Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
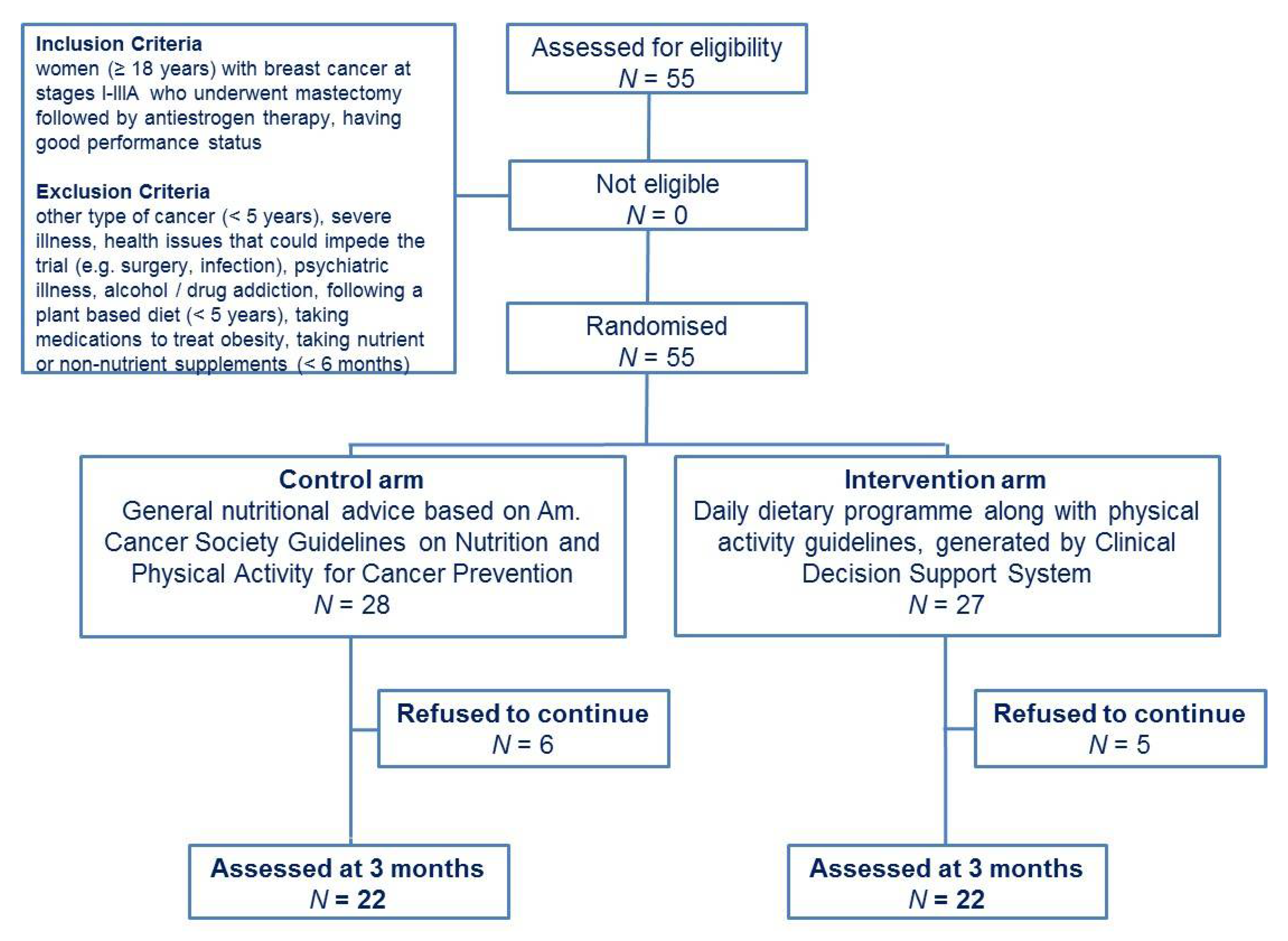
2.2. Participants
2.3. Study Design
2.4. Assessments
2.5. Primary Outcome and Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Dietary Intake and Circulating Vitamin C
3.2. Anthropometrics and Physical Activity
3.3. Health Related Quality of Life and Psychological Distress
3.4. Blood Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Breast Cancer Now Most Common Form of Cancer: WHO Taking Action. Available online: https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common-form-of-cancer-who-taking-action (accessed on 3 February 2021).
- Dhankhar, R.; Vyas, S.P.; Jain, A.K.; Arora, S.; Rath, G.; Goyal, A.K. Advances in novel drug delivery strategies for breast cancer therapy. Artif. Cells Blood Substit. Immobil. Biotechnol. 2010, 38, 230–249. [Google Scholar] [CrossRef]
- Suzuki, R.; Orsini, N.; Saji, S.; Key, T.J.; Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status—A meta-analysis. Int. J. Cancer 2009, 124, 698–712. [Google Scholar] [CrossRef]
- Chan, D.S.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.-B.; Gunnarsdóttir, K.Á.; Højris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef]
- Ioannides, S.J.; Barlow, P.L.; Elwood, J.M.; Porter, D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: A systematic review. Breast Cancer Res. Treat. 2014, 147, 237–248. [Google Scholar] [CrossRef]
- Kalezic, A.; Udicki, M.; Srdic Galic, B.; Aleksic, M.; Korac, A.; Jankovic, A.; Korac, B. Redox profile of breast tumor and associated adipose tissue in premenopausal women—Interplay between obesity and malignancy. Redox Biol. 2021, 41, 101939. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Bracken, M.B.; Sanft, T.B.; Ligibel, J.A.; Harrigan, M.; Irwin, M.L. Weight gain after breast cancer diagnosis and all-cause mortality: Systematic review and meta-analysis. J. Natl. Cancer Inst. 2015, 107, djv275. [Google Scholar] [CrossRef] [PubMed]
- Makari-Judson, G.; Braun, B.; Jerry, D.J.; Mertens, W.C. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J. Clin. Oncol. 2014, 5, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Prout, M.; Geiger, A.M.; Kamineni, A.; Thwin, S.S.; Avila, C.; Silliman, R.A.; Quinn, V.; Yood, M.U. Comorbidities and cardiovascular disease risk in older breast cancer survivors. Am. J. Manag. Care 2014, 20, 86–92. [Google Scholar]
- Parkin, D.M.; Boyd, L.; Walker, L.C. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Nho, J.H. Lifestyle intervention for breast cancer women. J. Lifestyle Med. 2019, 9, 12–14. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef]
- Wayne, S.J.; Baumgartner, K.; Baumgartner, R.N.; Bernstein, L.; Bowen, D.J.; Ballard-Barbash, R. Diet quality is directly associated with quality of life in breast cancer survivors. Breast Cancer Res. Treat. 2006, 96, 227–232. [Google Scholar] [CrossRef]
- Braakhuis, A.; Campion, P.; Bishop, K. The effects of dietary nutrition education on weight and health biomarkers in breast cancer survivors. Med. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Skouroliakou, M.; Grosomanidis, D.; Massara, P.; Kostara, C.; Papandreou, P.; Ntountaniotis, D.; Xepapadakis, G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur. J. Nutr. 2018, 57, 2133–2145. [Google Scholar] [CrossRef]
- Matalas, A.L. Disparities within traditional Mediterranean food patterns: An historical approach of the Greek diet. Int. J. Food Sci. Nutr. 2006, 57, 529–536. [Google Scholar] [CrossRef]
- Nounou, M.I.; ElAmrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast cancer: Conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer 2015, 9, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Mazo, C.; Kearns, C.; Mooney, C.; Gallagher, W.M. Clinical Decision Support Systems in breast cancer: A systematic review. Cancers 2020, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.R.; Samsa, G.; Hasselblad, V.; Williams, J.W.; Musty, M.D.; et al. Effect of clinical decision-support systems: A systematic review. Ann. Intern. Med. 2012, 157, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Jacob, V.; Thota, A.B.; Chattopadhyay, S.K.; Njie, G.J.; Proia, K.K.; Hopkins, D.P.; Ross, M.N.; Pronk, N.P.; Clymer, J.M. Cost and economic benefit of clinical decision support systems for cardiovascular disease prevention: A community guide systematic review. JAMIA 2017, 24, 669–676. [Google Scholar] [CrossRef]
- Ajami, S.; Amini, F. Reduce medication errors with clinical decision support systems. J. Inf. Technol. Softw. Eng. 2013, 7, e001. [Google Scholar] [CrossRef]
- Njie, G.J.; Proia, K.K.; Thota, A.B.; Finnie, R.K.C.; Hopkins, D.P.; Banks, S.M.; Callahan, D.B.; Pronk, N.P.; Rask, K.J.; Lackland, D.T.; et al. Community Preventive Services Task Force. Clinical decision support systems and prevention: A community guide cardiovascular disease systematic review. Am. J. Prev. Med. 2015, 49, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Skiba, D.J.; Gance-Cleveland, B.; Gilbert, K.; Gilbert, L.; Dandreaux, D. Comparing the Effectiveness of CDSS on provider’s behaviors to implement obesity prevention guidelines. NI 2012 2012, 2012, 376. [Google Scholar] [PubMed]
- Naureckas, S.M.; Zweigoron, R.; Haverkamp, K.S.; Kaleba, E.O.; Pohl, S.J.; Ariza, A.J. Developing an electronic clinical decision support system to promote guideline adherence for healthy weight management and cardiovascular risk reduction in children: A progress update. Transl. Behav. Med. 2011, 1, 103–107. [Google Scholar] [CrossRef][Green Version]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- WHO|Global Database on Body Mass Index (BMI). Available online: https://www.who.int/nutrition/databases/bmi/en (accessed on 26 April 2019).
- Sabounchi, N.S.; Rahmandad, H.; Ammerman, A. Best-fitting prediction equations for basal metabolic rate: Informing obesity interventions in diverse populations. Int. J. Obes. 2013, 37, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Boléo-Tomé, C.; Monteiro-Grillo, I.; Camilo, M.; Ravasco, P. Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br. J. Nutr. 2012, 108, 343–348. [Google Scholar] [CrossRef]
- Gioxari, A.; Tzanos, D.; Kostara, C.; Papandreou, P.; Mountzios, G.; Skouroliakou, M. Mediterranean diet implementation to protect against advanced lung cancer index (Ali) rise: Study design and preliminary results of a randomised controlled trial. IJERPH 2021, 18, 3700. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar]
- Mourouti, N.; Papavagelis, C.; Psaltopoulou, T.; Aravantinos, G.; Samantas, E.; Filopoulos, E.; Manousou, A.; Plytzanopoulou, P.; Vassilakou, T.; Malamos, N.; et al. Aims, design and methods of a case-control study for the assessment of the role of dietary habits, eating behaviors and environmental factors, on the development of breast cancer. Maturitas 2013, 74, 31–36. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults, the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Kontodimopoulos, N.; Ntinoulis, K.; Niakas, D. Validity of the Greek EORTC QLQ-C30 and QLQ-BR23 for measuring health-related quality of life in breast cancer patients. Eur. J. Cancer Care 2011, 20, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Spinhoven, P.; Ormel, J.; Sloekers, P.P.; Kempen, G.I.; Speckens, A.E.; Van Hemert, A.M. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol. Med. 1997, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Valentini, A.; Perrone, M.A.; Cianfarani, M.A.; Tarantino, U.; Massoud, R.; Merra, G.; Bernardini, S.; Morris, H.A.; Bertoli, A. Obesity, vitamin D status and physical activity: 1,25(OH)2D as a potential marker of vitamin D deficiency in obese subjects. Panminerva Med. 2020, 62, 83–92. [Google Scholar] [CrossRef]
- Johnston, C.S.; Corte, C. People with marginal vitamin C status are at high risk of developing vitamin C deficiency. J. Am. Diet. Assoc. 1999, 99, 854–856. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Boucher, P.; Mamelle, N. Mediterranean dietary pattern in a randomized trial: Prolonged survival and possible reduced cancer rate. Arch. Intern. Med. 1998, 158, 1181–1187. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000, 9, 869–873. [Google Scholar]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean diet and breast cancer risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef]
- van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Cottet, V.; González, C.A.; Luján-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the Mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Hu, F.B.; McCullough, M.L.; Newby, P.K.; Willett, W.C.; Holmes, M.D. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J. Nutr. 2006, 136, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Di Maso, M.; Dal Maso, L.; Augustin, L.S.A.; Puppo, A.; Falcini, F.; Stocco, C.; Mattioli, V.; Serraino, D.; Polesel, J. Adherence to the Mediterranean diet and mortality after breast cancer. Nutrients 2020, 12, 3649. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Blackburn, G.L.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J. Natl. Cancer Inst. 2006, 98, 1767–1776. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and breast cancer: A literature review on prevention, treatment and recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Jayedi, A.; Emadi, A.; Khan, T.A.; Abdolshahi, A.; Shab-Bidar, S. Dietary fiber and survival in women with breast cancer: A dose-response meta-analysis of prospective cohort studies. Nutr. Cancer 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A meta-analysis. Eur. J. Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Knobf, M.T.; Jeon, S. Metabolic syndrome, exercise, and cardiovascular fitness in breast cancer survivors. J. Adv. Pract. Oncol. 2020, 11, 98–102. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-Gonzalez, M.Á.; Sánchez, J.M.; et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals: A randomized, controlled trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, C.; Porciello, G.; Vitale, S.; Palumbo, E.; Crispo, A.; Grimaldi, M.; Calabrese, I.; Pica, R.; Prete, M.; Falzone, L.; et al. Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications. Nutrients 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.; Brauer, P.; Royall, D.; Keller, H.; Hanning, R.M.; DiCenso, A. Use of electronic dietary assessment tools in primary care: An interdisciplinary perspective. BMC Med. Inform. Decis. Mak. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Pramesh, C.S.; Rakesh Aggarwal, R. Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. Perspect. Clin. Res. 2016, 7, 144–146. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef]
| Characteristics | Enrolled Patients (N = 44) | Control Group (N = 22) | CDSS Group (N = 22) | p |
|---|---|---|---|---|
| Females | 44 | 22 | 22 | - |
| Age (years) | 49.7 ± 8.1 | 49.8 ± 8.4 | 49.6 ± 7.9 | 0.956 |
| Body weight (Kg) | 75.8 ± 13.9 | 73.2 ± 13.1 | 78.4 ± 14.6 | 0.218 |
| BMI (kg/m2) <18.5 18.5–24.9 25–29.9 >30 | 28.8 ± 5.6 0 14 (31.8) 13 (29.5) 17 (38.6) | 28.3 ± 6.0 0 9 (40.9) 6 (27.3) 7 (31.8) | 29.3 ± 5.4 0 5 (22.7) 7 (31.8) 10 (45.4) | 0.556 |
| % BFM | 39.4 ± 8.2 | 38.3 ± 8.4 | 40.6 ± 8.0 | 0.326 |
| WC (cm) | 97.9 ± 11.7 | 96.9 ± 10.6 | 98.8 ± 12.9 | 0.593 |
| Hormone Therapy Aromatase inhibitors Tamoxifen | 30 (68.2) 14 (31.8) | 16 (72.7) 6 (27.3) | 14 (63.6) 8 (36.4) | - |
| Current smokers | 13 (29.5) | 6 (27.3) | 7 (31.8) | - |
| ECOG performance status Score 0 Score 1 | 44 (100.0) 0 | 22 (100.0) 0 | 22 (100.0) 0 | - |
| METs-min/week | 767.9 ± 410.7 | 841.5 ± 445.9 | 694.4 ± 367.8 | 0.239 |
| Glucose (mg/dL) | 94.8 ± 10.1 | 95.5 ± 10.3 | 94.1 ± 10.1 | 0.655 |
| Total cholesterol (mg/dL) | 192.3 ± 40.7 | 189.1 ± 47.5 | 195.4 ± 33.4 | 0.611 |
| HDL (mg/dL) | 58.2 ± 19.1 | 58.4 ± 17.1 | 58.0 ± 21.3 | 0.942 |
| LDL (mg/dL) | 114.8 ± 31.8 | 115.6 ± 35.9 | 113.9 ± 27.8 | 0.856 |
| Triacylglycerols (mg/dL) | 94.5 ± 38.9 | 93.4 ± 36.3 | 95.6 ± 42.1 | 0.850 |
| MDA (nmol/mL) | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.5 | 0.669 |
| Vitamin C (mg/L) | 4.4 ± 2.1 | 4.1 ± 2.2 | 4.7 ± 2.1 | 0.285 |
| Vitamin 1,25(OH)2D (ng/L) | 31.9 ± 6.8 | 32.9 ± 8.9 | 31.0 ± 3.6 | 0.363 |
| MedDietScore | 31.0 ± 4.0 | 31.3 ± 3.5 | 30.6 ± 4.5 | 0.552 |
| Fibers (g/day) | 18.7 ± 2.6 | 18.7 ± 2.8 | 18.7± 2.6 | 0.987 |
| SFAs (g/day) | 16.8 ± 3.2 | 17.3 ± 3.7 | 16.3 ± 2.7 | 0.312 |
| MUFAs (g/day) | 29.8 ± 4.7 | 30.1 ± 5.2 | 29.5 ± 4.4 | 0.689 |
| Vitamin C (mg/day) | 267.2 ± 81.6 | 281.8 ± 99.5 | 252.7 ± 57.4 | 0.242 |
| Scales | Control Group (N = 22) | CDSS Group (N = 22) | p |
|---|---|---|---|
| EORTC-QLQ-C30: functional scales | |||
| Physical | 75.8 ± 20.3 | 73.9 ± 13.9 | 0.732 |
| Role | 56.8 ± 31.6 | 53.0 ± 35.5 | 0.710 |
| Emotional | 64.0 ± 22.8 | 64.4 ± 26.6 | 0.959 |
| Cognitive | 74.2 ± 18.3 | 72.0 ± 31.4 | 0.770 |
| Social | 78.8 ± 29.6 | 75.8 ± 17.6 | 0.683 |
| EORTC-QLQ-C30: symptom scales | |||
| Fatigue | 46.5 ± 20.2 | 48.5 ± 28.4 | 0.787 |
| Nausea / vomiting | 1.5 ± 7.1 | 1.5 ± 4.9 | 0.998 |
| Pain | 31.1 ± 31.4 | 31.8 ± 25.7 | 0.931 |
| Dyspnoea | 28.8 ± 25.8 | 24.2 ± 27.6 | 0.576 |
| Insomnia | 34.8 ± 34.9 | 33.3 ± 38.5 | 0.892 |
| Appetite loss | 10.6 ± 15.9 | 7.6 ± 14.3 | 0.510 |
| Constipation | 12.1 ± 28.3 | 13.6 ± 24.5 | 0.850 |
| Diarrhoea | 12.1 ± 26.3 | 12.1 ± 21.9 | 0.999 |
| EORTC-QLQ-C30: global health, QoL | 61.0 ± 22.6 | 62.9 ± 18.5 | 0.762 |
| EORTC-QLQ-BR23: functional scales | |||
| Body image | 67.0 ± 26.9 | 69.7 ± 27.6 | 0.748 |
| Sexual functioning | 79.5 ± 24.1 | 79.5 ± 26.2 | 0.999 |
| Future perspective | 36.4 ± 32.4 | 40.9 ± 32.4 | 0.644 |
| EORTC-QLQ-BR23: symptoms | |||
| Systemic therapy side effects | 14.5 ± 9.8 | 15.8 ± 13.3 | 0.715 |
| Breast symptoms | 28.0 ± 26.8 | 31.1 ± 26.4 | 0.707 |
| Arm symptoms | 26.8 ± 23.2 | 28.8 ± 26.7 | 0.789 |
| HADS: depression 0 to 7 (%) 8 to 10 (%) 11 to 21 (%) | 6.5 ± 4.0 13 (59.1) 6 (27.3) 3 (13.6) | 6.6 ± 3.1 11 (50.0) 10 (45.5) 1 (4.5) | 0.866 |
| HADS: anxiety 0 to 7 (%) 8 to 10 (%) 11 to 21 (%) | 9.4 ± 5.1 10 (45.5) 3 (13.6) 9 (40.9) | 8.5 ± 4.9 10 (45.5) 4 (18.2) 8 (36.4) | 0.567 |
| Characteristics. | Group | Baseline (N = 22) | 3 Months (N = 22) | p | * p |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Body weight (kg) | control | 73.2 ± 13.1 | 74.1 ± 13.5 | 0.223 | <0.001 |
| CDSS | 78.4 ± 14.6 | 74.8 ± 13.4 | <0.001 | ||
| BMI (kg/m2) <18.5 18.5–24.99 25–30 >30 | control | 28.3 ± 6.00 9 (40.9) 6 (27.3) 7 (31.8) | 28.6 ± 6.00 6 (27.3) 10 (45.4) 6 (27.3) | 0.262 | <0.001 |
| CDSS | 29.3 ± 5.40 5 (22.7) 7 (31.8) 10 (45.4) | 28.0 ± 4.90 7 (31.8) 8 (36.4) 7 (31.8) | <0.001 | ||
| % BFM | control | 38.3 ± 8.4 | 39.0 ± 8.1 | 0.183 | <0.001 |
| CDSS | 40.6 ± 8.0 | 37.2 ± 8.0 | <0.001 | ||
| WC (cm) | control | 96.9 ± 10.6 | 97.3 ± 10.8 | 0.579 | <0.001 |
| CDSS | 98.8 ± 12.9 | 95.7 ± 12.2 | <0.001 | ||
| METs-min/week | control | 841.5 ± 445.9 | 798.2 ± 668.8 | 0.696 | 0.001 |
| CDSS | 694.4 ± 367.8 | 1393.4 ± 895.9 | 0.001 | ||
| Current smokers | control | 6 (27.3) | 4 (18.2) | - | - |
| CDSS | 7 (31.8) | 4 (18.2) | - | ||
| Glucose (mg/dL) | control | 95.5 ± 10.3 | 104.6 ± 23.5 | 0.046 | 0.043 |
| CDSS | 94.1 ± 10.1 | 93.6 ± 7.0 | 0.745 | ||
| Cholesterol (mg/dL) | control | 189.1 ± 47.5 | 205.3 ± 48.9 | 0.045 | 0.091 |
| CDSS | 195.4 ± 33.4 | 193.1 ± 35.8 | 0.758 | ||
| HDL (mg/dL) | control | 58.4 ± 17.1 | 60.2 ± 15.1 | 0.571 | 0.114 |
| CDSS | 58.0 ± 21.3 | 69.1 ± 20.7 | 0.034 | ||
| LDL (mg/dL) | control | 115.6 ± 35.9 | 127.6 ± 37.7 | 0.037 | 0.215 |
| CDSS | 113.9 ± 27.8 | 114.5 ± 39.4 | 0.936 | ||
| Triacylglycerols (mg/dL) | control | 93.4 ± 36.3 | 112.8 ± 49.0 | 0.016 | 0.008 |
| CDSS | 95.6 ± 42.1 | 89.8 ± 36.3 | 0.276 | ||
| MDA (nmol/mL) | control | 1.5 ± 0.5 | 1.8 ± 0.6 | 0.017 | 0.007 |
| CDSS | 1.6 ± 0.5 | 1.3 ± 0.5 | 0.144 | ||
| Vitamin C (mg/L) | control | 4.1 ± 2.2 | 4.2 ± 1.4 | 0.837 | 0.021 |
| CDSS | 4.7 ± 2.1 | 6.1 ± 1.9 | <0.001 | ||
| Vitamin 1,25(OH)2D (ng/L) | control | 32.9 ± 8.9 | 30.9 ± 8.8 | 0.163 | 0.066 |
| CDSS | 31.0 ± 3.6 | 33.0 ± 7.5 | 0.224 | ||
| MedDietScore | control | 31.3 ± 3.5 | 31.9 ± 4.0 | 0.409 | 0.002 |
| CDSS | 30.6 ± 4.5 | 34.6 ± 4.3 | <0.001 | ||
| Fibers (g/day) | control | 18.7 ± 2.8 | 19.1 ± 3.1 | 0.089 | 0.003 |
| CDSS | 18.7 ±2.6 | 20.8 ± 3.5 | <0.001 | ||
| SFAs (g/day) | control | 17.3 ± 3.7 | 18.1 ± 3.8 | <0.001 | 0.001 |
| CDSS | 16.3 ± 2.7 | 14.6 ± 2.5 | 0.017 | ||
| MUFAs (g/day) | control | 30.1 ± 5.2 | 29.6 ± 5.5 | 0.628 | <0.001 |
| CDSS | 29.5 ± 4.4 | 33.3 ± 3.1 | <0.001 | ||
| Vitamin C (mg/day) | control | 281.8 ± 99.5 | 236.8 ± 67.6 | 0.005 | 0.001 |
| CDSS | 252.7 ± 57.4 | 298.2 ± 73.6 | 0.018 |
| Scales | Group | Baseline (N = 22) | 3 Months (N = 22) | p | * p |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| EORTC-QLQ-C30: functional scales | |||||
| Physical | control | 75.8 ± 20.3 | 74.8 ± 19.3 | 0.836 | 0.361 |
| CDSS | 73.9 ± 13.9 | 77.9 ± 11.5 | 0.200 | ||
| Role | control | 56.8 ± 31.6 | 56.8 ± 28.0 | 0.999 | 0.186 |
| CDSS | 53.0 ± 35.5 | 68.9 ± 21.4 | 0.047 | ||
| Emotional | control | 64.0 ± 22.8 | 70.1 ± 18.5 | 0.292 | 0.542 |
| CDSS | 64.4 ± 26.6 | 75.0 ± 12.9 | 0.037 | ||
| Cognitive | control | 74.2 ± 18.3 | 75.8 ± 21.7 | 0.808 | 0.212 |
| CDSS | 72.0 ± 31.4 | 84.8 ± 11.4 | 0.060 | ||
| Social | control | 78.8 ± 29.6 | 78.8 ± 21.3 | 0.999 | 0.830 |
| CDSS | 75.8 ± 17.6 | 77.3 ± 15.0 | 0.677 | ||
| EORTC-QLQ-C30: symptoms | |||||
| Fatigue | control | 46.5 ± 20.2 | 41.4 ± 28.1 | 0.370 | 0.662 |
| CDSS | 48.5 ± 28.4 | 39.9 ± 24.6 | 0.157 | ||
| Nausea / vomiting | control | 1.5 ± 7.1 | 2.3 ± 7.8 | 0.747 | 0.999 |
| CDSS | 1.5 ± 4.9 | 2.3 ± 5.9 | 0.329 | ||
| Pain | control | 31.1 ± 31.4 | 24.2 ± 32.0 | 0.387 | 0.802 |
| CDSS | 31.8 ± 25.7 | 22.7 ± 28.0 | 0.062 | ||
| Dyspnoea | control | 28.8 ± 25.8 | 22.7 ± 23.9 | 0.296 | 0.609 |
| CDSS | 24.2 ± 27.6 | 22.7 ± 28.0 | 0.825 | ||
| Insomnia | control | 34.8 ± 34.9 | 30.3 ± 27.0 | 0.601 | 0.357 |
| CDSS | 33.3 ± 38.5 | 18.2 ± 24.6 | 0.057 | ||
| Appetite loss | control | 10.6 ± 15.9 | 9.1 ± 15.2 | 0.329 | 0.481 |
| CDSS | 7.6 ± 14.3 | 3.0 ± 9.8 | 0.266 | ||
| Constipation | control | 12.1 ± 28.3 | 10.6 ± 21.5 | 0.747 | 0.216 |
| CDSS | 13.6 ± 24.5 | 3.0 ± 9.8 | 0.069 | ||
| Diarrhoea | control | 12.1 ± 26.3 | 6.1 ± 22.1 | 0.406 | 0.870 |
| CDSS | 12.1 ± 21.9 | 4.5 ± 11.7 | 0.203 | ||
| EORTC-QLQ-C30: Global health, QoL | control | 61.0 ± 22.6 | 67.0 ± 16.6 | 0.179 | 0.613 |
| CDSS | 62.9 ± 18.5 | 72.0 ± 11.9 | 0.035 | ||
| EORTC-QLQ-BR23: functional scales | |||||
| Body image | control | 67.0 ± 26.9 | 67.8 ± 23.6 | 0.891 | 0.745 |
| CDSS | 69.7 ± 27.6 | 68.2 ± 23.1 | 0.725 | ||
| Sexual functioning | control | 79.5 ± 24.1 | 70.5 ± 29.1 | 0.063 | 0.127 |
| CDSS | 79.5 ± 26.2 | 81.1 ± 23.2 | 0.765 | ||
| Future perspective | control | 36.4 ± 32.4 | 36.4 ± 27.0 | 0.999 | 0.137 |
| CDSS | 40.9 ± 32.4 | 56.1 ± 21.5 | 0.116 | ||
| EORTC-QLQ-BR23: symptoms | |||||
| Systemic therapy side effects | control | 14.5 ± 9.8 | 13.9 ± 13.2 | 0.846 | 0.900 |
| CDSS | 15.8 ± 13.3 | 14.7 ± 12.9 | 0.056 | ||
| Breast symptoms | control | 28.0 ± 26.8 | 25.4 ± 22.6 | 0.050 | 0.901 |
| CDSS | 31.1 ± 26.4 | 27.7 ± 20.1 | 0.568 | ||
| Arm symptoms | control | 26.8 ± 23.2 | 28.8 ± 18.4 | 0.296 | 0.395 |
| CDSS | 28.8 ± 26.7 | 26.3 ± 22.1 | 0.613 | ||
| HADS: depression 0 to 7 (%) 8 to 10 (%) 11 to 21 (%) | control | 6.5 ± 4.0 13 (59.1) 6 (27.3) 3 (13.6) | 5.5 ± 3.7 19 (86.4) 2 (9.1) 1 (4.5) | 0.338 | 0.330 |
| CDSS | 6.6 ± 3.1 11 (50.0) 10 (45.5) 1 (4.5) | 4.4 ± 3.8 16 (72.7) 4 (18.2) 2 (9.1) | 0.022 | ||
| HADS: anxiety 0 to 7 (%) 8 to 10 (%) 11 to 21 (%) | control | 9.4 ± 5.1 10 (45.5) 3 (13.6) 9 (40.9) | 7.1 ± 5.5 12 (54.5) 4 (18.2) 6 (27.3) | 0.089 | 0.848 |
| CDSS | 8.5 ± 4.9 10 (45.5) 4 (18.2) 8 (36.4) | 6.0 ± 3.6 15 (68.2) 4 (18.2) 3 (13.6) | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, P.; Gioxari, A.; Nimee, F.; Skouroliakou, M. Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial. Nutrients 2021, 13, 2115. https://doi.org/10.3390/nu13062115
Papandreou P, Gioxari A, Nimee F, Skouroliakou M. Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial. Nutrients. 2021; 13(6):2115. https://doi.org/10.3390/nu13062115
Chicago/Turabian StylePapandreou, Panos, Aristea Gioxari, Frantzeska Nimee, and Maria Skouroliakou. 2021. "Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial" Nutrients 13, no. 6: 2115. https://doi.org/10.3390/nu13062115
APA StylePapandreou, P., Gioxari, A., Nimee, F., & Skouroliakou, M. (2021). Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial. Nutrients, 13(6), 2115. https://doi.org/10.3390/nu13062115






