through downregulation of PI3K/AKT/gsk3β pathway | Xue et al. (2017a) [28] | |||
| - | DMBA (rats) | 200 * and 400 * mg/kg | Immunosuppression of treg through PD1/PDL1 pathway | Xue et al. (2017b) [29] | |||
| MCF-7 with ex vivo sample (blood from Sprague Dawley Rats) | - | 200 * and 400 * mg/kg | Inhibit proliferation, induce apoptosis, and inhibit migration through downregulation of MMP-9 and upregulation of e-cadherin | He et al. (2019) [30] | |||
| Laminaria japonica | Fucoidan | MDA-MB-231 | - | 0.125, 0.25, 0.5, 1 *, and 2 * mg/mL | Inhibit angiogenesis and proliferation through activation of MAPK and PI3K followed by inhibition of ap-1 and NF-κB signaling | Hsu et al. (2020) [31] | |
| Cladosiphon novae-caledoniae | Fucoidan (LMWF) | MCF-7 MDA-MB-231 | - | 82, 410, and 820 * µg/mL | Apoptosis via mitochondria pathway associated with ROS-dependent JNK phosphorylation | Zhang et al. (2011) [32] | |
| Cladosiphon novae-caledoniae | Fucoidan (LMWF) | MDA-MB-231 | - | 200 * and 400 * µg/mL | Apoptosis through caspase activation and mitochondrial dysfunction | Zhang et al. (2013) [33] | |
| Sargassum hemiphyllum | Fucoidan (LMWF) | MCF-7 MDA-MB-231 | - | 50, 100, and 200 * µg/mL | Inhibit tumor progression through mir-29c/adam12 and mir-17-5p/PTEN axes | Wu et al. (2016) [34] | |
| - | Fucoidan | MCF-7 | - | 100 * µg/mL | Apoptosis through β1-integrin-caspase-8 complex | Yamasaki et al. (2012) [35] | |
| Laurencia papillosa | Aspe | MDA-MB-231 | - | 10 *, 50 *, and 100 * µg/mL | Inhibit proliferation through g1-phase arrest and induce apoptosis through caspase activation | Murad et al. (2015) [36] | |
| Laurencia papillosa | Carrageenan | MCF-7 | - | 25, 50 *, 100 *, 150 *, and 200 * µg/mL | Inhibit proliferation and induce apoptosis | Ghannam et al. (2018) [37] | |
| Undaria pinnatifida | Spup | - | DMBA (rats) | 100, 200, and 300 * mg/kg | Reduce tumor growth, have immunomodulatory activity, and modulate sex hormones | Han et al. (2016) [38] | |
| Undaria pinnatifida | Spup | MCF-7 | - | 25 *, 100 *, and 200 * µg/mL | Inhibit migration and proliferation and induce apoptosis | Wu et al. (2019) [39] | |
| BACTERIA | Halomonas smyrnensis aad6 | Levan | MCF-7 | - | 10, 25, 50, 75, 100 *, 250, 500, 750, 1000, and 1500 µg/mL | Inhibit proliferation, induce apoptosis and oxidative stress | Queiroz et al. (2017) [40] |
| PLANTS | Ruyiping | Pcspp | MDA-MB-231 MDA-MB-468 | - | 1, 2.5, 5, 10, 20, 30, 40 *, 45, and 50% | Inhibit proliferation and emt-marker | Li et al. (2019) [41] |
| Astragalus membranaceus | APS | MCF-7 MDA-MB-231 | - | 0.25 *, 0.5 *, 0.75, 1, and 2 mg/mL | Inhibit proliferation through inhibition of CCNDB1, CDC6, and p53 | Liu et al. (2019) [42] | |
| Millettia pulchra Kurz var. | Yulangsan | 4T1 with ex vivo samples (Blood from Sprague Dawley Rats) | 4T1 (mice) | 750 *, 1500 *, and 3000 * mg/kg 150 *, 300 *, and 600 * mg/kg | Inhibit angiogenesis through inhibition of VEGF, induce apoptosis through caspase activation and inhibit metastasis | Qin et al. (2019) [43] | |
| Sparganii Rhizoma | SpaTA | ZR-75-1 MDA-MB-231 | - | 76.4, 152.8, 305.6 *, and 611.2 * mg/L | Induce apoptosis through regulating ERα | Wu, Sun, and Wang (2017) [44] | |
| Pyracantha fortunean | Se-PFPs | MDA-MB-231 | MDA-MB-231 (mice) | 50, 100 *, 200, and 400 * µg/mL 100 * and 400 *, mg/kg | Inhibit proliferation by arresting cells at G2 phase via inhibiting CDC25C-CyclinB1/CDC2 pathway and induce apoptosis through p53-mediated cytochrome c-caspase pathway | Yuan et al. (2016) [45] | |
| FRUITS | Lycium barbarum (wolfberry) | WFP | MCF-7 | - | 25, 50, 100 *, and 200 * µg/mL | Induce apoptosis and oxidative stress and inhibit proliferation through the g0/g1 cell cycle arrest | He et al. (2012) [46] |
| Lycium barbarum (wolberry) | LBP | MCF-7 | - | 0.05, 0.1, 0.25, 0.5 *, and 1 mg/mL | Inhibit angiogenesis through IGF-1 and PI3K/HIF-1A/VEGF pathway | Huang et al. (2011) [47] | |
| Citrus sphaerocarpa (hallabong peels) | Hbe-ii | MDA-MB-231 | - | 1.56, 3.12, 6.25, 12.5, 25 *, 50, and 100 µg/mL | Inhibit metastasis through inhibition of tube formation and MMP-9 | Park et al. (2016) [48] | |
| Apple | Pectin | 4T1 | 4T1 (mice) | 0.01, 0.1, 0.5, and 1 * % w/v | Induce apoptosis and inhibit metastasis through up-regulation of p53 | Delphi et al. (2016) [49] | |
| Capsicum annuum | CAP | MCF-7 MDA-MB-231 MDA-MB-436 | Ehrlich (mice) | 0.025, 0.05, 0.1 *, 0.2, 0.4 mg/mL 50, 100 *, and 150 mg/kg | Inhibit proliferation and angiogenesis | Adami et al. (2018) [50] | |
| FUNGUS | S. Cerevisae | Β-(1-3)-d-glucan | MCF-7 LCC9 | - | 1, 10 *, 50 *, 100, 200, 300, and 400 µg/mL | Inhibit proliferation, induce apoptosis and increase genes rassf1, IGFBP3, CTNNB1, and ERβ | Jafaar et al. (2014) [51] |
| Botryosphaeria rhodina mamb-05 Lasiodiplodia theobromae MMPI | (1-3)(1-6)-β-d-glucan (1-6)-β-d-glucan | MCF-7 | - | 10, 25, 50, 75, 100 *, 250, 750, 1000, and 1500 µg/mL | Inhibit proliferation, induce apoptosis, necrosis, and oxidative stress mediated by amp-activated protein-kinase and forkhead transcription factor foxo3a | Queiroz et al. (2015) [52] | |
| Trichoderma pseudokoningii | EPS | MCF-7 | - | 0.10, 0.25 *, 0.50 *, 0.75, and 1 * mg/mL | Induce oxidative stress and apoptosis through an intrinsic mitochondrial pathway | Wang et al. (2015) [53] | |
| Aureobasidium pullulan | Pullulan (PS) | - | 4T1 (mice) | 2.4 * mg/kg | Immunostimulant of macrophages m1 | Xie et al. (2018) [54] | |
| MUSHROOMS | Pholiota dinghuensis bi | PDP3 | MCF-7 | - | 13.5, 28.2 *, and 52.1 * µg/mL | Inhibit proliferation and induce apoptosis through p38/MAPK pathway | Gan et al. (2015) [55] |
| Trametes robiniophila murr (hauier) | SP1 | MCF-7 | - | 100 *, 200 *, and 400 * µg/mL | Induce apoptosis through downregulation bax and mtdh protein | Luo et al. (2016) [56] | |
| Mortierella hepialid | MHP-1 | MCF-7 | MDA-MB-231 (mice) | 0.1 *, 1 *, and 10 * µm 20, 40, and 60 * mg/kg | Inhibit metastasis through inhibition of TGF-β signaling | Lin et al. (2016) [57] | |
| Lentinus edodes | Lentinan [β-(1-3)(1-6)-d-glucan] | MCF-7 T47D Mda-mb-231 MAA-MB-468 | MCF-7 (mice) | 12.5, 25, 50, 100 *, 200 *, and 400 * µg/mL 1 * mg/kg | Inhibited tumor growth through suppressing cell proliferation and enhancing apoptosis via PI3K/AKT/mTOR, NF-κB -, ERK-, ERα-, caspase-, and p53-dependent pathways | Xu, Zou, and Xu (2017) [58] | |
| Lentinus edodes | Lentinan [β-(1-3)(1-6)-d-glucan] | MCF-7 | MCF-7 (mice) | 15.6, 31.3, 62.5, 125, 250 *, 500 * and 1000 * µg/mL 5 *, 10 *, and 20 * mg/kg | Induce autophagy through LC3 conversion and apoptosis through caspase-7-mediated mitochondrial pathway | Li et al. (2018) [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corso, C.R.; Mulinari Turin de Oliveira, N.; Moura Cordeiro, L.; Sauruk da Silva, K.; da Silva Soczek, S.H.; Frota Rossato, V.; Fernandes, E.S.; Maria-Ferreira, D. Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies. Nutrients 2021, 13, 2008. https://doi.org/10.3390/nu13062008
Corso CR, Mulinari Turin de Oliveira N, Moura Cordeiro L, Sauruk da Silva K, da Silva Soczek SH, Frota Rossato V, Fernandes ES, Maria-Ferreira D. Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies. Nutrients. 2021; 13(6):2008. https://doi.org/10.3390/nu13062008
Chicago/Turabian StyleCorso, Claudia Rita, Natalia Mulinari Turin de Oliveira, Leonardo Moura Cordeiro, Karien Sauruk da Silva, Suzany Hellen da Silva Soczek, Virgilio Frota Rossato, Elizabeth Soares Fernandes, and Daniele Maria-Ferreira. 2021. "Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies" Nutrients 13, no. 6: 2008. https://doi.org/10.3390/nu13062008
APA StyleCorso, C. R., Mulinari Turin de Oliveira, N., Moura Cordeiro, L., Sauruk da Silva, K., da Silva Soczek, S. H., Frota Rossato, V., Fernandes, E. S., & Maria-Ferreira, D. (2021). Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies. Nutrients, 13(6), 2008. https://doi.org/10.3390/nu13062008







