Up-Regulation of Specific Bioactive Lipids in Celiac Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical and Sociodemographics
2.3. Anthropometric Measures
2.4. Blood Samples
2.5. Untargeted Metabolomics
2.5.1. Chemicals
2.5.2. Sample Processing
2.5.3. Analysis Conditions
2.6. Data Analyses
3. Results
3.1. Clinical Traits
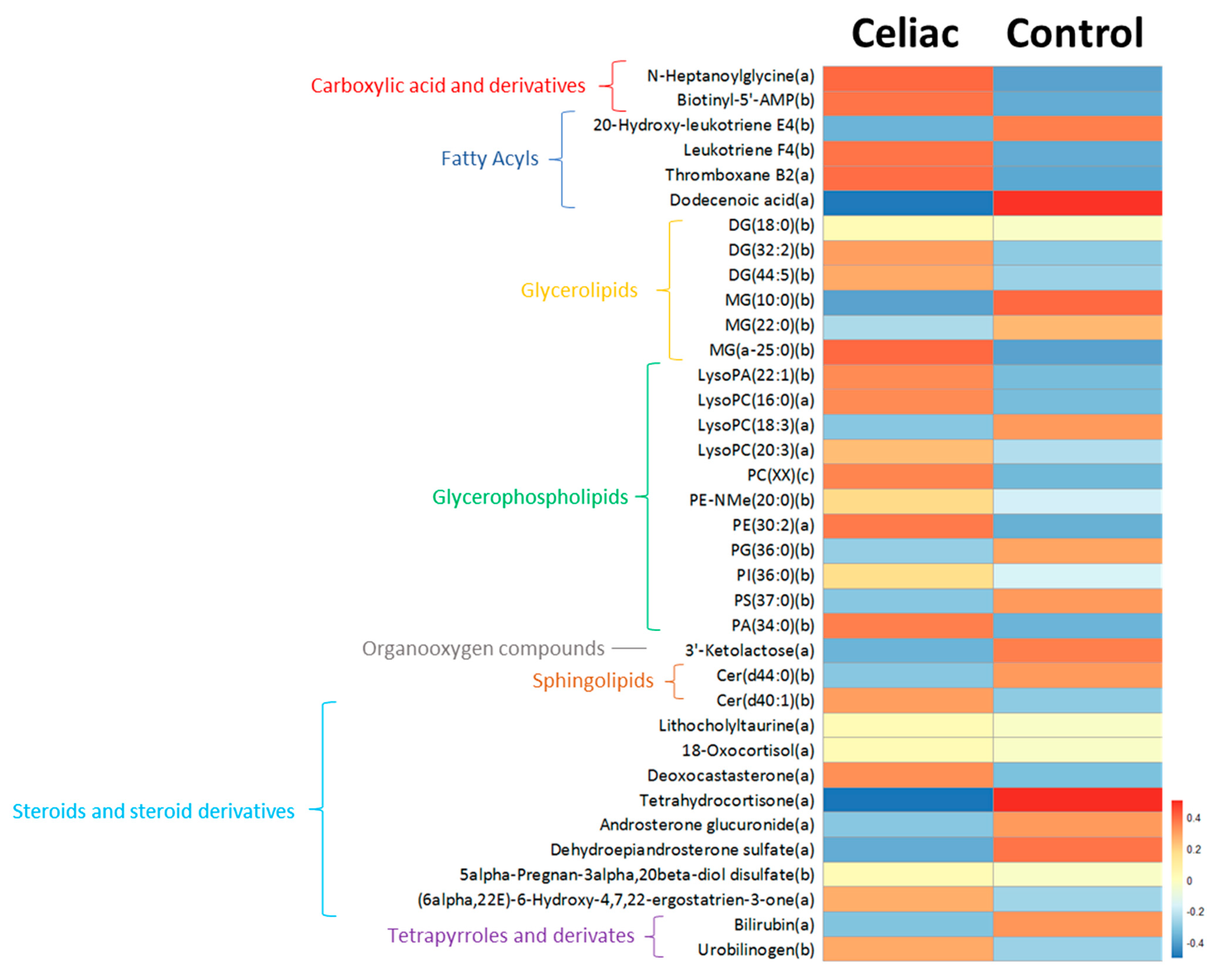
3.2. Untargeted Metabolomics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrell, R.J.; Kelly, C.P. Celiac Sprue. N. Engl. J. Med. 2002, 346, 180–188. [Google Scholar] [CrossRef]
- Green, P.H.; Jabri, B. Coeliac disease. Lancet 2003, 362, 383–391. [Google Scholar] [CrossRef]
- Schuppan, D. Current concepts of celiac disease pathogenesis. Gastroenterology 2000, 119, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kagnoff, M.F. Celiac disease: Pathogenesis of a model immunogenetic disease. J. Clin. Investig. 2007, 117, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrao, G.; Corazza, G.R.; Bagnardi, V.; Brusco, G.; Ciacci, C.; Cottone, M.; Guidetti, C.S.; Usai, P.; Cesari, P.; Pelli, M.A.; et al. Mortality in patients with coeliac disease and their relatives: A cohort study. Lancet 2001, 358, 356–361. [Google Scholar] [CrossRef]
- Mäki, M.; Mustalahti, K.; Kokkonen, J.; Kulmala, P.; Haapalahti, M.; Karttunen, T.; Ilonen, J.; Laurila, K.; Dahlbom, I.; Hansson, T.; et al. Prevalence of Celiac Disease among Children in Finland. N. Engl. J. Med. 2003, 348, 2517–2524. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A.; Catassi, C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology 2001, 120, 636–651. [Google Scholar] [CrossRef]
- Kivelä, L.; Kurppa, K. Screening for coeliac disease in children. Acta Paediatr. 2018, 107, 1879–1887. [Google Scholar] [CrossRef] [Green Version]
- Romanos, J.; van Diemen, C.C.; Nolte, I.M.; Trynka, G.; Zhernakova, A.; Fu, J.; Bardella, M.T.; Barisani, D.; McManus, R.; van Heel, D.; et al. Analysis of HLA and Non-HLA Alleles Can Identify Individuals at High Risk for Celiac Disease. Gastroenterology 2009, 137, 834–840.e3. [Google Scholar] [CrossRef] [Green Version]
- Kårhus, L.L.; Thuesen, B.; Skaaby, T.; Rumessen, J.J.; Linneberg, A. The distribution of HLA DQ2 and DQ8 haplotypes and their association with health indicators in a general Danish population. United Eur. Gastroenterol. J. 2018, 6, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calabrò, A.; De Carli, V.; Luchinat, C.; Nepi, S.; Porfirio, B.; Renzi, D.; Saccenti, E.; Tenori, L. The Metabonomic Signature of Celiac Disease. J. Proteome Res. 2009, 8, 170–177. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Calabrò, A.; La Marca, G.; Lami, G.; Luchinat, C.; Renzi, D.; Tenori, L. Are Patients with Potential Celiac Disease Really Potential? The Answer of Metabonomics. J. Proteome Res. 2011, 10, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, E.M.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellitto, M.; Bai, G.; Serena, G.; Fricke, W.F.; Sturgeon, C.; Gajer, P.; White, J.R.; Koenig, S.S.K.; Sakamoto, J.; Boothe, D.; et al. Proof of Concept of Microbiome-Metabolome Analysis and Delayed Gluten Exposure on Celiac Disease Autoimmunity in Genetically At-Risk Infants. PLoS ONE 2012, 7, e33387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchberg, F.F.; Werkstetter, K.J.; Uhl, O.; Auricchio, R.; Castillejo, G.; Korponay-Szabo, I.R.; Polanco, I.; Ribes-Koninckx, C.; Vriezinga, S.L.; Koletzko, B.; et al. Investigating the early metabolic fingerprint of celiac disease—A prospective approach. J. Autoimmun. 2016, 72, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Sen, P.; Carlsson, C.; Virtanen, S.M.; Simell, S.; Hyoty, H.; Ilonen, J.; Toppari, J.; Veijola, R.; Hyötyläinen, T.; Knip, M.; et al. Persistent Alterations in Plasma Lipid Profiles Before Introduction of Gluten in the Diet Associated With Progression to Celiac Disease. Clin. Transl. Gastroenterol. 2019, 10, e00044. [Google Scholar] [CrossRef]
- Martín-Masot, R.; Mota-Martorell, N.; Jové, M.; Maldonado, J.; Pamplona, R.; Nestares, T. Alterations in One-Carbon Metabolism in Celiac Disease. Nutrients 2020, 12, 3723. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [Green Version]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; De La Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burla, B.; Arita, M.; Arita, M.; Bendt, A.K.; Cazenave-Gassiot, A.; Dennis, E.A.; Ekroos, K.; Han, X.; Ikeda, K.; Liebisch, G.; et al. MS-based lipidomics of human blood plasma: A community-initiated position paper to develop accepted guidelines. J. Lipid Res. 2018, 59, 2001–2017. [Google Scholar] [CrossRef] [Green Version]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Mearin, M.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabré, R.; Jové, M.; Naudí, A.; Ayala, V.; Piñol-Ripoll, G.; Gil-Villar, M.P.; Dominguez-Gonzalez, M.; Obis, È.; Berdun, R.; Mota-Martorell, N.; et al. Specific Metabolomics Adaptations Define a Differential Regional Vulnerability in the Adult Human Cerebral Cortex. Front. Mol. Neurosci. 2016, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 6 July 2019).
- Kolde, R. pheatmap: Pretty Heatmaps. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 15 September 2020).
- Gordon, M.; Lumley, T. Forestplot: Advanced Forest Plot Using ’Grid’ Graphics. R Package Version 1.10. 2020. Available online: https://CRAN.R-project.org/package=forestplot (accessed on 12 April 2021).
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Allison, P.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Tenori, L.; Oakman, C.; Morris, P.G.; Gralka, E.; Turner, N.; Cappadona, S.; Fornier, M.; Hudis, C.; Norton, L.; Luchinat, C.; et al. Serum metabolomic profiles evaluated after surgery may identify patients with oestrogen receptor negative early breast cancer at increased risk of disease recurrence. Results from a retrospective study. Mol. Oncol. 2015, 9, 128–139. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG). Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Freeman, H.J. Endocrine manifestations in celiac disease. World J. Gastroenterol. 2016, 22, 8472–8479. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.; Merida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Almena, M.; Merida, I. Shaping up the membrane: Diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 2011, 36, 593–603. [Google Scholar] [CrossRef]
- Sakane, F.; Hoshino, F.; Murakami, C. New Era of Diacylglycerol Kinase, Phosphatidic Acid and Phosphatidic Acid-Binding Protein. Int. J. Mol. Sci. 2020, 21, 6794. [Google Scholar] [CrossRef] [PubMed]
- Sedda, S.; DiNallo, V.; Marafini, I.; Franzè, E.; Paoluzi, O.A.; Izzo, R.; Giuffrida, P.; Di Sabatino, A.; Corazza, G.R.; Monteleone, G. mTOR sustains inflammatory response in celiac disease. Sci. Rep. 2020, 10, 10798. [Google Scholar] [CrossRef]
- Johnson, C.; Gonzalez, F.J. Challenges and opportunities of metabolomics. J. Cell. Physiol. 2011, 227, 2975–2981. [Google Scholar] [CrossRef] [PubMed]

| Trait | Healthy Siblings (n = 17) | Celiac Children (n = 17) | p-Value |
|---|---|---|---|
| Sex (female, n (%)) | 10 (58.8) | 13 (76.4) | 0.271 |
| Age (years) | 11.25 (4.23) | 9.39 (2.77) | 0.145 |
| Weight (kg) | 38.54 (16.9) | 30 (9.63) | 0.082 |
| Height (cm) | 140.66 (20.32) | 131.2 (19.56) | 0.178 |
| BMI (kg/m2) | 18.5 (3.98) | 17 (1.58) | 0.166 |
| Mediterranean diet (MD) adherence (n (%)) | |||
| Low | 1 (5.9) | 1 (5.9) | |
| Medium | 8 (47.1) | 7 (41.2) | 0.936 |
| High | 7 (41.2) | 8 (47.1) | |
| Gluten-free diet (GFD) | 0 | 17 | |
| Moderate physical activity (min/week) | 69.64 (37) | 81.4 (56.9) | 0.52 |
| HLA DR-DQ genotype | |||
| Negative | 5 | 0 | |
| HLA-DQ2+ | 11 | 14 | |
| HLA DQ8+ | 0 | 0 | |
| HLA-DQ2 + DQ8+ | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Masot, R.; Galo-Licona, J.D.; Mota-Martorell, N.; Sol, J.; Jové, M.; Maldonado, J.; Pamplona, R.; Nestares, T. Up-Regulation of Specific Bioactive Lipids in Celiac Disease. Nutrients 2021, 13, 2271. https://doi.org/10.3390/nu13072271
Martín-Masot R, Galo-Licona JD, Mota-Martorell N, Sol J, Jové M, Maldonado J, Pamplona R, Nestares T. Up-Regulation of Specific Bioactive Lipids in Celiac Disease. Nutrients. 2021; 13(7):2271. https://doi.org/10.3390/nu13072271
Chicago/Turabian StyleMartín-Masot, Rafael, Jose Daniel Galo-Licona, Natàlia Mota-Martorell, Joaquim Sol, Mariona Jové, José Maldonado, Reinald Pamplona, and Teresa Nestares. 2021. "Up-Regulation of Specific Bioactive Lipids in Celiac Disease" Nutrients 13, no. 7: 2271. https://doi.org/10.3390/nu13072271
APA StyleMartín-Masot, R., Galo-Licona, J. D., Mota-Martorell, N., Sol, J., Jové, M., Maldonado, J., Pamplona, R., & Nestares, T. (2021). Up-Regulation of Specific Bioactive Lipids in Celiac Disease. Nutrients, 13(7), 2271. https://doi.org/10.3390/nu13072271






