Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases
Abstract
1. Introduction
2. Methods
2.1. Study Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Experimental Protocol
2.4. Endpoints
2.5. Sample Size and Statistical Analysis
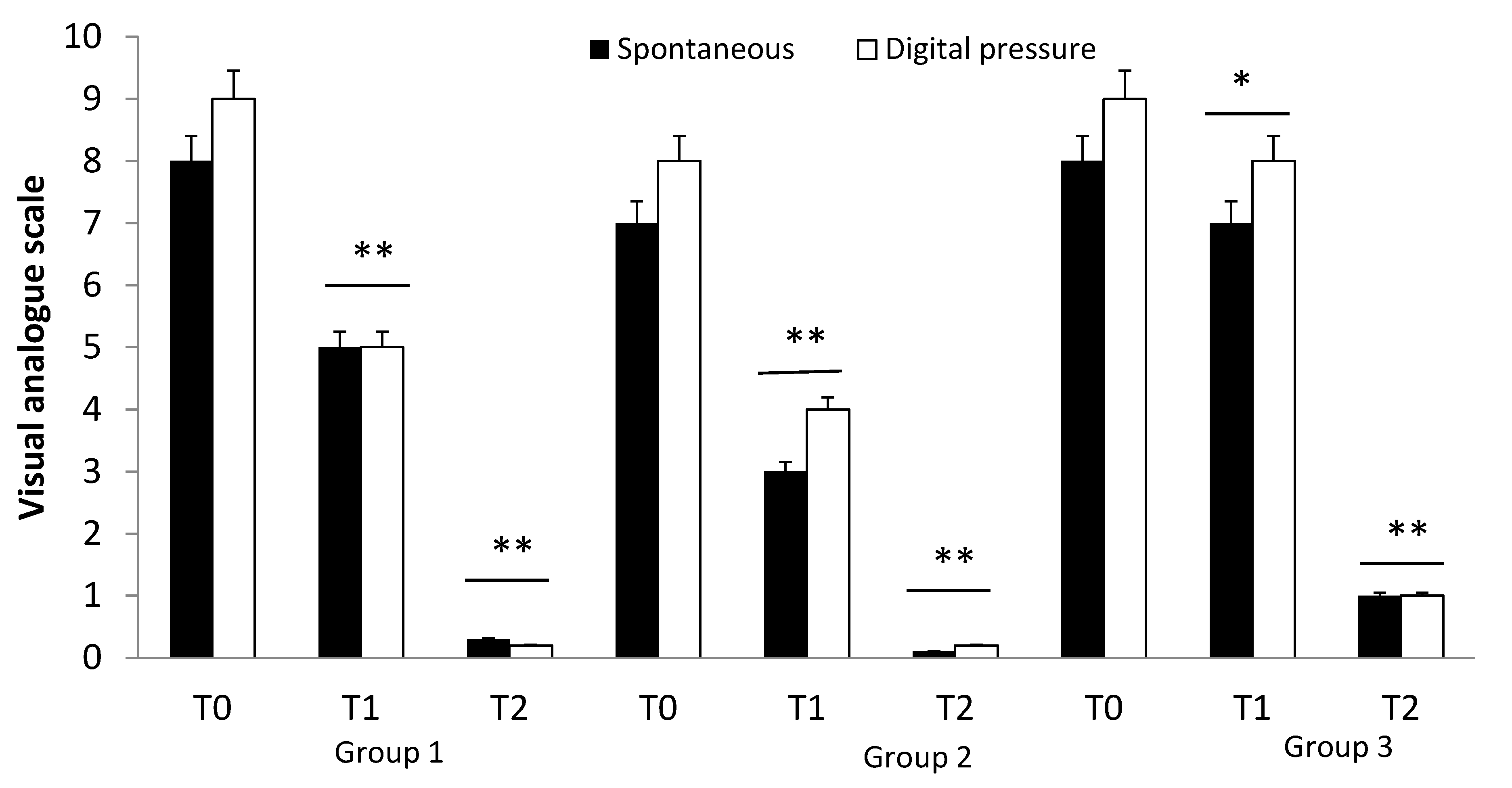
3. Results
3.1. Population
3.2. Effects of Drug Treatment
4. Discussion
5. Clinical Risk Management Consideration and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto: A typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef]
- Yatagai, C.; Maruyama, M.; Kawahara, T.; Sumi, H. Nattokinase-promoted tissue plasminogen activator release from human cells. Pathophysiol. Haemost. Thromb. 2008, 36, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Ihara, H.; Umemura, K.; Suzuki, Y.; Oike, M.; Akita, S.; Takada, A.; Tsukamoto, Y.; Suzuki, I. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis Cleaves and inactivates plasminogen activator inhibitor type 1. J. Biol. Chem. 2001, 276, 24690–24696. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, F.; Zhang, T.; Wang, J. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox. Biol. 2020, 32, 101500. [Google Scholar] [CrossRef]
- Ren, N.; Chen, H.; Li, Y.; McGowan, E.; Lin, Y. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Nat. Med. J. China 2017, 97, 2038–2042. [Google Scholar]
- Suzuki, Y.; Kondo, K.; Matsumoto, Y.; Zhao, B.Q.; Otsuguro, K.; Maeda, T.; Umemura, K.; Tsukamoto, Y.; Urano, T. Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life Sci. 2003, 73, 1289–1298. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, X.; Jiang, H.; Zhang, S.; Dong, M.; Zhao, X. Study on the antioxidative activity and effects on experimental hyperlipidemia of natto extract. Acta Nutr. Sin. 2003, 26, 296–299. [Google Scholar]
- Jang, J.Y.; Kim, T.S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, Y.B.; Kim, S.K.; Park, S.K.; Lee, S.P.; et al. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab. Anim. Res. 2013, 29, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Fadl, N.; Ahmed, H.; Booles, H.; Sayed, A. Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum. Exp. Toxicol. 2013, 32, 721–735. [Google Scholar] [CrossRef]
- Ji, H.; Yu, L.; Liu, K.; Yu, Z.; Zhang, Q.; Zou, F.; Liu, B. Mechanisms of nattokinase in protection of cerebral ischemia. Eur. J. Pharmacol. 2014, 745, 144–151. [Google Scholar] [CrossRef]
- Sharma, D.; Shekhar, S.K.; Kumar, A.; Godheja, J. Isolation, characterization, production and purification of fibrinolytic enzyme nattokinase from Bacillus subtilis. Int. J. Pharm. Sci. Res. 2020, 11, 1768–1776. [Google Scholar]
- Lan, G.; Li, C.; He, L.; Zeng, X.; Zhu, Q. Effects of different strains and fermentation method on nattokinase activity, biogenic amines, and sensory characteristics of natto. J. Food Sci. Technol. 2020, 57, 4414–4423. [Google Scholar] [CrossRef]
- Ero, M.P.; Ng, C.M.; Mihailovski, T.; Harvey, N.R.; Lewis, B.H. A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Altern. Ther. Health. Med. 2013, 19, 16–19. [Google Scholar]
- Lampe, B.J.; English, J.C. Toxicological assessment of nattokinase derived from Bacillus subtilis var. natto. Food Chem. Toxicol. 2016, 88, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Le Gal, G.; Bates, S.M.; Righini, M.; Haramati, L.B.; Lang, E.; Mustafa, R.A.; Kline, J.A.; Chasteen, S.; Snyder, M.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018, 2, 3226–3256. [Google Scholar] [CrossRef]
- Brodovicz, K.G.; McNaughton, K.; Uemura, N.; Meininger, G.; Girman, C.J.; Yale, S.H. Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin. Med. Res. 2009, 7, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Ferreri, G.; Colosimo, M.; Pirritano, D.; Guadagnino, L.; Pelaia, G.; De Sarro, G.B.; Maselli, R. Adverse drug reactions to antibiotics ob-served in two pulmonology divisions of Catanzaro, Italy: A six-year retrospective study. Pharm. Res. 2002, 46, 395–400. [Google Scholar] [CrossRef]
- Gareri, P.; De Fazio, P.; Gallelli, L.; De Fazio, S.; Davoli, A.; Seminara, G.; Sarro, G.D.; Cotroneo, A. Venlafaxine-propafenone interaction resulting in hallucinations and psychomotor agitation. Ann. Pharmacother. 2008, 42, 434–438. [Google Scholar] [CrossRef]
- Gallelli, L.; Ferraro, M.; Spagnuolo, V.; Rende, P.; Mauro, G.F.; De Sarro, G. Rosuvastatin-induced rhabdomyolysis probably via CYP2C9 saturation. Drug Metabol. Drug Interact. 2009, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Staltari, O.; Palleria, C.; Di Mizio, G.; De Sarro, G.; Caroleo, B. A case of adverse drug reaction induced by dispensing error. J. Forensic Leg. Med. 2012, 19, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Chirchiglia, D.; Cione, E.; Carollo, M.C.; Wang, M.; Di Mizio, G.; Faedda, N.; Gallelli, L.; Giacolini, T.; Siviglia, S.; Guidetti, V. Effects of Add-On Ultramicronized N-Palmitoyl Ethanol Amide in Patients Suffering of Migraine with Aura: A Pilot Study. Front. Neurol. 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Gum, S.N.; Paik, J.K.; Lim, H.H.; Kim, K.C.; Ogasawara, K.; Lee, J.H.; Inoue, K.; Park, S.; Park, Y. Effects of nattokinase on blood pressure: A randomized, controlled trial. Hypertens. Res. 2008, 31, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Lenninger, M.; Ero, M.P.; Benson, K.F. Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: Results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial. Integr. Blood Press. Control. 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Lin, Y.; Qu, X. Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, M.; Yang, X.; Chen, Q.; Chen, H.; Lin, D.H. Thrombolytic effects in vivo of nattokinase in a carrageenan-induced rat model of thrombosis. Acta Haematol. 2014, 132, 247–253. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Nirengi, S.; Homma, T.; Esaki, K.; Ohta, M.; Clark, J.F.; Hamaoka, T. A single-dose of oral nattokinase potentiates throm-bolysis and anti-coagulation profiles. Sci. Rep. 2015, 5, 11601. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Juritsch, A.F.; Moreau, R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr. Rev. 2018, 76, 618–638. [Google Scholar] [CrossRef]
- Serra, R.; Buffone, G.; Falcone, D.; Molinari, V.; Scaramuzzino, M.; Gallelli, L.; de Franciscis, S. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. 2013, 21, 395–401. [Google Scholar] [CrossRef]
- Serra, R.; Gallelli, L.; Buffone, G.; Molinari, V.; Stillitano, D.M.; Palmieri, C.; de Franciscis, S. Doxycycline speeds up healing of chronic venous ulcers. Int. Wound J. 2015, 12, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Buffone, G.; Calio, F.G.; Squillace, A.; de Franciscis, S.; Rizzo, B.A.; Massara, M.; Spinelli, F.; et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int. Wound J. 2016, 13, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Ielapi, N.; Bitonti, A.; Candido, S.; Fregola, S.; Gallo, A.; Gallelli, L.; Muraca, L.; Raimondo, L.; Velcean, L.; et al. Efficacy of a Low-Dose Diosmin Therapy on Improving Symptoms and Quality of Life in Patients with Chronic Venous Disease: Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 999. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Montemurro, R.; Butrico, L.; Calio, F.G.; Mastrangelo, D.; de Franciscis, S.; Scarcello, S.E.; Gallelli, L.; Buffone, G. The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015, 157, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, J.; Linhardt, R.J. Interactions between nattokinase and heparin/GAGs. Glycoconj J. 2015, 32, 695–702. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Wang, H.; Li, W.; Zhang, C.; Liu, Y.; Xu, F.; Zhang, F.; Chen, J.; Duan, L. Nattokinase-heparin exhibits beneficial efficacy and safety—An optimal strategy for CKD patients on hemodialysis. Glycoconj J. 2019, 36, 93–101. [Google Scholar] [CrossRef]
- Taniguchi, A.; Yamanaka-Okumura, H.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese style meal suppress postprandial glucose and insulin responses. Asia Pac. J. Clin. Nutr. 2008, 17, 663–668. [Google Scholar]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese-style breakfast improved insulin sensitivity, lipid metabolism, and oxidative stress in over-weight subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef]
- Jamilian, M.; Asemi, Z. The Effect of Soy Intake on Metabolic Profiles of Women with Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Zhang, Y.; Ross, S.; Schünemann, H.J.; Wiercioch, W.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Li, Y.-L.; Zhang, Y. Toxicological safety assessment on safety of nattokinase capsule. Prac. Prev. Med. 2012, 19, 1714–1716. [Google Scholar]
| Clinical Records | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Females | 32 | 34 | 26 |
| Males | 18 | 23 | 20 |
| VAS | 9 | 7 | 7 |
| ESR | 22 | 21 | 19 |
| Local Skin Redness | + yes | yes | yes |
| Local shinny skin | yes | yes | yes |
| Deep pit edema | Grade 2 | Grade 2 | Grade 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallelli, G.; Di Mizio, G.; Palleria, C.; Siniscalchi, A.; Rubino, P.; Muraca, L.; Cione, E.; Salerno, M.; De Sarro, G.; Gallelli, L. Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases. Nutrients 2021, 13, 2031. https://doi.org/10.3390/nu13062031
Gallelli G, Di Mizio G, Palleria C, Siniscalchi A, Rubino P, Muraca L, Cione E, Salerno M, De Sarro G, Gallelli L. Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases. Nutrients. 2021; 13(6):2031. https://doi.org/10.3390/nu13062031
Chicago/Turabian StyleGallelli, Giuseppe, Giulio Di Mizio, Caterina Palleria, Antonio Siniscalchi, Paolo Rubino, Lucia Muraca, Erika Cione, Monica Salerno, Giovambattista De Sarro, and Luca Gallelli. 2021. "Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases" Nutrients 13, no. 6: 2031. https://doi.org/10.3390/nu13062031
APA StyleGallelli, G., Di Mizio, G., Palleria, C., Siniscalchi, A., Rubino, P., Muraca, L., Cione, E., Salerno, M., De Sarro, G., & Gallelli, L. (2021). Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases. Nutrients, 13(6), 2031. https://doi.org/10.3390/nu13062031










