AB-Kefir Reduced Body Weight and Ameliorated Inflammation in Adipose Tissue of Obese Mice Fed a High-Fat Diet, but Not a High-Sucrose Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotic Strains
2.2. Experimental Design of the Animal Model
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Fecal Short Chain Fatty Acid (SCFA) Levels
2.5. Histological Staining
2.6. Real-Time Polymerase Chain Reaction (qPCR) for mRNA Quantitation
2.7. 16S rRNA Sequencing
2.8. Statistical Analyses
3. Results
3.1. AB-Kefir Ameliorated Body Weight Gain in HFD-Fed, but Not in WD-Fed, Mice
3.2. AB-Kefir Improved Glucose Intolerance in HFD-Fed, but Not in WD-Fed, Mice
3.3. AB-Kefir Ameliorated Hepatic Steatosis in HFD-Fed, but Not in WD-Fed, Mice
3.4. AB-Kefir Attenuated Adipocyte Hypertrophy and Regulated the Expression of Genes Related to Lipid Metabolism and Inflammation in the WAT of HFD-Fed Mice
3.5. Administration of AB-Kefir Resulted in a Change in SCFA Production in WD-Fed Mice but Not in HFD-Fed Mice
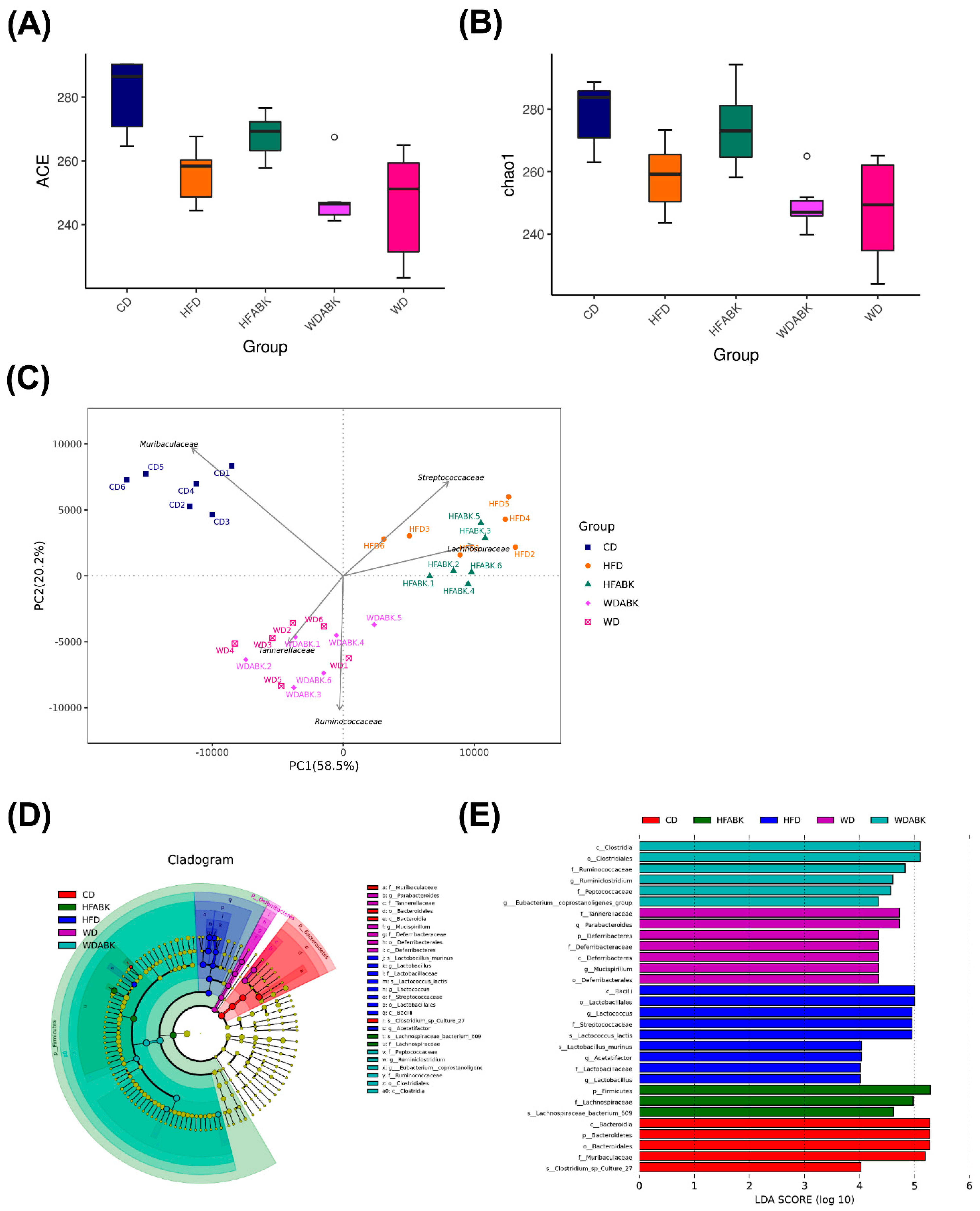
3.6. AB-Kefir Impacted the Gut Microbiota Composition in HFD- and WD-Induced Obese Mice
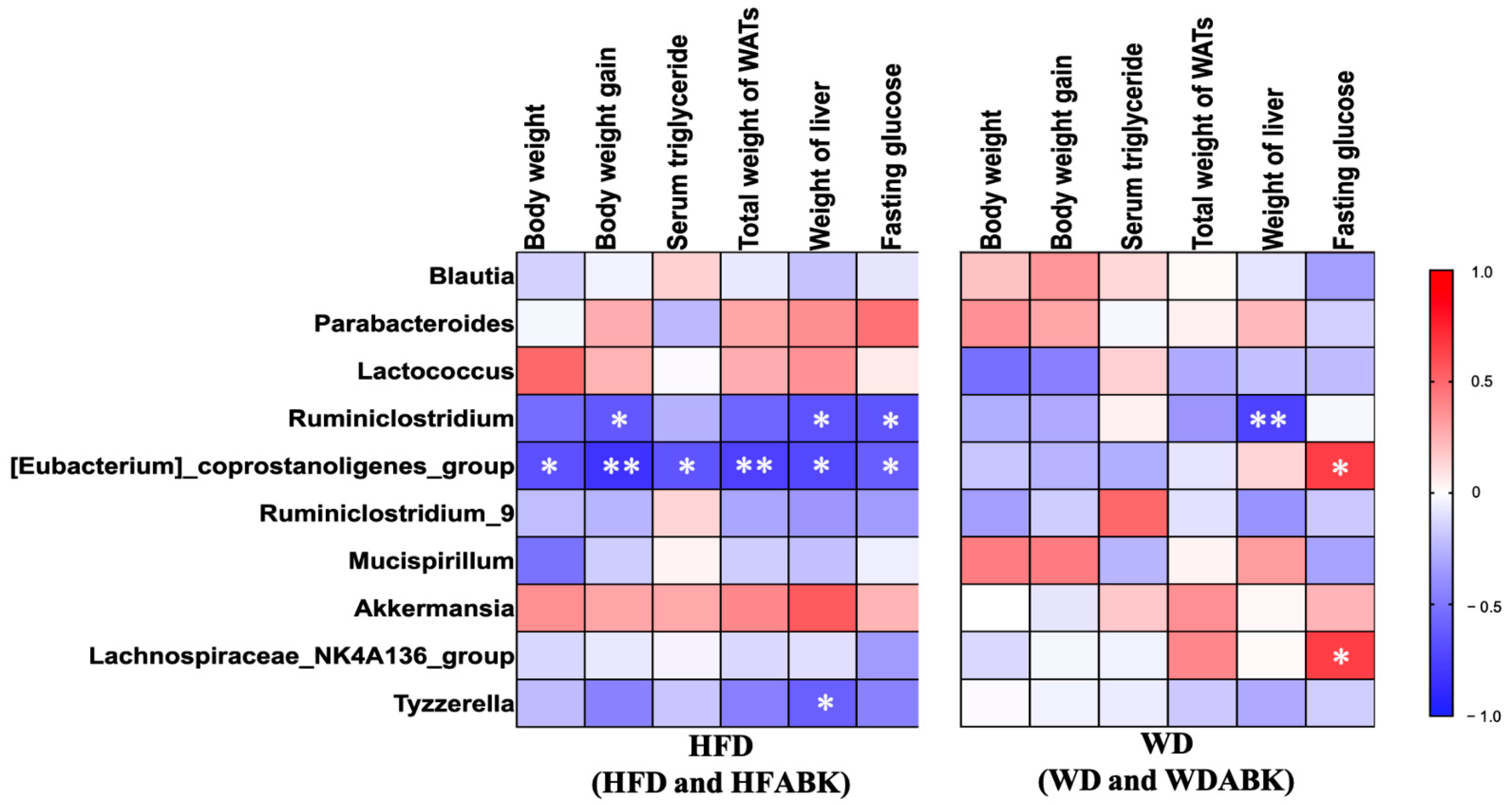
3.7. Correlation between Gut Microbiota and Characteristics of Obesity Affected by AB-Kefir Administration in HFD- and WD-Fed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, A.; San Mauro, M.; Sanchez, A.; Torres, J.M.; Marquina, D. The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst. Appl. Microbiol. 2003, 26, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Diniz, R.O.; Garla, L.K.; Schneedorf, J.M.; Carvalho, J.C.T. Study of anti-inflammatory activity of Tibetan mushroom, a symbiotic culture of bacteria and fungi encapsulated into a polysaccharide matrix. Pharmacol. Res. 2003, 47, 49–52. [Google Scholar] [CrossRef]
- Liu, J.-R.; Wang, S.-Y.; Lin, Y.-Y.; Lin, C.-W. Antitumor activity of milk kefir and soy milk kefir in tumor-bearing mice. Nutr. Cancer 2002, 44, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Clancy, S.M. Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. J. Am. Diet. Assoc. 2003, 103, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Tamai, Y.; Yoshimitsu, N.; Watanabe, Y.; Kuwabara, Y.; Nagai, S. Effects of milk fermented by culturing with various lactic acid bacteria and a yeast on serum cholesterol level in rats. J. Ferment. Bioeng. 1996, 81, 181–182. [Google Scholar] [CrossRef]
- Chen, H.-L.; Tung, Y.-T.; Tsai, C.-L.; Lai, C.-W.; Lai, Z.-L.; Tsai, H.-C.; Lin, Y.-L.; Wang, C.-H.; Chen, C.-M. Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. Int. J. Obes. 2014, 38, 1172–1179. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, H.; Jeong, D.; Kang, I.-B.; Chon, J.-W.; Kim, H.-S.; Song, K.-Y.; Seo, K.-H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Ju, T.; Fouhse, J.M.; Forgie, A.J.; Sergi, C.; Cotter, P.D.; Willing, B.P. Kefir microbial composition is a deciding factor in the physiological impact of kefir in a mouse model of obesity. Br. J. Nutr. 2021, 125, 129–138. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Huang, W.-C.; Lin, J.-S.; Chen, Y.-M.; Ho, S.-T.; Huang, C.-C.; Tung, Y.-T. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.-C.; Zaydi, A.I.; Lin, W.-H.; Lin, J.-S.; Liong, M.-T.; Wu, J.-J. Putative probiotic strains isolated from kefir improve gastrointestinal health parameters in adults: A randomized, single-blind, placebo-controlled study. Probiotics Antimicrob. Proteins 2020, 12, 840–850. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Davies, M.J.; Prewitt, R.L.; Lauterio, T. Development of hypertension in a rat model of diet-induced obesity. Hypertension 2000, 35, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Ahrén, B.; Scheurink, A.J. Marked hyperleptinemia after high-fat diet associated with severe glucose intolerance in mice. Eur. J. Endocrinol. 1998, 139, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Brenner, R.R.; Rimoldi, O.J.; Lombardo, Y.B.; González, M.S.; Bernasconi, A.M.; Chicco, A.; Basabe, J.C. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids 2003, 38, 733–742. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Chronic Intake of High-Fat and High-Sucrose Diets Differentially Affects Glucose Intolerance in Mice. J. Nutr. 2006, 136, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.-H.; Miyahara, H.; Takeo, J.; Katayama, M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol. Metab. Syndr. 2012, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, F. The host-microbe interface within the gut. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 915–931. [Google Scholar] [CrossRef]
- Shan, K.; Qu, H.; Zhou, K.; Wang, L.; Zhu, C.; Chen, H.; Gu, Z.; Cui, J.; Fu, G.; Li, J.; et al. Distinct gut microbiota induced by different fat-to-sugar-ratio high-energy diets share similar pro-obesity genetic and metabolite profiles in prediabetic mice. mSystems 2019, 4, e00219-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.Y. Lifestyle modifications result in alterations in the gut microbiota in obese children. BMC Microbiol. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Yang, N.-S.; Lin, Y.-C.; Ho, S.-T.; Li, K.-Y.; Lin, J.-S.; Liu, J.-R.; Chen, M.-J. A combination of Lactobacillus mali APS1 and dieting improved the efficacy of obesity treatment via manipulating gut microbiome in mice. Sci. Rep. 2018, 8, 6153. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes. Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Mulder, P.; Morrison, M.C.; Wielinga, P.Y.; van Duyvenvoorde, W.; Kooistra, T.; Kleemann, R. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int. J. Obes. 2016, 40, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wu, W.; Li, X.; Xin, X.; Liu, D. Daily supplementation with fresh angelica keiskei juice alleviates high-fat diet-induced obesity in mice by modulating gut microbiota composition. Mol. Nutr. Food Res. 2019, 63, 1900248. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.; Salbaum, J.M.; Luo, M.; Blanchard, E.E.; Berthoud, H. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruin, J.E.; Saber, N.; Braun, N.; Fox, J.K.; Mojibian, M.; Asadi, A.; Drohan, C.; O’Dwyer, S.; Rosman-Balzer, D.S.; Swiss, V.A.; et al. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem. Cell Rep. 2015, 4, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Le Barz, M.; Daniel, N.; Varin, T.V.; Naimi, S.; Demers-Mathieu, V.; Pilon, G.; Audy, J.; Laurin, É.; Roy, D.; Urdaci, M.C.; et al. In vivo screening of multiple bacterial strains identifies Lactobacillus rhamnosus Lb102 and Bifidobacterium animalis ssp. lactis Bf141 as probiotics that improve metabolic disorders in a mouse model of obesity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 4921–4935. [Google Scholar] [CrossRef]
- Kang, J.-H.; Yun, S.-I.; Park, H.-O. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J. Microbiol. 2010, 48, 712–714. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-H.; Shin, N.R.; Lim, S.-K.; Im, U.; Song, E.-J.; Nam, Y.-D.; Kim, H. Diet control more intensively disturbs gut microbiota than genetic background in wild type and ob/ob Mice. Front. Microbiol. 2019, 10, 1292. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Guimarães, V.H.D.; de Farias Lelis, D.; Oliveira, L.P.; Borém, L.M.A.; Guimarães, F.A.D.; Farias, L.C.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Comparative study of dietary fat: Lard and sugar as a better obesity and metabolic syndrome mice model. Arch. Physiol. Biochem. 2020, 1–11. [Google Scholar] [CrossRef]
- Kübeck, R.; Bonet-Ripoll, C.; Hoffmann, C.; Walker, A.; Müller, V.M.; Schüppel, V.L.; Lagkouvardos, I.; Scholz, B.; Engel, K.-H.; Daniel, H.; et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol. Metab. 2016, 5, 1162–1174. [Google Scholar] [CrossRef]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Volat, F.; Sandhow, L.; Galitzky, J.; Nguyen, T.; Esteve, D.; Åström, G.; Mejhert, N.; Ledoux, S.; Thalamas, C.; et al. CD36 is a marker of human adipocyte progenitors with pronounced adipogenic and triglyceride accumulation potential. Stem. Cells 2017, 35, 1799–1814. [Google Scholar] [CrossRef] [Green Version]
- Liss, K.H.H.; Lutkewitte, A.J.; Pietka, T.; Finck, B.N.; Franczyk, M.; Yoshino, J.; Klein, S.; Hall, A.M. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J. Lipid Res. 2018, 59, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.R.; Kim, H.J.; Xu, X.; Ferrante, A.W.J. Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis during metabolic stresses. Obesity 2016, 24, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, S.; Mepani, R.J.; Laznik, D.; Ye, L.; Jurczak, M.J.; Jornayvaz, F.R.; Estall, J.L.; Chatterjee Bhowmick, D.; Shulman, G.I.; Spiegelman, B.M. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 9635. [Google Scholar] [CrossRef] [Green Version]
- Hajri, T.; Hall, A.M.; Jensen, D.R.; Pietka, T.A.; Drover, V.A.; Tao, H.; Eckel, R.; Abumrad, N.A. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 2007, 56, 1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.S.; Okamoto, K.; Kim, Y.-S.; Takeda, Y.; Bortner, C.D.; Dang, H.; Wada, T.; Xie, W.; Yang, X.-P.; Liao, G.; et al. Nuclear orphan receptor tak1/tr4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance. Diabetes 2011, 60, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, J.; Mori, M.A.; Lee, K.Y.; Smyth, G.; Liew, C.W.; Macotela, Y.; Rourk, M.; Bluher, M.; Russell, S.J.; Kahn, C.R. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat. Commun. 2012, 3, 902. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J.; Healy, S.; O’Toole, P.W.; Murphy, E.F.; Cotter, P.D. The probiotic L. casei LC-XCALTM improves metabolic health in a diet-induced obesity mouse model without altering the microbiome. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef] [PubMed]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef] [Green Version]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Li, L.; Batt, S.M.; Wannemuehler, M.; Dispirito, A.; Beitz, D.C. Effect of feeding of a cholesterol-reducing bacterium, Eubacterium coprostanoligenes, to germ-free mice. Lab. Anim. Sci. 1999, 48, 253–255. [Google Scholar]
- Hu, S.; Du, M.; Su, L.; Yang, H. Phosphatidylserine from portunus trituberculatus eggs alleviates insulin resistance and alters the gut microbiota in high-fat-diet-fed mice. Mar. Drugs 2020, 18, 483. [Google Scholar] [CrossRef]
- Chen, L.; van den Munckhof, I.C.L.; Schraa, K.; Ter Horst, R.; Koehorst, M.; van Faassen, M.; van der Ley, C.; Doestzada, M.; Zhernakova, D.V.; Kurilshikov, A.; et al. Genetic and microbial associations to plasma and fecal bile acids in obesity relate to plasma lipids and liver fat content. Cell Rep. 2020, 33, 108212. [Google Scholar] [CrossRef]
- Freier, T.A.; Beitz, D.C.; Li, L.; Hartman, P.A. Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int. J. Syst. Bacteriol. 1994, 44, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamura, R.; Nakamura, K.; Kitada, N.; Aizawa, T.; Shimizu, Y.; Nakamura, K.; Ayabe, T.; Kimura, T.; Tamakoshi, A. Associatins of gut microbiota, dietary intake, and serum short-chain fatty acids with fecal short-chain fatty acids. Biosci. Microbiota Food Health 2020, 39, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Hsu, A.-H.; Chiou, S.-Y.; Lin, Y.-C.; Lin, J.-S. AB-Kefir Reduced Body Weight and Ameliorated Inflammation in Adipose Tissue of Obese Mice Fed a High-Fat Diet, but Not a High-Sucrose Diet. Nutrients 2021, 13, 2182. https://doi.org/10.3390/nu13072182
Chen Y-T, Hsu A-H, Chiou S-Y, Lin Y-C, Lin J-S. AB-Kefir Reduced Body Weight and Ameliorated Inflammation in Adipose Tissue of Obese Mice Fed a High-Fat Diet, but Not a High-Sucrose Diet. Nutrients. 2021; 13(7):2182. https://doi.org/10.3390/nu13072182
Chicago/Turabian StyleChen, Yung-Tsung, Ai-Hua Hsu, Shiou-Yun Chiou, Yu-Chun Lin, and Jin-Seng Lin. 2021. "AB-Kefir Reduced Body Weight and Ameliorated Inflammation in Adipose Tissue of Obese Mice Fed a High-Fat Diet, but Not a High-Sucrose Diet" Nutrients 13, no. 7: 2182. https://doi.org/10.3390/nu13072182





