Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization
2.3. Study Protocol
2.4. Data Collection
2.5. Data Analysis and Sample Size Calculation
3. Results
3.1. Baseline Characteristics of Participants
3.2. Primary Endpoints
3.3. Secondary Endpoints: Clinical Characteristics Overtime
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19). JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. COVID-19 Vaccines: No Time for Complacency. Lancet 2020, 396, 1607. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A., Jr.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D Supplementation for Prevention of Mortality in Adults. Cochrane Database Syst. Rev. 2014, CD007470. [Google Scholar] [CrossRef]
- Sarhan, T.S.; Elrifai, A. Serum Level of Vitamin D as a Predictor for Severity and Outcome of Pneumonia. Clin. Nutr. 2021, 40, 2389–2393. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhou, T.; Heianza, Y.; Qi, L. Habitual Use of Vitamin D Supplements and Risk of Coronavirus Disease 2019 (COVID-19) Infection: A Prospective Study in UK Biobank. Am. J. Clin. Nutr. 2021, 113, 1275–1281. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Alguwaihes, A.M.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alzahrani, S.H.; Sabico, S.; Al-Daghri, N.M.; Jammah, A.A. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: A single-centre retrospective study. Cardiovasc. Diabetol. 2020, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Musavi, H.; Abazari, O.; Barartabar, Z.; Kalaki-Jouybari, F.; Hemmati-Dinarvand, M.; Esmaeili, P.; Mahjoub, S. The Benefits of Vitamin D in the COVID-19 Pandemic: Biochemical and Immunological Mechanisms. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Amer, O.E.; Alotaibi, N.H.; Aldisi, D.A.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Alshingetti, N.; Alomar, S.Y.; Alfawaz, H.; et al. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: A multi-centre case-control study. J. Transl. Med. 2021, 19, 166. [Google Scholar] [CrossRef]
- Waldron, J.L.; Ashby, H.L.; Cornes, M.P.; Bechervaise, J.; Razavi, C.; Thomas, O.L.; Chugh, S.; Deshpande, S.; Ford, C.; Gama, R. Vitamin D: A Negative Acute Phase Reactant. J. Clin. Pathol. 2013, 66, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Goncalves de Carvalho, C.M.; Ribeiro, S.M. Aging, low-grade systemic inflammation and vitamin D: A mini-review. Eur. J. Clin Nutr. 2017, 71, 434–440. [Google Scholar] [CrossRef]
- Bassatne, A.; Basbous, M.; Chakhtoura, M.; El Zein, O.; Rahme, M.; El-Hajj Fuleihan, G. The link between COVID-19 and Vitamin D (VIVID): A systematic review and meta-analysis. Metabolism 2021, 119, 154753. [Google Scholar] [CrossRef] [PubMed]
- Alguwaihes, A.M.; Sabico, S.; Hasanato, R.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alyusuf, E.Y.; Alzahrani, S.H.; et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: A retrospective case-control study in an Arab Gulf country. Aging Clin. Exp. Res. 2021, 33, 1415–1422. [Google Scholar] [CrossRef]
- Ministry of Health. Saudi Arabia COVID-19 Dashboard. Available online: https://covid19.moh.gov.sa (accessed on 8 December 2020).
- Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19 (Version 2.1 July 2020). Available online: https://www.moh.gov.sa/en/Ministry/MediaCenter/Publications/Pages/covid19.aspx (accessed on 9 December 2020).
- Saudi MoH Hospital Admission Criteria for COVID-19 Patients (May 2020). Available online: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/admission-criteria.pdf (accessed on 5 March 2021).
- Saudi Clinical Trials Registry. Available online: https://old.sfda.gov.sa/en/drug/clinical_trials/pages/registered-clinical.aspx (accessed on 24 May 2021).
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef] [Green Version]
- VDSCP Vitamin D Certified Assays. Certifications from 2020 (Updated September 2020). Available online: https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures-508.pdf (accessed on 8 December 2020).
- Al-Saleh, Y.; Sulimani, R.; Sabico, S.; Raef, H.; Fouda, M.; Alshahrani, F.; Al Shaker, M.; Al Wahabi, B.; Sadat-Ali, M.; Al Rayes, H.; et al. 2015 Guidelines for Osteoporosis in Saudi Arabia: Recommendations from the Saudi Osteoporosis Society. Ann. Saudi Med. 2015, 35, 1–12. [Google Scholar] [CrossRef]
- Al Saleh, Y.; Beshyah, S.A.; Hussein, W.; Almadani, A.; Hassoun, A.; Al Mamari, A.; Ba-Essa, E.; Al-Dhafiri, E.; Hassanein, M.; Fouda, M.A.; et al. Diagnosis and management of vitamin D deficiency in the Gulf Cooperative Council (GCC) countries: An expert consensus summary statement from the GCC vitamin D advisory board. Arch. Osteoporos. 2020, 15, 35. [Google Scholar] [CrossRef]
- Simpson, S.; van der Mei, I.; Stewart, N.; Blizzard, L.; Tettey, P.; Taylor, B. Weekly cholecalciferol supplementation results in significant reductions in infection risk among the vitamin D deficient: Results from the CIPRIS pilot RCT. BMC Nutr. 2015, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcala Diaz, J.F.; Lopez Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid. Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Swalih, M.; Patel, P.; Smith, M.A.; Perrin, R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am. J. Ther. 2020, 27, e485–e490. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubee, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Bjorkhem-Bergman, L. Vitamin D supplementation improves well-being in patients with frequent respiratory tract infections: A post hoc analysis of a randomized, placebo-controlled trial. BMC Res. Notes 2015, 8, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minakshi, R.; Padhan, K.; Rani, M.; Khan, N.; Ahmad, F.; Jameel, S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS ONE 2009, 4, e8342. [Google Scholar] [CrossRef]
- Jakovac, H. COVID-19 and vitamin D-Is there a link and an opportunity for intervention? Am. J. Physiol. Endocrinol. Metab. 2020, 318, e589. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Chen, X.; Geiger, J.D. Role of Endolysosomes in Severe Acute Respiratory Syndrome Coronavirus-2 Infection and Coronavirus Disease 2019 Pathogenesis: Implications for Potential Treatments. Front. Pharmacol. 2020, 11, 595888. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.D.; Khan, N.; Murugan, M.; Boison, D. Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities. Front. Pharmacol. 2020, 11, 594487. [Google Scholar] [CrossRef]
- Rawson, N.E.; Huang, L. Symposium overview: Impact of oronasal inflammation on taste and smell. Ann. N. Y. Acad. Sci. 2009, 1170, 581–584. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Brand, J.; Huang, L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. N. Y. Acad. Sci. 2009, 1170, 596–603. [Google Scholar] [CrossRef]
- Do, J.E.; Kwon, S.Y.; Park, S.; Lee, E.S. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet’s disease. Rheumatology 2008, 47, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Ojaimi, S.; Skinner, N.A.; Strauss, B.J.; Sundararajan, V.; Woolley, I.; Visvanathan, K. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: A pilot study. J. Transl. Med. 2013, 11, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Jiang, X.; Ji, R. Role of neurotrophin in the taste system following gustatory nerve injury. Metab. Brain. Dis. 2015, 30, 605–613. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2021, 40, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Sasdelli, A.S.; Ravaioli, F.; Baracco, B.; Battaiola, C.; Bocedi, G.; Brodosi, L.; Leoni, L.; Mari, G.A.; Musio, A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin. Nutr. 2021, 40, 1330–1337. [Google Scholar] [CrossRef]
- Zhou, Y.; Frey, T.K.; Yang, J.J. Viral calciomics: Interplays between Ca2+ and virus. Cell Calcium 2009, 46, 1–17. [Google Scholar] [CrossRef]
- Di Filippo, L.; Doga, M.; Frara, S.; Giustina, A. Hypocalcemia in COVID-19: Prevalence, clinical significance and therapeutic implications. Rev. Endocr. Metab. Disord. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Martha, J.W.; Wibowo, A.; Pranata, R. Hypocalcemia is associated with severe COVID-19: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; Frara, S.; Giustina, A. The emerging osteo-metabolic phenotype of COVID-19: Clinical and pathophysiological aspects. Nat. Rev. Endocrinol. 2021, 1–2. [Google Scholar] [CrossRef]


| Parameters | All | 1000 IU | 5000 IU | p-Value |
|---|---|---|---|---|
| n | 69 | 33 | 36 | |
| Anthropometrics/Vital Signs | ||||
| Age | 49.8 ± 14.3 | 53.5 ± 12.3 | 46.3 ± 15.2 | 0.03 |
| BMI | 30.7 ± 7.8 | 32.0 ± 6.5 | 28.2 ± 7.1 | 0.02 |
| Male/Female | 34/35 | 13/20 | 21/15 | 0.12 |
| WHR | 0.91 ± 0.11 | 0.91 ± 0.11 | 0.90 ± 0.14 | 0.45 |
| Systolic BP (mmHg) | 128.2 ± 17.2 | 128.3 ± 20.7 | 128.1 ± 13.4 | 0.96 |
| Diastolic BP (mmHg) | 74.0 ± 13.7 | 72.8 ± 16.5 | 75.1 ± 10.6 | 0.47 |
| Temperature (°C) | 37.5 ± 0.9 | 37.3 ± 0.9 | 37.7 ± 0.9 | 0.06 |
| Pulse Rate | 93.9 ± 17.2 | 93.2 ± 17.4 | 94.5 ± 17.4 | 0.76 |
| Respiratory Rate | 23.9 ± 4.7 | 24.7 ± 5.0 | 23.2 ± 4.2 | 0.19 |
| Medical History (%) | ||||
| Hypertension | 38 (5) | 18 (54) | 20 (56) | 0.61 |
| T2DM | 35 (51) | 17 (52) | 18 (50) | 0.76 |
| Obesity | 23 (33) | 12 (36) | 11 (31) | 0.54 |
| Hyperlipidaemia | 9 (13) | 4 (12) | 5 (14) | 1.0 |
| CKD | 5 (7) | 4 (12) | 1 (3) | 0.19 |
| Cardiovascular Disease | 4 (6) | 3 (9) | 1 (3) | 0.34 |
| Asthma | 3 (4) | 2 (6) | 1 (3) | 0.60 |
| Rheumatoid | 2 (3) | 1 (3) | 1 (3) | 1.0 |
| Thyroid | 2 (3) | 1 (3) | 1 (3) | 1.0 |
| Epilepsy | 1 (1) | 1 (3) | -- | 1.0 |
| Supplements (%) | ||||
| Vitamin C | 34 (47) | 14 (40) | 20 (53) | 0.28 |
| Symptoms (%) | ||||
| Fever | 56 (77) | 24 (69) | 32 (84) | 0.18 |
| Dyspnea | 52 (71) | 26 (74) | 26 (68) | 0.58 |
| Fatigue | 43 (59) | 22 (63) | 21 (55) | 0.51 |
| Cough | 37 (51) | 21 (60) | 16 (42) | 0.28 |
| Headache | 33 (45) | 13 (37) | 20 (53) | 0.17 |
| Joint pain | 24 (33) | 12 (34) | 12 (32) | 0.85 |
| Nausea | 18 (25) | 9 (26) | 9 (24) | 0.31 |
| Diarrhea | 16 (22) | 8 (23) | 8 (21) | 0.17 |
| Sore throat | 14 (19) | 5 (14) | 9 (24) | 0.17 |
| Vomiting | 14 (19) | 8 (23) | 6 (16) | 0.42 |
| Outcomes (N) | ||||
| ICU Admission | 5 | 3 | 2 | 1.0 |
| Mortality | 1 | -- | 1 | -- |
| Days to Discharge | 7 (5–9) | 7 (0–10) | 6 (5–8) | 0.14 |
| Symptoms | 1000 IU | 5000 IU | p-Value |
|---|---|---|---|
| Fever | 9.9 ± 1.7 | 8.5 ± 0.9 | 0.97 |
| Dyspnea | 11.2 ± 1.6 | 8.9 ± 1.1 | 0.24 |
| Fatigue | 8.9 ± 0.5 | 7.7 ± 0.8 | 0.27 |
| Cough | 9.1 ± 0.8 | 6.2 ± 0.8 | 0.007 |
| Headache | 10.6 ± 0.9 | 8.7 ± 0.8 | 0.24 |
| GI symptoms | 9.7 ± 1.2 | 7.6 ± 0.7 | 0.89 |
| Sore throat | 9.5 ± 0.6 | 12.5 ± 0.7 | 0.15 |
| Body Aches | 9.2 ± 0.9 | 9.6 ± 0.9 | 0.68 |
| Chills | 17.6 ± 1.2 | 11.2 ± 1.1 | 0.14 |
| Anosmia | 16.3 ± 1.7 | 11.2 ± 1.1 | 0.14 |
| Ageusia | 16.9 ± 1.7 | 11.4 ± 1.0 | 0.035 |
| Parameters | 1000 IU (n = 33) | 5000 IU (n = 36) | Between Group p-Value | ||||
|---|---|---|---|---|---|---|---|
| Pre- | Post | p-Value | Pre- | Post | p-Value | ||
| Anthropometrics | |||||||
| BMI (kg/m2) | 32.0 ± 6.5 | 31.6 ± 6.0 | 0.04 | 28.2 ± 7.1 | 27.9 ± 5.4 | 0.049 | 0.08 |
| WHR | 0.91 ± 0.11 | 0.91 ± 0.1 | 0.84 | 0.9 ± 0.14 | 0.9 ± 0.1 | 0.65 | 0.73 |
| Complete Blood Count | |||||||
| Hemoglobin (g/L) | 12.7 ± 1.8 | 13.2 ± 2.2 | 0.17 | 13.0 ± 2.8 | 13.4 ± 2.4 | 0.03 | 0.88 |
| Hematocrit (%) | 38.5 ± 5.5 | 40.2 ± 7.2 | 0.04 | 40.3 ± 5.7 | 40.5 ± 6.4 | 0.66 | 0.51 |
| RBC count | 4.6 ± 0.6 | 4.8 ± 0.9 | 0.18 | 4.8 ± 0.5 | 4.8 ± 0.7 | 0.53 | 0.43 |
| WBC count # | 8.5 ± 1.0 | 9.4 ± 0.9 | 0.03 | 6.9 ± 0.4 | 9.5 ± 0.8 | 0.001 | 0.74 |
| Platelet count # | 269 ± 29 | 403 ± 24 | <0.001 | 241 ± 16 | 380 ± 27 | <0.001 | 0.53 |
| Lymphocyte # | 1.0 ± 0.1 | 1.7 ± 0.2 | 0.03 | 2.4 ± 1.1 | 1.5 ± 0.2 | 0.95 | 0.37 |
| Monocyte # | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.01 | 0.4 ± 0.0 | 0.6 ± 0.1 | <0.001 | 0.37 |
| Eosinophil # | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.85 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.35 | 0.30 |
| Neutrophil # | 6.2 ± 0.7 | 6.3 ± 0.5 | 0.56 | 5.3 ± 0.5 | 7.1 ± 0.8 | 0.03 | 0.80 |
| Prothrombin Time | 13.6 ± 1.6 | 13.0 ± 1.3 | 0.05 | 13.1 ± 1.3 | 12.9 ± 1.7 | 0.79 | 0.76 |
| APTT | 32.7 ± 4.8 | 33.8 ± 7.9 | 0.85 | 31.9 ± 4.7 | 33.8 ± 11.4 | 0.24 | 0.74 |
| INR | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.06 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.80 | 0.78 |
| Bicarbonate (mEq/L) | 20.8 ± 3.6 | 22.5 ± 3.1 | 0.36 | 21.8 ± 2.7 | 21.9 ± 6.1 | 0.51 | 0.79 |
| Liver Profile | |||||||
| Bilirubin # | 7.1 ± 1.2 | 6.2 ± 0.7 | 0.65 | 9.1 ± 1.2 | 8.8 ± 0.7 | 0.86 | 0.06 |
| Bilirubin (direct) # | 4.1 ± 0.4 | 3.9 ± 0.5 | 0.55 | 5.3 ± 0.6 | 4.5 ± 0.3 | 0.10 | 0.12 |
| ALP (U/L) # | 97.5 ± 16.2 | 85.9 ± 13.5 | 0.22 | 88.5 ± 11.0 | 106.4 ± 18.6 | 0.48 | 0.67 |
| ALT (U/L) # | 62.1 ± 17.6 | 84.7 ± 20.8 | 0.02 | 65.3 ± 14.6 | 114.9 ± 33.5 | 0.002 | 0.73 |
| LDH (U/L) # | 564 ± 56 | 484 ± 40 | 0.32 | 487 ± 36 | 410 ± 28 | 0.16 | 0.32 |
| Renal Profile | |||||||
| Creatinine (µmol/L) | 71.6 ± 16.2 | 70.9 ± 12.1 | 0.68 | 67.0 ± 19.1 | 66.8 ± 6.3 | 0.50 | 0.46 |
| Urea (mg/dl) # | 9.1 ± 1.8 | 8.6 ± 1.7 | 0.89 | 5.1 ± 0.5 | 8.0 ± 1.6 | <0.001 | 0.14 |
| Lipid Profile | |||||||
| Triglycerides (mmol/L) # | 1.5 ± 0.1 | 2.0 ± 0.2 | 0.48 | 1.4 ± 0.1 | 2.0 ± 0.2 | 0.36 | 0.52 |
| Total Cholesterol (mmol/L) | 4.0 ± 1.4 | 4.4 ± 1.4 | 0.86 | 4.0 ± 0.9 | 4.5 ± 1.4 | 0.97 | 0.75 |
| HDL-Cholesterol (mmol/L) | 1.0 ± 0.2 | 1.1 ± 0.4 | 0.39 | 1.0 ± 0.3 | 1.1 ± 0.4 | 0.52 | 0.48 |
| LDL-Cholesterol (mmol/L) | 2.4 ± 1.2 | 2.4 ± 1.1 | 0.30 | 2.3 ± 0.8 | 2.4 ± 1.1 | 0.81 | 0.58 |
| Inflammatory Markers | |||||||
| D-Dimer (µg/mL) # | 3.4 ± 2.0 | 1.9 ± 0.5 | 0.26 | 0.6 ± 0.1 | 1.3 ± 0.6 | 0.08 | 0.02 |
| Ferritin (µg/mL) # | 784 ± 112 | 526 ± 76 | 0.004 | 733 ± 153 | 519 ± 96 | 0.19 | 0.69 |
| CRP (mg/L) # | 47.9 ± 6.8 | 33.1 ± 7.1 | 0.10 | 33.7 ± 5.7 | 34.2 ± 6.4 | 0.58 | 0.25 |
| IL-6 (pg/mL) # | 23.9 ± 5.9 | 19.2 ± 5.6 | 0.03 | 18.6 ± 4.6 | 10.5 ± 2.9 | 0.01 | 0.83 |
| Glycemic Profile | |||||||
| Fasting Glucose (mmol/L) # | 10.3 ± 1.1 | 11.2 ± 1.2 | 0.38 | 10.4 ± 1.1 | 11.4 ± 1.0 | 0.13 | 0.91 |
| Vitamin D | |||||||
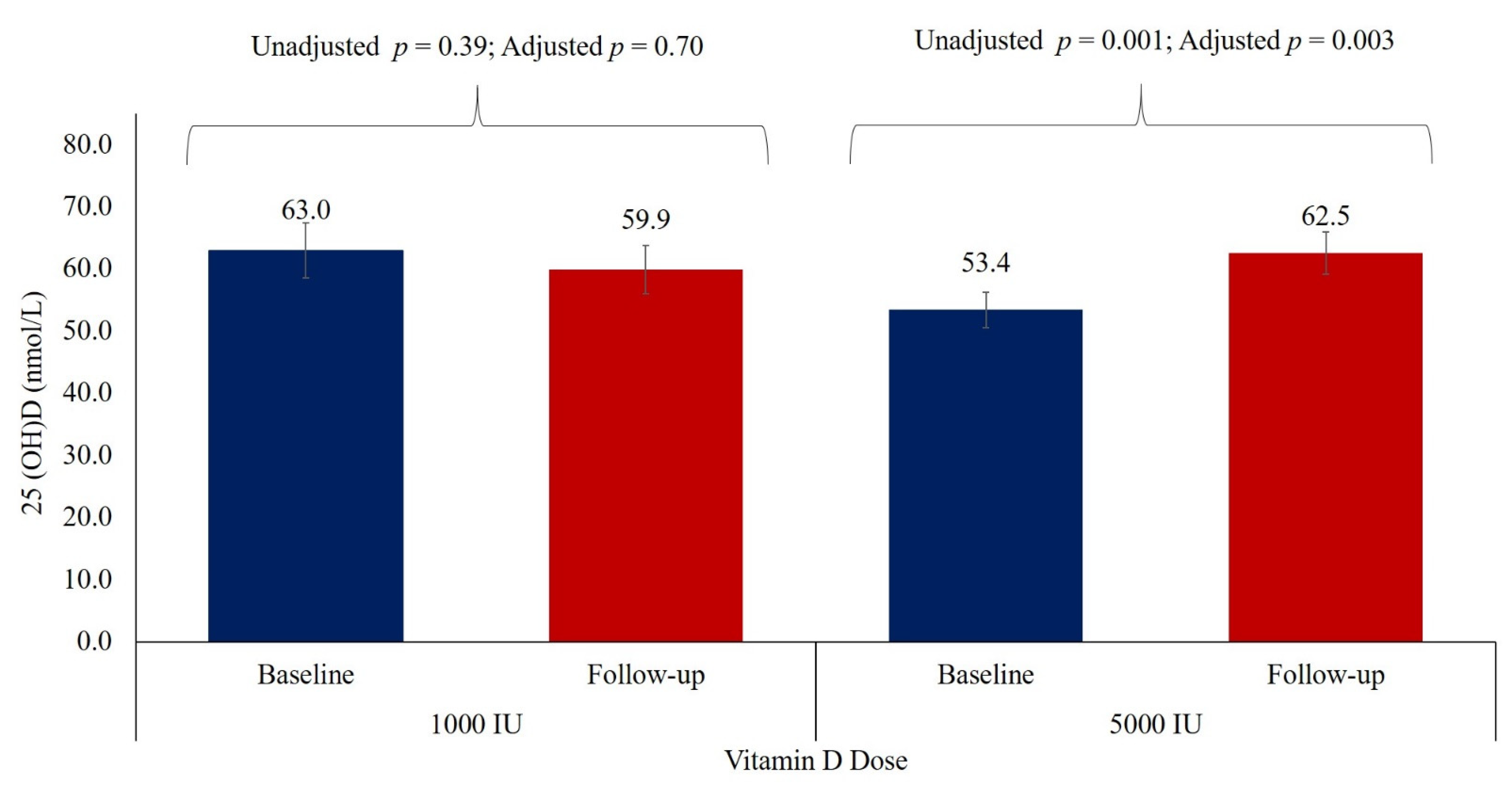
| 25(OH)D (nmol/L) (75–250) # | 63.0 ± 2.5 | 59.9 ± 3.9 | 0.66 | 53.4 ± 2.9 | 62.5 ± 3.4 | 0.001 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. https://doi.org/10.3390/nu13072170
Sabico S, Enani MA, Sheshah E, Aljohani NJ, Aldisi DA, Alotaibi NH, Alshingetti N, Alomar SY, Alnaami AM, Amer OE, et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients. 2021; 13(7):2170. https://doi.org/10.3390/nu13072170
Chicago/Turabian StyleSabico, Shaun, Mushira A. Enani, Eman Sheshah, Naji J. Aljohani, Dara A. Aldisi, Naif H. Alotaibi, Naemah Alshingetti, Suliman Y. Alomar, Abdullah M. Alnaami, Osama E. Amer, and et al. 2021. "Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial" Nutrients 13, no. 7: 2170. https://doi.org/10.3390/nu13072170
APA StyleSabico, S., Enani, M. A., Sheshah, E., Aljohani, N. J., Aldisi, D. A., Alotaibi, N. H., Alshingetti, N., Alomar, S. Y., Alnaami, A. M., Amer, O. E., Hussain, S. D., & Al-Daghri, N. M. (2021). Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients, 13(7), 2170. https://doi.org/10.3390/nu13072170







