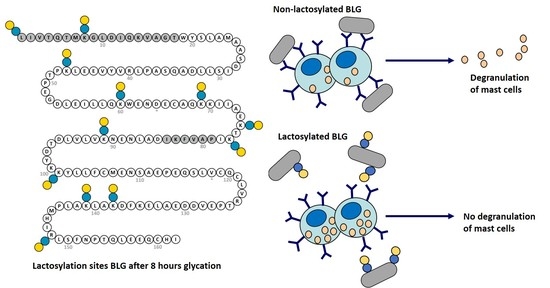
Limited Lactosylation of Beta-Lactoglobulin from Cow’s Milk Exerts Strong Influence on Antigenicity and Degranulation of Mast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sodium Dodecylsulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting (WB)
2.4. Capillary Electrophoresis—Mass Spectrometry
2.5. Intact Protein Analysis
2.6. Peptide Identification
2.7. Antigenicity Assessment of BLG
2.8. Degranulation of Rat Basophilic Leukemia-huFcεRI Cells (RBL-hεIa-2B12 Cells)
2.9. Statistics
3. Results
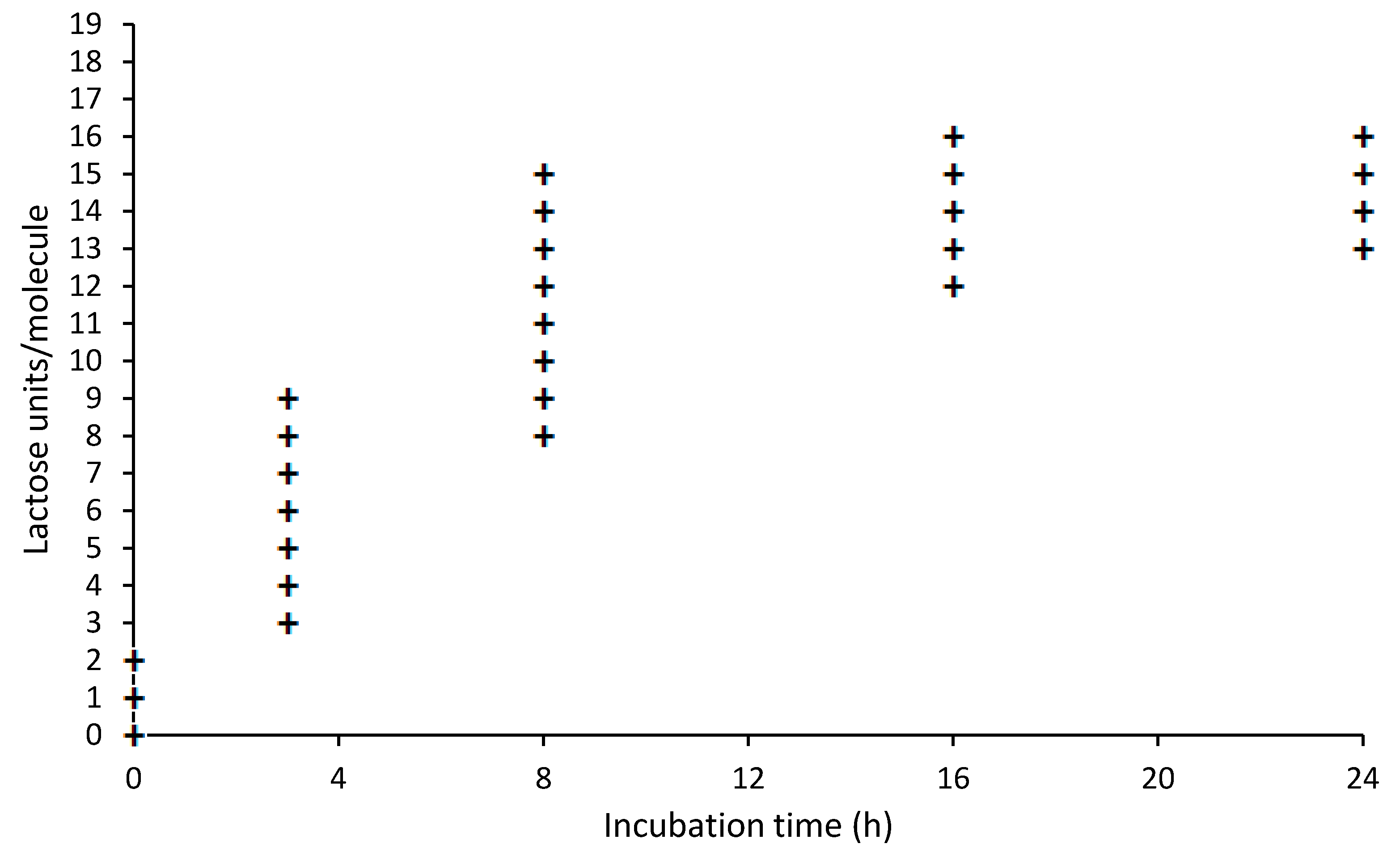
3.1. Lactosylation of BLG and Characterization by WB
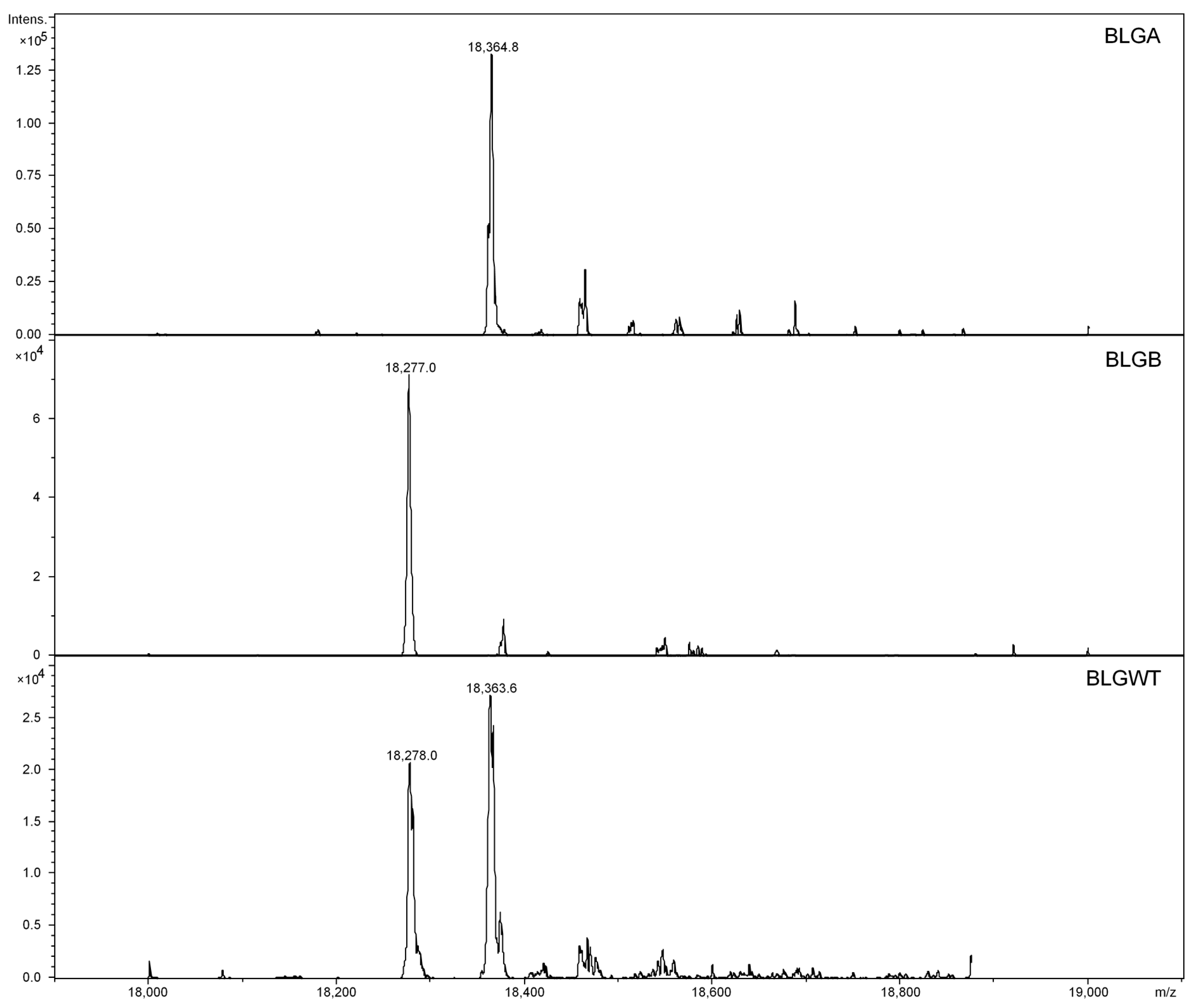
3.2. CE-MS Analyses of Intact BLGWT, BLGA and BLGB
3.3. Analyses of Tryptic Digest of BLG
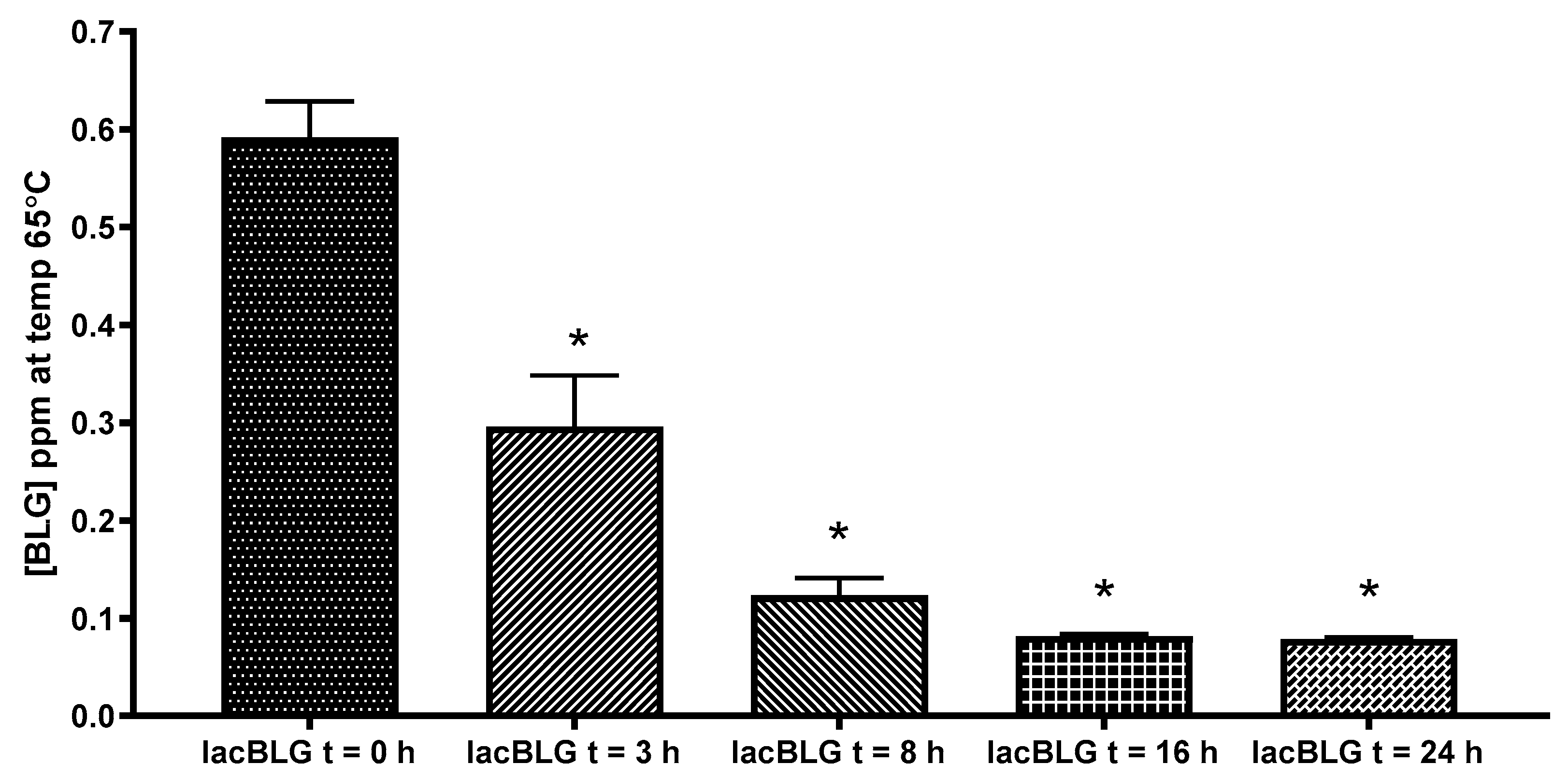
3.4. Antigenicity of Lactosylated BLG Using ELISA
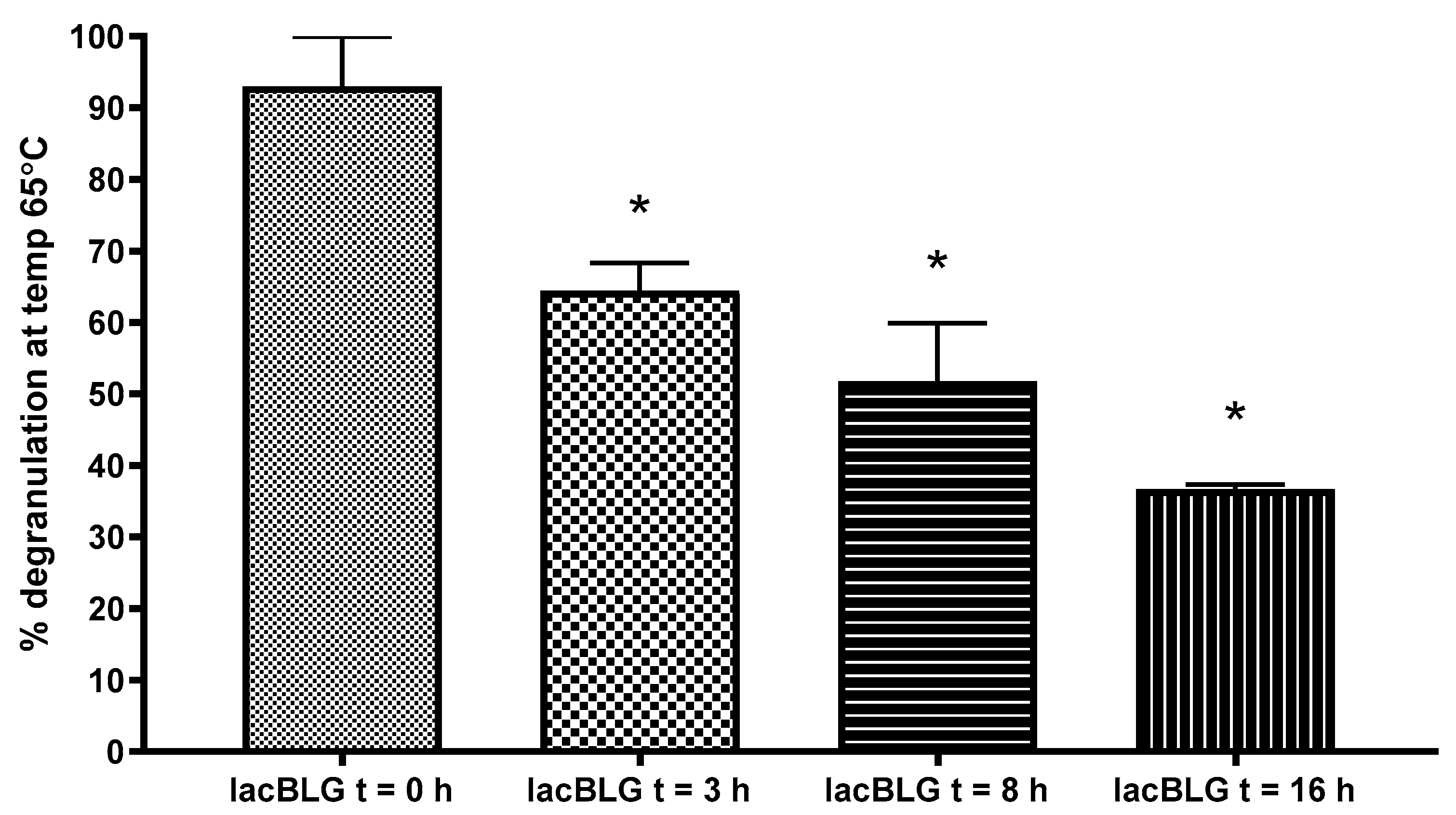
3.5. Degranulation Capacity of Lactosylated BLG Using RBL-hεIa-2B12 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical changes of bioactive phytochemicals during thermal processing. Food Sci. 2016. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Gaudin, J.C.; Rabesona, H.; Nioi, C.; Agarwal, D.; Drouet, M.; Chobert, J.M.; Bordbar, A.K.; Haertle, T. Effects of heating and glycation of beta-lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. J. Agric. Food Chem. 2009, 57, 4974–4982. [Google Scholar] [CrossRef]
- Perusko, M.; van Roest, M.; Stanic-Vucinic, D.; Simons, P.J.; Pieters, R.; Velickovic, T.C.; Smit, J.J. Glycation of the major milk allergen beta-lactoglobulin changes its allergenicity by alterations in cellular uptake and degradation. Mol. Nutr. Food Res. 2018, 62, e1800341. [Google Scholar] [CrossRef]
- Yang, Z.H.; Li, C.; Li, Y.Y.; Wang, Z.H. Effects of Maillard reaction on allergenicity of buckwheat allergen Fag t 3 during thermal processing. J. Sci. Food Agric. 2013, 93, 1510–1515. [Google Scholar] [CrossRef]
- Toda, M.; Hellwig, M.; Henle, T.; Vieths, S. Influence of the Maillard reaction on the allergenicity of food proteins and the development of allergic inflammation. Curr. Allergy Asthma Rep. 2019, 19, 4. [Google Scholar] [CrossRef]
- Ma, X.J.; Chen, H.B.; Gao, J.Y.; Hu, C.Q.; Li, X. Conformation affects the potential allergenicity of ovalbumin after heating and glycation. Food Addit. Contam. Part A 2013, 30, 1684–1692. [Google Scholar] [CrossRef]
- Vissers, Y.M.; Blanc, F.; Skov, P.S.; Johnson, P.E.; Rigby, N.M.; Przybylski-Nicaise, L.; Bernard, H.; Wal, J.M.; Ballmer-Weber, B.; Zuidmeer-Jongejan, L.; et al. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS ONE 2011, 6, e23998. [Google Scholar] [CrossRef]
- Zhong, J.; Yu, H.; Tu, Y.; Zhou, L.; Liu, W.; Luo, S.; Liu, C.; Prakash, S. Comparison of antigenicity and conformational changes to beta-lactoglobulin following kestose glycation reaction with and without dynamic high-pressure microfluidization treatment. Food Chem. 2019, 278, 491–496. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Maillard reaction in food allergy: Pros and cons. Crit. Rev. Food Sci. Nutr. 2018, 58, 208–226. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wu, C.H.; Lu, C.H.; Kuo, Y.M.; Li, K.J.; Hsieh, S.C.; Yu, C.L. Advanced glycation end products of bovine serum albumin suppressed Th1/Th2 cytokine but enhanced monocyte IL-6 gene expression via MAPK-ERK and MyD88 transduced NF-kappaB p50 signaling pathways. Molecules 2019, 24, 2461. [Google Scholar] [CrossRef]
- Iwan, M.; Vissers, Y.M.; Fiedorowicz, E.; Kostyra, H.; Kostyra, E.; Savelkoul, H.F.; Wichers, H.J. Impact of Maillard reaction on immunoreactivity and allergenicity of the hazelnut allergen Cor a 11. J. Agric. Food Chem. 2011, 59, 7163–7171. [Google Scholar] [CrossRef]
- Gupta, R.K.; Raghav, A.; Sharma, A.; Gupta, K.; Neelabh; Mandal, P.; Tripathi, A.; Ansari, I.A.; Das, M.; Dwivedi, P.D. Glycation of clinically relevant chickpea allergen attenuates its allergic immune response in Balb/c mice. Food Chem. 2017, 235, 244–256. [Google Scholar] [CrossRef]
- Enomoto, H.; Hayashi, Y.; Li, C.P.; Ohki, S.; Ohtomo, H.; Shiokawa, M.; Aoki, T. Glycation and phosphorylation of alpha-lactalbumin by dry heating: Effect on protein structure and physiological functions. J. Dairy Sci. 2009, 92, 3057–3068. [Google Scholar] [CrossRef]
- Yousefi, R.; Ferdowsi, L.; Tavaf, Z.; Sadeghian, T.; Tamaddon, A.M.; Moghtaderi, M.; Pourpak, Z. Evaluation of structure, chaperone-like activity and allergenicity of reduced glycated adduct of bovine beta-casein. Protein Pept. Lett. 2017, 24, 46–55. [Google Scholar] [CrossRef]
- Morisawa, Y.; Kitamura, A.; Ujihara, T.; Zushi, N.; Kuzume, K.; Shimanouchi, Y.; Tamura, S.; Wakiguchi, H.; Saito, H.; Matsumoto, K. Effect of heat treatment and enzymatic digestion on the B cell epitopes of cow’s milk proteins. Clin. Exp. Allergy 2009, 39, 918–925. [Google Scholar] [CrossRef]
- Chen, Y.; Tu, Z.; Wang, H.; Zhang, L.; Sha, X.; Pang, J.; Yang, P.; Liu, G.; Yang, W. Glycation of beta-lactoglobulin under dynamic high pressure microfluidization treatment: Effects on IgE-binding capacity and conformation. Food Res. Int. 2016, 89, 882–888. [Google Scholar] [CrossRef]
- Jimenez-Saiz, R.; Belloque, J.; Molina, E.; Lopez-Fandino, R. Human immunoglobulin E (IgE) binding to heated and glycated ovalbumin and ovomucoid before and after in vitro digestion. J. Agric. Food Chem. 2011, 59, 10044–10051. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Wellner, A.; Gadermaier, G.; Ilchmann, A.; Briza, P.; Krause, M.; Nagai, R.; Burgdorf, S.; Scheurer, S.; Vieths, S.; et al. Ovalbumin modified with pyrraline, a Maillard reaction product, shows enhanced T-cell immunogenicity. J. Biol. Chem. 2014, 289, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, H.; Zhang, X.; Zhou, P. Insight into the effects of deglycosylation and glycation of shrimp tropomyosin on in vivo allergenicity and mast cell function. Food Funct. 2019, 10, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Fenaille, F.; Morgan, F.; Parisod, V.; Tabet, J.C.; Guy, P.A. Solid-state glycation of beta-lactoglobulin by lactose and galactose: Localization of the modified amino acids using mass spectrometric techniques. J. Mass Spectrom. 2004, 39, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Knipping, K.; Simons, P.J.; Buelens-Sleumer, L.S.; Cox, L.; den Hartog, M.; de Jong, N.; Teshima, R.; Garssen, J.; Boon, L.; Knippels, L.M. Development of beta-Lactoglobulin-Specific Chimeric Human IgEkappa Monoclonal Antibodies for In Vitro Safety Assessment of Whey Hydrolysates. PLoS ONE 2014, 9, e106025. [Google Scholar] [CrossRef]
- Knipping, K.; van Roest, M.; Kruijssen, L.; Smits, M.; Teunis, M.; Cox, L.; de Jong, N.; Simons, P.J.; Boon, L.; Teshima, R.; et al. Intra- and inter-laboratory validation of an innovative huFcepsilonRIalpha-RBL-2H3 degranulation assay for in vitro allergenicity assessment of whey hydrolysates. Toxicol. In Vitro 2016, 33, 29–34. [Google Scholar] [CrossRef]
- Vijayalakshmi, L.; Krishna, R.; Sankaranarayanan, R.; Vijayan, M. An asymmetric dimer of beta-lactoglobulin in a low humidity crystal form—Structural changes that accompany partial dehydration and protein action. Proteins 2008, 71, 241–249. [Google Scholar] [CrossRef]
- Kuwata, K.; Hoshino, M.; Forge, V.; Era, S.; Batt, C.A.; Goto, Y. Solution structure and dynamics of bovine beta-lactoglobulin A. Protein Sci. 1999, 8, 2541–2545. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, S.; Morais Cabral, J.H.; Cooper, R.; Flower, D.R.; Yewdall, S.J.; Polikarpov, I.; North, A.C.; Sawyer, L. Bovine beta-lactoglobulin at 1.8 A resolution—Still an enigmatic lipocalin. Structure 1997, 5, 481–495. [Google Scholar] [CrossRef]
- Yang, W.; Tu, Z.; Wang, H.; Zhang, L.; Song, Q. Glycation of ovalbumin after high-intensity ultrasound pretreatment: Effects on conformation, IgG/IgE binding ability and antioxidant activity. J. Sci. Food Agric. 2018, 98, 3767–3773. [Google Scholar] [CrossRef]
- Xu, L.; Gong, Y.; Gern, J.E.; Ikeda, S.; Lucey, J.A. Glycation of whey protein with dextrans of different molar mass: Effect on immunoglobulin E-binding capacity with blood sera obtained from patients with cow milk protein allergy. J. Dairy Sci. 2018, 101, 6823–6834. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosman, G.P.; Oliveira, S.; Simons, P.J.; Sastre Torano, J.; Somsen, G.W.; Knippels, L.M.J.; Haselberg, R.; Pieters, R.J.; Garssen, J.; Knipping, K. Limited Lactosylation of Beta-Lactoglobulin from Cow’s Milk Exerts Strong Influence on Antigenicity and Degranulation of Mast Cells. Nutrients 2021, 13, 2041. https://doi.org/10.3390/nu13062041
Bosman GP, Oliveira S, Simons PJ, Sastre Torano J, Somsen GW, Knippels LMJ, Haselberg R, Pieters RJ, Garssen J, Knipping K. Limited Lactosylation of Beta-Lactoglobulin from Cow’s Milk Exerts Strong Influence on Antigenicity and Degranulation of Mast Cells. Nutrients. 2021; 13(6):2041. https://doi.org/10.3390/nu13062041
Chicago/Turabian StyleBosman, Gerlof P., Sergio Oliveira, Peter J. Simons, Javier Sastre Torano, Govert W. Somsen, Leon M. J. Knippels, Rob Haselberg, Roland J. Pieters, Johan Garssen, and Karen Knipping. 2021. "Limited Lactosylation of Beta-Lactoglobulin from Cow’s Milk Exerts Strong Influence on Antigenicity and Degranulation of Mast Cells" Nutrients 13, no. 6: 2041. https://doi.org/10.3390/nu13062041
APA StyleBosman, G. P., Oliveira, S., Simons, P. J., Sastre Torano, J., Somsen, G. W., Knippels, L. M. J., Haselberg, R., Pieters, R. J., Garssen, J., & Knipping, K. (2021). Limited Lactosylation of Beta-Lactoglobulin from Cow’s Milk Exerts Strong Influence on Antigenicity and Degranulation of Mast Cells. Nutrients, 13(6), 2041. https://doi.org/10.3390/nu13062041