Diagnostic Power of Circulatory Metabolic Biomarkers as Metabolic Syndrome Risk Predictors in Community-Dwelling Older Adults in Northwest of England (A Feasibility Study)
Abstract
:1. Introduction
1.1. Metabolic Syndrome
1.2. Prevalence of Age-Related Metabolic Syndrome
1.3. Biomarkers Associated with Age-Related Metabolic Syndrome
1.3.1. Interleukin-6 (IL-6)
1.3.2. Insulin
1.3.3. Tumor Necrosis Factor-Alpha (TNF-α)
1.3.4. Adiponectin
1.3.5. Leptin
1.3.6. Plasminogen Activator Inhibitor-I (PAI-I)
1.3.7. Resistin
1.3.8. C-Reactive Protein (CRP)
1.3.9. Ferritin
1.3.10. Cystatin-C
1.4. Biomarkers Associated with Age-Related Metabolic Syndrome—Opportunities and Challenges
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.4. Outcome Measures
2.4.1. Body Composition and Blood Pressure
2.4.2. Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics and Prevalence of Metabolic Syndrome
3.2. Prevalence of Metabolic Syndrome
3.3. Association with Cardiometabolic Risk
3.4. Discriminatory Diagnostic Power
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Geissler, C.E.; Powers, H.J.E. Human Nutrition, 13th ed.; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Gropper, S.A.S.; Smith, J.L.; Groff, J.L. Advanced Nutrition and Human Metabolism, 6th ed.; Wadsworth/Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirabdollahian, F.; Haghighatdoost, F. Anthropometric Indicators of Adiposity Related to Body Weight and Body Shape as Cardiometabolic Risk Predictors in British Young Adults: Superiority of Waist-to-Height Ratio. J. Obes. 2018, 2018, 8370304. [Google Scholar] [CrossRef] [Green Version]
- van Vliet, M.; Heymans, M.W.; von Rosenstiel, I.A.; Brandjes, D.P.; Beijnen, J.H.; Diamant, M. Cardiometabolic risk variables in overweight and obese children: A worldwide comparison. Cardiovasc. Diabetol. 2011, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vliet, M.; von Rosenstiel, I.A.; Schindhelm, R.K.; Brandjes, D.P.; Beijnen, J.H.; Diamant, M. Ethnic differences in cardiometabolic risk profile in an overweight/obese paediatric cohort in the Netherlands: A cross-sectional study. Cardiovasc. Diabetol. 2009, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.J.; Glaesmer, H.; Klotsche, J.; Bohler, S.; Lehnert, H.; Zeiher, A.M.; Marz, W.; Pittrow, D.; Stalla, G.K.; Wittchen, H.U.; et al. Accuracy of anthropometric indicators of obesity to predict cardiovascular risk. J. Clin. Endocrinol. Metab. 2007, 92, 589–594. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Assuncao, N.; Sudo, F.K.; Drummond, C.; de Felice, F.G.; Mattos, P. Metabolic Syndrome and cognitive decline in the elderly: A systematic review. PLoS ONE 2018, 13, e0194990. [Google Scholar] [CrossRef]
- NHS. Metabolic Syndrome. Available online: https://www.nhs.uk/conditions/metabolic-syndrome/ (accessed on 31 March 2021).
- Liu, P.; Sui, S.; Xu, D.; Xing, X.; Liu, C. Clinical analysis of the relationship between cystatin C and metabolic syndrome in the elderly. Rev. Port. Cardiol. 2014, 33, 411–416. [Google Scholar] [CrossRef]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management (NG136); Clinical Guidelines; National Institute for Health and Care Excellence: London, UK, 2019. [Google Scholar]
- Monastero, R.N.; Pentyala, S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int J. Inflamm. 2017, 2017, 4309485. [Google Scholar] [CrossRef] [Green Version]
- Diabetes UK. Diabetes in the UK 2010: Key Statistics on Diabetes. Available online: https://www.diabetes.org.uk/resources-s3/2017-11/diabetes_in_the_uk_2010.pdf (accessed on 31 March 2021).
- British Heart Foundation. UK Factsheet. Available online: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics (accessed on 31 March 2021).
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and regional differences in fat cell progenitors—A mini-review. Gerontology 2011, 57, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a biomarker of the metabolic syndrome. Circ. J. 2004, 68, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Robberecht, H.; Hermans, N. Biomarkers of Metabolic Syndrome: Biochemical Background and Clinical Significance. Metab. Syndr. Relat. Disord. 2016, 14, 47–93. [Google Scholar] [CrossRef]
- Aroor, A.R.; McKarns, S.; Demarco, V.G.; Jia, G.; Sowers, J.R. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism 2013, 62, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Emanuela, F.; Grazia, M.; de Marco, R.; Maria Paola, L.; Giorgio, F.; Marco, B. Inflammation as a Link between Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 476380. [Google Scholar] [CrossRef]
- Weiss, T.W.; Arnesen, H.; Seljeflot, I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism 2013, 62, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Chedraui, P.; Escobar, G.S.; Perez-Lopez, F.R.; Palla, G.; Montt-Guevara, M.; Cecchi, E.; Genazzani, A.R.; Simoncini, T.; Research Group for the Omega Women’s Health Project. Angiogenesis, inflammation and endothelial function in postmenopausal women screened for the metabolic syndrome. Maturitas 2014, 77, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Indulekha, K.; Surendar, J.; Mohan, V. High sensitivity C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and vascular cell adhesion molecule-1 levels in Asian Indians with metabolic syndrome and insulin resistance (CURES-105). J. Diabetes Sci. Technol. 2011, 5, 982–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiras, D.D. Human Biology, 8th ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2015. [Google Scholar]
- Insel, P.M. Nutrition, 5th ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2014. [Google Scholar]
- Folsom, A.R.; Kaye, S.A.; Sellers, T.A.; Hong, C.P.; Cerhan, J.R.; Potter, J.D.; Prineas, R.J. Body fat distribution and 5-year risk of death in older women. JAMA 1993, 269, 483–487. [Google Scholar] [CrossRef]
- Musialik, K. The influence of chosen adipocytokines on blood pressure values in patients with metabolic syndrome. Kardiol. Pol. 2012, 70, 1237–1242. [Google Scholar]
- Aprahamian, T.R.; Sam, F. Adiponectin in cardiovascular inflammation and obesity. Int. J. Inflamm. 2011, 2011, 376909. [Google Scholar] [CrossRef] [Green Version]
- Balasoiu, M.; Balasoiu, A.T.; Stepan, A.E.; Dinescu, S.N.; Avramescu, C.S.; Dumitrescu, D.; Cernea, D.; Alexandru, D. Proatherogenic adipocytokines levels in metabolic syndrome. Rom. J. Morphol. Embryol. 2014, 55, 29–33. [Google Scholar] [PubMed]
- Gormez, S.; Demirkan, A.; Atalar, F.; Caynak, B.; Erdim, R.; Sozer, V.; Gunay, D.; Akpinar, B.; Ozbek, U.; Buyukdevrim, A.S. Adipose tissue gene expression of adiponectin, tumor necrosis factor-alpha and leptin in metabolic syndrome patients with coronary artery disease. Intern. Med. 2011, 50, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Hirose, H.; Takayama, T.; Hozawa, S.; Hibi, T.; Saito, I. Prediction of metabolic syndrome using artificial neural network system based on clinical data including insulin resistance index and serum adiponectin. Comput. Biol. Med. 2011, 41, 1051–1056. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernandez, C.; Pino, J.; Mera, A.; Gonzalez-Gay, M.A.; Gomez, R.; Lago, F.; Mobasheri, A.; Gualillo, O. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharm. 2019, 165, 196–206. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Mohlig, M.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003, 361, 226–228. [Google Scholar] [CrossRef]
- Santaniemi, M.; Kesaniemi, Y.A.; Ukkola, O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur. J. Endocrinol. 2006, 155, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Gannage-Yared, M.H.; Khalife, S.; Semaan, M.; Fares, F.; Jambart, S.; Halaby, G. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur. J. Endocrinol. 2006, 155, 167–176. [Google Scholar] [CrossRef]
- Baratta, R.; Amato, S.; Degano, C.; Farina, M.G.; Patane, G.; Vigneri, R.; Frittitta, L. Adiponectin relationship with lipid metabolism is independent of body fat mass: Evidence from both cross-sectional and intervention studies. J. Clin. Endocrinol. Metab. 2004, 89, 2665–2671. [Google Scholar] [CrossRef] [Green Version]
- Falahi, E.; Khalkhali Rad, A.H.; Roosta, S. What is the best biomarker for metabolic syndrome diagnosis? Diabetes Metab. Syndr. 2015, 9, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, C.M.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential Role of Leptin and Adiponectin in Cardiovascular System. Int. J. Endocrinol. 2015, 2015, 534320. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Jo, H.H.; Kim, M.R.; You, Y.O.; Kim, J.H. Association between metabolic syndrome and serum leptin levels in postmenopausal women. J. Obs. Gynaecol. 2012, 32, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Martins Mdo, C.; Lima Faleiro, L.; Fonseca, A. Relationship between leptin and body mass and metabolic syndrome in an adult population. Rev. Port. Cardiol. 2012, 31, 711–719. [Google Scholar] [CrossRef]
- Yun, J.E.; Kimm, H.; Jo, J.; Jee, S.H. Serum leptin is associated with metabolic syndrome in obese and nonobese Korean populations. Metabolism 2010, 59, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.R.; Christ, G.; Gruber, F.; Grubic, N.; Hufnagl, P.; Krebs, M.; Mihaly, J.; Prager, G.W. Plasminogen activator inhibitor 1: Physiological and pathophysiological roles. News Physiol. Sci. 2002, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Paganelli, F.; Alessi, M.C.; Morange, P.; Maixent, J.M.; Levy, S.; Vague, I.J. Relationship of plasminogen activator inhibitor-1 levels following thrombolytic therapy with rt-PA as compared to streptokinase and patency of infarct related coronary artery. Thromb. Haemost. 1999, 82, 104–108. [Google Scholar] [CrossRef]
- Kruithof, E.K.; Gudinchet, A.; Bachmann, F. Plasminogen activator inhibitor 1 and plasminogen activator inhibitor 2 in various disease states. Thromb. Haemost. 1988, 59, 7–12. [Google Scholar] [CrossRef]
- Kraja, A.T.; Province, M.A.; Arnett, D.; Wagenknecht, L.; Tang, W.; Hopkins, P.N.; Djousse, L.; Borecki, I.B. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr. Metab. 2007, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkinen, P.A.; Wahl, P.; Cushman, M.; Lewis, M.R.; Tracy, R.P. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am. J. Epidemiol. 2000, 152, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Alessi, M.C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arter. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef]
- Aquilante, C.L.; Kosmiski, L.A.; Knutsen, S.D.; Zineh, I. Relationship between plasma resistin concentrations, inflammatory chemokines, and components of the metabolic syndrome in adults. Metabolism 2008, 57, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Gatselis, N.K.; Makaritsis, K.; Dalekos, G.N. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013, 227, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, J.K.; Sakata, S.; Liang, I.; Park, W.; Hajjar, R.J.; Lebeche, D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell Cardiol. 2008, 45, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Zahary, M.N.; Harun, N.S.; Yahaya, R.; Nik Him, N.A.S.; Rohin, M.A.K.; Ridzwan, N.H.; Jumli, M.N.; Wan Jusoh, A.F. Serum adiponectin and resistin: Correlation with metabolic syndrome and its associated criteria among temiar subtribe in Malaysia. Diabetes Metab. Syndr. 2019, 13, 2015–2019. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Lopez, V.; Lazzaro, V.; Pino, J.; Gomez-Reino, J.J.; Gualillo, O. Adipokines, metabolic syndrome and rheumatic diseases. J. Immunol. Res. 2014, 2014, 343746. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, H.S. Human resistin in cardiovascular disease. J. Smooth Muscle Res. 2012, 48, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Melone, M.; Wilsie, L.; Palyha, O.; Strack, A.; Rashid, S. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J. Am. Coll. Cardiol. 2012, 59, 1697–1705. [Google Scholar] [CrossRef] [Green Version]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef] [Green Version]
- Lazar, M.A. Resistin- and Obesity-associated metabolic diseases. Horm. Metab. Res. 2007, 39, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Muse, E.D.; Feldman, D.I.; Blaha, M.J.; Dardari, Z.A.; Blumenthal, R.S.; Budoff, M.J.; Nasir, K.; Criqui, M.H.; Cushman, M.; McClelland, R.L.; et al. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2015, 239, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffler, A.; Scholmerich, J.; Buchler, C. Mechanisms of disease: Adipocytokines and visceral adipose tissue--emerging role in nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 273–280. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.S.; Guerrero, D.B.; Soto, M.B.; Diaz, S.P.; Martinez-Olmos, M.; Vidal, O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur. J. Clin. Nutr. 2006, 60, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, R.; Velmurugan, K.; Arvind, K.; Sivaram, P.; Sientay, C.; Uday, S.; Mohan, V. Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance—The Chennai Urban Rural Epidemiology Study (CURES). Metabolism 2006, 55, 1232–1238. [Google Scholar] [CrossRef]
- Guldiken, S.; Demir, M.; Arikan, E.; Turgut, B.; Azcan, S.; Gerenli, M.; Tugrul, A. The levels of circulating markers of atherosclerosis and inflammation in subjects with different degrees of body mass index: Soluble CD40 ligand and high-sensitivity C-reactive protein. Thromb. Res. 2007, 119, 79–84. [Google Scholar] [CrossRef]
- Ridker, P.M.; Buring, J.E.; Cook, N.R.; Rifai, N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation 2003, 107, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Imagama, S.; Ito, Z.; Wakao, N.; Seki, T.; Hirano, K.; Muramoto, A.; Sakai, Y.; Matsuyama, Y.; Hamajima, N.; Ishiguro, N.; et al. Influence of spinal sagittal alignment, body balance, muscle strength, and physical ability on falling of middle-aged and elderly males. Eur. Spine J. 2013, 22, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Abril-Ulloa, V.; Flores-Mateo, G.; Sola-Alberich, R.; Manuel-y-Keenoy, B.; Arija, V. Ferritin levels and risk of metabolic syndrome: Meta-analysis of observational studies. BMC Public Health 2014, 14, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Li, S.; Liu, G.; Yan, F.; Ma, X.; Huang, Z.; Tian, H. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2012, 7, e41641. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Montonen, J.; Boeing, H.; Steffen, A.; Lehmann, R.; Fritsche, A.; Joost, H.G.; Schulze, M.B.; Pischon, T. Body iron stores and risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia 2012, 55, 2613–2621. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.J.; Poulton, R.; Williams, S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis 2002, 165, 179–184. [Google Scholar] [CrossRef]
- Nassar, B.A.; Zayed, E.M.; Title, L.M.; O’Neill, B.J.; Bata, I.R.; Kirkland, S.A.; Dunn, J.; Dempsey, G.I.; Tan, M.H.; Johnstone, D.E. Relation of HFE gene mutations, high iron stores and early onset coronary artery disease. Can. J. Cardiol. 1998, 14, 215–220. [Google Scholar]
- Eftekhari, M.H.; Mozaffari-Khosravi, H.; Shidfar, F.; Zamani, A. Relation between Body Iron Status and Cardiovascular Risk Factors in Patients with Cardiovascular Disease. Int. J. Prev. Med. 2013, 4, 911–916. [Google Scholar] [PubMed]
- Suarez-Ortegon, M.F.; Ensaldo-Carrasco, E.; Shi, T.; McLachlan, S.; Fernandez-Real, J.M.; Wild, S.H. Ferritin, metabolic syndrome and its components: A systematic review and meta-analysis. Atherosclerosis 2018, 275, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Real, J.M.; McClain, D.; Manco, M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care 2015, 38, 2169–2176. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Real, J.M.; Lopez-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [Green Version]
- Piperno, A.; Trombini, P.; Gelosa, M.; Mauri, V.; Pecci, V.; Vergani, A.; Salvioni, A.; Mariani, R.; Mancia, G. Increased serum ferritin is common in men with essential hypertension. J. Hypertens. 2002, 20, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.; Konig, D.; Berg, A.; Keul, J.; Baumstark, M.W. Relationship of serum ferritin concentrations with metabolic cardiovascular risk factors in men without evidence for coronary artery disease. Atherosclerosis 1997, 128, 235–240. [Google Scholar] [CrossRef]
- Mojiminiyi, O.A.; Marouf, R.; Abdella, N.A. Body iron stores in relation to the metabolic syndrome, glycemic control and complications in female patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R.F. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men--the Third National Health and Nutrition Examination Survey. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubb, A.; Horio, M.; Hansson, L.O.; Bjork, J.; Nyman, U.; Flodin, M.; Larsson, A.; Bokenkamp, A.; Yasuda, Y.; Blufpand, H.; et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin. Chem. 2014, 60, 974–986. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.A.; Ko, S.H.; Yim, H.W.; Ahn, Y.B.; Yoon, K.H.; Cha, B.Y.; Son, H.Y.; Kwon, H.S. Insulin resistance and inflammation may have an additional role in the link between cystatin C and cardiovascular disease in type 2 diabetes mellitus patients. Metabolism 2010, 59, 241–246. [Google Scholar] [CrossRef]
- Battistoni, A.; Rubattu, S.; Volpe, M. Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int. J. Cardiol. 2012, 157, 160–168. [Google Scholar] [CrossRef]
- Servais, A.; Giral, P.; Bernard, M.; Bruckert, E.; Deray, G.; Isnard Bagnis, C. Is serum cystatin-C a reliable marker for metabolic syndrome? Am. J. Med. 2008, 121, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, N.; Fernandez-Berges, D.J.; Koenig, W.; Consuegra-Sanchez, L.; Fernandez, J.M.; Robles, N.R.; Sanchez, P.L.; Beiras, A.C.; Orbe, P.M.; Kaski, J.C.; et al. Plasma cystatin C for prediction of 1-year cardiac events in Mediterranean patients with non-ST elevation acute coronary syndrome. Atherosclerosis 2010, 209, 300–305. [Google Scholar] [CrossRef]
- Luc, G.; Bard, J.M.; Lesueur, C.; Arveiler, D.; Evans, A.; Amouyel, P.; Ferrieres, J.; Juhan-Vague, I.; Fruchart, J.C.; Ducimetiere, P.; et al. Plasma cystatin-C and development of coronary heart disease: The PRIME Study. Atherosclerosis 2006, 185, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnusson, M.; Hedblad, B.; Engstrom, G.; Persson, M.; Nilsson, P.; Melander, O. High levels of cystatin C predict the metabolic syndrome: The prospective Malmo Diet and Cancer Study. J. Intern. Med. 2013, 274, 192–199. [Google Scholar] [CrossRef]
- Retnakaran, R.; Connelly, P.W.; Harris, S.B.; Zinman, B.; Hanley, A.J. Cystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youth. Pediatr. Nephrol. 2007, 22, 1007–1013. [Google Scholar] [CrossRef]
- Naour, N.; Fellahi, S.; Renucci, J.F.; Poitou, C.; Rouault, C.; Basdevant, A.; Dutour, A.; Alessi, M.C.; Bastard, J.P.; Clement, K.; et al. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity 2009, 17, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.-C.; Roux-Lombard, P.; Tamisier, R.; Arnaud, C.; Monneret, D.; Arnol, N.; Baguet, J.-P.; Levy, P.; Pepin, J.-L. Endothelial dysfunction and specific inflammation in obesity hypoventilation syndrome. PLoS ONE 2009, 4, e6733. [Google Scholar] [CrossRef] [Green Version]
- Demircan, N.; Gurel, A.; Armutcu, F.; Unalacak, M.; Aktunc, E.; Atmaca, H. The evaluation of serum cystatin C, malondialdehyde, and total antioxidant status in patients with metabolic syndrome. Med. Sci. Monit. 2008, 14, CR97-101. [Google Scholar] [PubMed]
- Liu, M.Y.; Xydakis, A.M.; Hoogeveen, R.C.; Jones, P.H.; Smith, E.O.; Nelson, K.W.; Ballantyne, C.M. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin. Chem. 2005, 51, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Saluk, J.; Hoppensteadt, D.; Syed, D.; Liles, J.; Abro, S.; Walborn, A.; Bansal, V.; Fareed, J. Biomarker profiling of plasma samples utilizing RANDOX biochip array technology. Int. Angiol. 2017, 36, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Mooney, K.; Cousins, R.; Angell, P.; Jackson, M.; Pugh, J.N.; Coyles, G.; Amirabdollahian, F.; Khaiyat, O. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: A secondary analysis of the Liverpool Hope University-Sarcopenia Ageing Trial (LHU-SAT). Eur. J. Appl. Physiol. 2020, 120, 493–503. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness: Liverpool Hope University-Sarcopenia Aging Trial (LHU-SAT). Front. Physiol. 2019, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Roche, D.M.; Jackson, M.; Amirabdollahian, F.; Khaiyat, O. Clustered cardiometabolic risk and arterial stiffness of recreational adult tennis players. J. Sports Med. Phys. Fit. 2020. [Google Scholar] [CrossRef]
- Mooney, K.; Kirk, B.; Khaiyat, O.; Amirabdollahian, F. Effects of protein supplementation alone and in combination with exercise on cardiometabolic health markers in older adults. Proc. Nutr. Soc. 2019, 78, E39. [Google Scholar] [CrossRef] [Green Version]
- Onat, A.; Can, G.; Kaya, H.; Hergenc, G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J. Clin. Lipidol. 2010, 4, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mooney, K. The Effects of Protein Supplementation Alone and in Combination with Exercise Training on Musculoskeletal Strength and Functioning, Cardio-Metabolic Health and Nutritional Status in Older Adults. Ph.D. Thesis, Liverpool Hope University, Liverpool, UK, 2020. [Google Scholar]
- Skorepa, P.; Sobotka, O.; Vanek, J.; Ticha, A.; Fortunato, J.; Manak, J.; Blaha, V.; Horacek, J.M.; Sobotka, L. The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients. Nutrients 2020, 12, 1373. [Google Scholar] [CrossRef]
- Molloy, R.M.; Mc Connell, R.I.; Lamont, J.V.; FitzGerald, S.P. Automation of biochip array technology for quality results. Clin. Chem. Lab. Med. 2005, 43, 1303–1313. [Google Scholar] [CrossRef]
- Park, Y.; Park, J.Y.; Han, K.H.; Kim, H.S. Serum cytokine levels in chronic hepatitis B patients receiving peginterferon alpha-2a therapy. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 499–506. [Google Scholar] [CrossRef]
- Gonen, M. Analyzing Receiver Operating Characteristic Curves with SAS; SAS Pub.: Cary, NC, USA, 2007. [Google Scholar]
- Bendzala, M.; Sabaka, P.; Caprnda, M.; Komornikova, A.; Bisahova, M.; Baneszova, R.; Petrovic, D.; Prosecky, R.; Rodrigo, L.; Kruzliak, P.; et al. Atherogenic index of plasma is positively associated with the risk of all-cause death in elderly women: A 10-year follow-up. Wien. Klin. Wochenschr. 2017, 129, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.K.; Blaha, M.J.; Loprinzi, P.D. Atherogenic Index of Plasma and Triglyceride/High-Density Lipoprotein Cholesterol Ratio Predict Mortality Risk Better Than Individual Cholesterol Risk Factors, Among an Older Adult Population. Mayo Clin. Proc. 2017, 92, 680–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Zhou, S.; Feng, X.; Yang, J.; Qiao, J.; Zhao, Y.; Shi, D.; Zhou, Y. The sensibility of the new blood lipid indicator--atherogenic index of plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis. 2020, 19, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Association of ideal cardiovascular health metrics with serum uric acid, inflammation and atherogenic index of plasma: A population-based survey. Atherosclerosis 2019, 284, 44–49. [Google Scholar] [CrossRef]
- Wang, L.; Chen, F.; Chai, X.; Chen, Y.; Li, Z. Atherogenic Index of Plasma Is an Independent Risk Factor for Coronary Artery Disease and a Higher SYNTAX Score. Angiology 2021, 72, 181–186. [Google Scholar] [CrossRef]
- Won, K.B.; Jang, M.H.; Park, E.J.; Park, H.B.; Heo, R.; Han, D.; Chang, H.J. Atherogenic index of plasma and the risk of advanced subclinical coronary artery disease beyond traditional risk factors: An observational cohort study. Clin. Cardiol. 2020, 43, 1398–1404. [Google Scholar] [CrossRef]
- Wu, T.T.; Gao, Y.; Zheng, Y.Y.; Ma, Y.T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Lixandru, D.; Elena, V.B.; Bogdana, V.; Horia, V.; Petruta-Ramona, A.; Emil, B.M.; Paul, G.; Constantin, I.-T.; Cristian, S. Changes in the serum proinflammatory cytokines in patients with elevated HOMA-IR and type 2 diabetes mellitus. Farmacia 2015, 63, 132–139. [Google Scholar]
- Cho, N.H.; Ku, E.J.; Jung, K.Y.; Oh, T.J.; Kwak, S.H.; Moon, J.H.; Park, K.S.; Jang, H.C.; Kim, Y.J.; Choi, S.H. Estimated Association Between Cytokines and the Progression to Diabetes: 10-year Follow-Up From a Community-Based Cohort. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [Green Version]
- Bao, P.; Liu, G.; Wei, Y. Association between IL-6 and related risk factors of metabolic syndrome and cardiovascular disease in young rats. Int. J. Clin. Exp. Med. 2015, 8, 13491–13499. [Google Scholar] [PubMed]
- Knight, E.L.; Verhave, J.C.; Spiegelman, D.; Hillege, H.L.; de Zeeuw, D.; Curhan, G.C.; de Jong, P.E. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004, 65, 1416–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Ye, X.; Pei, X.; Wu, J.; Zhao, W. Reference intervals for serum cystatin C and factors influencing cystatin C levels other than renal function in the elderly. PLoS ONE 2014, 9, e86066. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ma, F.; Lou, H.; Zhu, Y. Relationship between cystatin C and metabolic syndrome among Chinese premenopausal and postmenopausal women without recognized chronic kidney disease. Menopause 2015, 22, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Pei, D. Association of cystatin C levels with metabolic syndrome incidence: A nested case-control study with propensity score matching. J. Int. Med. Res. 2021, 49, 300060520986311. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Jiang, Y.; Qin, G.; Qian, Y.; Shen, X.; Jiang, Z.; Zheng, S.; Song, Z. Association of body mass index, waist circumference, and metabolic syndrome with serum cystatin C in a Chinese population. Medicine 2017, 96, e6289. [Google Scholar] [CrossRef]
- Zhuo, Q.; Wang, Z.Q.; Fu, P.; Piao, J.H.; Tian, Y.; Xu, J.; Yang, X.G. Association between adiponectin and metabolic syndrome in older adults from major cities of China. Biomed. Environ. Sci. 2010, 23, 53–61. [Google Scholar] [CrossRef]
- Zhuo, Q.; Wang, Z.; Fu, P.; Piao, J.; Tian, Y.; Xu, J.; Yang, X. Comparison of adiponectin, leptin and leptin to adiponectin ratio as diagnostic marker for metabolic syndrome in older adults of Chinese major cities. Diabetes Res. Clin. Pract. 2009, 84, 27–33. [Google Scholar] [CrossRef]
- Soebijanto, N.; Waspadji, S. Adiponectin levels and its role in insulin resistance among adult women with metabolic syndrome. Acta Med. Indones. 2010, 42, 187–191. [Google Scholar]
- Christiana, U.I.; Casimir, A.E.; Nicholas, A.A.; Christian, M.C.; Obiefuna, A.I. Plasma levels of inflammatory cytokines in adult Nigerians with the metabolic syndrome. Niger. Med. J. 2016, 57, 64–68. [Google Scholar] [CrossRef]
- Esteghamati, A.; Khalilzadeh, O.; Anvari, M.; Rashidi, A.; Mokhtari, M.; Nakhjavani, M. Association of serum leptin levels with homeostasis model assessment-estimated insulin resistance and metabolic syndrome: The key role of central obesity. Metab. Syndr. Relat. Disord. 2009, 7, 447–452. [Google Scholar] [CrossRef]
- Chiu, F.H.; Chuang, C.H.; Li, W.C.; Weng, Y.M.; Fann, W.C.; Lo, H.Y.; Sun, C.; Wang, S.H. The association of leptin and C-reactive protein with the cardiovascular risk factors and metabolic syndrome score in Taiwanese adults. Cardiovasc. Diabetol. 2012, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babatsikou, F.; Zavitsanou, A. Epidemiology of hypertension in the elderly. Health Sci. J. 2010, 4, 24–30. [Google Scholar]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef] [Green Version]
- Joffres, M.; Falaschetti, E.; Gillespie, C.; Robitaille, C.; Loustalot, F.; Poulter, N.; McAlister, F.A.; Johansen, H.; Baclic, O.; Campbell, N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open 2013, 3, e003423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ambrosi, J.; Salvador, J.; Páramo, J.A.; Orbe, J.; de Irala, J.; Diez-Caballero, A.; Gil, M.a.J.; Cienfuegos, J.A.; Frühbeck, G. Involvement of leptin in the association between percentage of body fat and cardiovascular risk factors. Clin. Biochem. 2002, 35, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Makni, E.; Moalla, W.; Benezzeddine-Boussaidi, L.; Lac, G.; Tabka, Z.; Elloumi, M. Correlation of resistin with inflammatory and cardiometabolic markers in obese adolescents with and without metabolic syndrome. Obes. Facts 2013, 6, 393–404. [Google Scholar] [CrossRef]

| WHO | NCEP ATP III | IDF | EGIR | AHA | |
|---|---|---|---|---|---|
| Criteria | IR or diabetes plus 2/5 of criteria below | Any 3/5 of criteria below | Obesity plus two of criteria below | Hyperinsulinemia plus two of the criteria below | ≥3 of criteria below |
| Obesity | Waist/hip ratio >0.9 (M), >0.85 (W) or BMI > 30 kg/m2 | WC ≥ 102 cm (M) ≥88 cm (W) | WC > 94 cm (M) >80 cm (W) | WC ≥ 94 cm (M) ≥80 cm (W) | WC ≥ 102 cm (M) ≥88 cm (W) |
| Hyperglycemia | IR or FBG > 7.8 mmol/L | FBG ≥ 5.6 mmol/L | FBG > 5.6 mmol/L | IR | FBG ≥ 5.6 mmol/L |
| Elevated Triglycerides | TGs > 1.7 mmol/L | TGs ≥ 1.7 mmol/L | TGs > 1.7 mmol/L | TGs > 2.0 mmol/L | TGs ≥ 1.7 mmol/L |
| Decreased HDL-C | <0.9 mmol/L (M), <1.0 mmol/L (W) | <1.0 mmol/L (M), <1.3 mmol/L (W) | <1 mmol/L (M), <1.3 mmol/L (W) | <1.0 mmol/L | <1.0 mmol/L (M), <1.3 mmol/L (W) |
| Hypertension | BP ≥ 140/90 mmHg or taking medication for hypertension | BP ≥ 130/85 mmHg | BP ≥ 130/85 mmHg | BP ≥ 140/90 mmHg or taking medication for hypertension | BP > 130/85 mmHg |
| Other | Urine albumin ≥20 μg/min or albumin creatinine ratio ≥30 mg/g |
| Variable | Mean ± SE or % |
|---|---|
| Age (years) | 68.73 ± 0.58 |
| Sex (male: female) (%) | 48:52 |
| Height (cm) | 166.51 ± 0.94 |
| Weight (kg) | 75.33 ± 1.65 |
| BMI (kg/m2) | 27.06 ± 0.52 |
| Waist Circumference (cm) | 90.19 ± 1.45 |
| WHtR | 0.54 ± 0.008 |
| Body Fat | |
| Low (%) | 4.2 |
| Optimal (%) | 41.1 |
| Moderate (%) | 34.7 |
| High (%) | 20 |
| Skeletal Muscle Index | 8.92 ± 0.11 |
| Gait Speed | 1.30 ± 0.02 |
| Hand Grip | 28.59 ± 0.89 |
| Employed (%) | 15 |
| Married (%) | 66 |
| Live alone (%) | 28 |
| Education | |
| Primary or Secondary (%) | 19 |
| Higher occupational (%) | 36 |
| University (%) | 45 |
| SBP (mmHg) | 143.92 ± 1.62 |
| DBP (mmHg) | 82.73 ± 1.17 |
| MAP (mmHg) | 103.13 ± 1.18 |
| Pulse Wave Velocity | 8.89 ± 0.22 |
| HbA1c (%) | 5.47 ± 0.04 |
| Total Cholesterol (mmol/l) | 5.18 ± 0.11 |
| HDL-C (mmol/l) | 1.56 ± 0.04 |
| LDL-C (mmol/l) | 2.97 ± 0.10 |
| TGs (mmol/l) | 1.44 ± 0.10 |
| Fasting blood glucose (mmol/l) | 5.51 ± 0.07 |
| MetS prevalence | |
| Prevalence based on NCEP ATP III definition (%) | 26 |
| Prevalence based on IDF definition (%) | 33 |
| Prevalence based on CMDS definition (%) | 22 |
| Occurrence of individual MetS risk factors (as NCEP ATP III) | |
| Elevated Waist Circumference (%) | 27 |
| Reduced HDL-C (%) | 13.5 |
| Elevated TGs (%) | 26.5 |
| Elevated fasting blood glucose (%) | 38.7 |
| Hypertension (%) | 88 |
| At least one risk factor of MetS present | 97 |
| At least two risk factors of MetS present | 59 |
| Dependent | Blood Glucose (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) | TGs a (mmol/L) | HBA1C (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | ||||||
| IL-6 (pg/mL) | 0.128 | 0.016 | 0.226 | 0.039 | 0.001 | 0.721 | −0.122 | 0.015 | 0.246 | 0.094 | 0.009 | 0.370 | 0.083 | 0.007 | 0.422 | |
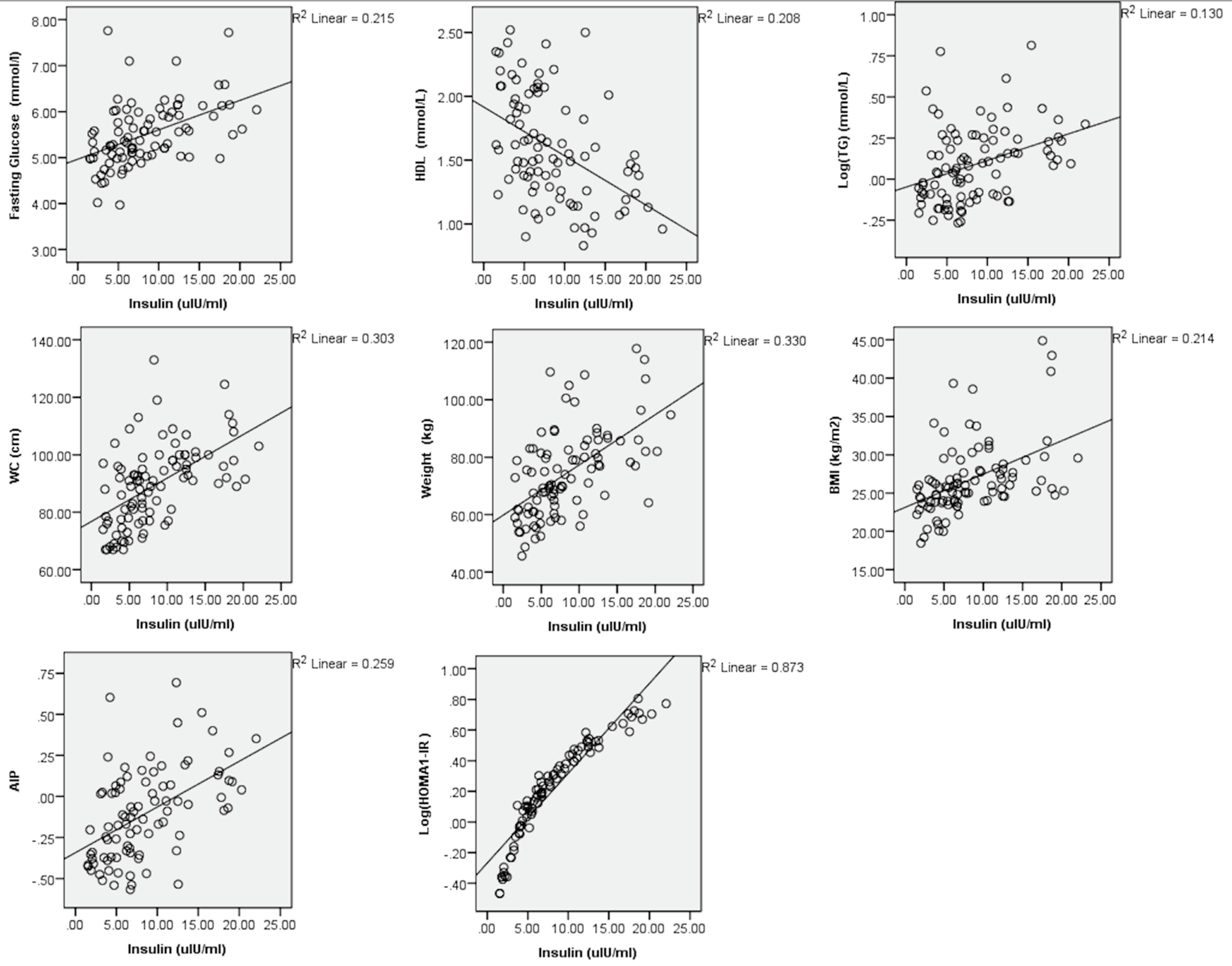
| Insulin (µlU/mL) | 0.464 | 0.215 | <0.0001 * | 0.114 | 0.013 | 0.301 | −0.457 | 0.208 | <0.0001 * | 0.361 | 0.130 | <0.0001 * | 0.201 | 0.040 | 0.055 | |
| TNF-α (pg/mL) | −0.045 | 0.002 | 0.668 | 0.182 | 0.033 | 0.089 | −0.240 | 0.058 | 0.021 | 0.207 | 0.043 | 0.045 | −0.115 | 0.013 | 0.268 | |
| Adiponectin (µg/mL) | −0.238 | 0.057 | 0.023 | 0.107 | 0.011 | 0.328 | 0.543 | 0.294 | <0.0001 * | −0.124 | 0.015 | 0.239 | −0.151 | 0.023 | 0.148 | |
| Leptin (ng/mL) | 0.013 | 0.0002 | 0.899 | 0.028 | 0.001 | 0.795 | −0.022 | 0.0005 | 0.835 | 0.166 | 0.028 | 0.110 | −0.046 | 0.002 | 0.658 | |
| PAI-1 (ng/mL) | 0.061 | 0.004 | 0.563 | 0.108 | 0.012 | 0.316 | −0.163 | 0.027 | 0.120 | 0.265 | 0.070 | 0.010 | 0.071 | 0.005 | 0.492 | |
| Resistin (ng/mL) | 0.203 | 0.041 | 0.053 | −0.058 | 0.003 | 0.591 | −0.167 | 0.028 | 0.111 | 0.078 | 0.006 | 0.457 | 0.011 | 0.0001 | 0.918 | |
| CRP (µg/mL) | 0.194 | 0.038 | 0.065 | −0.034 | 0.001 | 0.756 | −0.071 | 0.005 | 0.500 | 0.169 | 0.029 | 0.103 | 0.160 | 0.026 | 0.121 | |
| Ferritin (ng/mL) | 0.097 | 0.009 | 0.371 | 0.003 | 0.00001 | 0.982 | −0.211 | 0.045 | 0.051 | −0.125 | 0.016 | 0.247 | 0.045 | 0.002 | 0.673 | |
| Cystatin-C (µg/mL) | −0.054 | 0.003 | 0.612 | 0.146 | 0.021 | 0.174 | 0.069 | 0.005 | 0.514 | 0.106 | 0.011 | 0.308 | 0.012 | 0.0001 | 0.907 | |
| Dependent | WC (cm) | Weight (kg) | BMI (kg/m2) | Body Fat (%) | ||||||||||||
| Predictors | β | p-Value | β | p-Value | β | p-Value | β | p-Value | ||||||||
| IL-6 (pg/mL) | 0.309 | 0.095 | 0.002 | 0.298 | 0.089 | 0.003 | 0.374 | 0.140 | <0.0001 * | 0.386 | 0.149 | <0.0001 * | ||||
| Insulin (µlU/mL) | 0.550 | 0.303 | <0.0001 * | 0.574 | 0.330 | <0.0001 * | 0.462 | 0.214 | <0.0001 * | 0.283 | 0.080 | 0.008 | ||||
| TNF-α (pg/mL) | 0.094 | 0.009 | 0.364 | 0.125 | 0.016 | 0.224 | 0.094 | 0.009 | 0.360 | 0.099 | 0.010 | 0.350 | ||||
| Adiponectin (µg/mL) | −0.396 | 0.157 | <0.0001 * | −0.326 | 0.106 | 0.001 | −0.189 | 0.036 | 0.069 | 0.105 | 0.011 | 0.327 | ||||
| Leptin (ng/mL) | 0.248 | 0.062 | 0.015 | 0.346 | 0.119 | 0.001 | 0.539 | 0.291 | <0.0001 * | 0.632 | 0.400 | <0.0001 * | ||||
| PAI-1 (ng/mL) | 0.153 | 0.024 | 0.136 | 0.186 | 0.035 | 0.069 | 0.148 | 0.022 | 0.151 | 0.180 | 0.033 | 0.087 | ||||
| Resistin (ng/mL) | 0.201 | 0.041 | 0.049 | 0.198 | 0.039 | 0.053 | 0.247 | 0.061 | 0.015 | 0.195 | 0.038 | 0.064 | ||||
| CRP (µg/mL) | 0.321 | 0.103 | 0.001 | 0.403 | 0.162 | <0.0001 * | 0.526 | 0.277 | <0.0001 * | 0.512 | 0.262 | <0.0001 * | ||||
| Ferritin (ng/mL) | 0.259 | 0.067 | 0.014 | 0.278 | 0.082 | 0.006 | 0.119 | 0.014 | 0.263 | −0.090 | 0.008 | 0.411 | ||||
| Cystatin-C (µg/mL) | −0.028 | 0.001 | 0.784 | −0.006 | 0.00004 | 0.956 | 0.050 | 0.003 | 0.625 | 0.131 | 0.017 | 0.215 | ||||
| Dependent | MAP (mmHG) | PWV (m/s) | AIP | HOMA1-IR a | ||||||||||||
| Predictors | β | p-Value | β | p-Value | β | p-Value | β | p-Value | ||||||||
| IL-6 (pg/mL) | 0.224 | 0.050 | 0.030 | 0.261 | 0.068 | 0.012 | 0.124 | 0.015 | 0.241 | 0.281 | 0.079 | 0.007 | ||||
| Insulin (µlU/mL) | 0.129 | 0.017 | 0.221 | 0.173 | 0.030 | 0.102 | 0.509 | 0.259 | <0.0001 * | 0.934 | 0.873 | <0.0001 * | ||||
| TNF-α (pg/mL) | −0.087 | 0.008 | 0.402 | −0.009 | 0.0001 | 0.931 | 0.290 | 0.084 | 0.005 | 0.227 | 0.052 | 0.030 | ||||
| Adiponectin (µg/mL) | 0.006 | 0.00004 | 0.956 | −0.075 | 0.006 | 0.483 | −0.361 | 0.130 | 0.001 | −0.474 | 0.225 | <0.0001 * | ||||
| Leptin (ng/mL) | 0.196 | 0.038 | 0.059 | 0.177 | 0.031 | 0.089 | 0.145 | 0.021 | 0.172 | 0.223 | 0.050 | 0.033 | ||||
| PAI-1 (ng/mL) | −0.051 | 0.003 | 0.625 | 0.082 | 0.007 | 0.435 | 0.294 | 0.086 | 0.005 | 0.275 | 0.075 | 0.008 | ||||
| Resistin (ng/mL) | 0.011 | 0.0001 | 0.915 | 0.026 | 0.001 | 0.803 | 0.137 | 0.019 | 0.196 | 0.234 | 0.055 | 0.026 | ||||
| CRP (µg/mL) | 0.106 | 0.011 | 0.310 | 0.084 | 0.007 | 0.422 | 0.168 | 0.028 | 0.112 | 0.271 | 0.074 | 0.010 | ||||
| Ferritin (ng/mL) | 0.056 | 0.003 | 0.603 | 0.003 | 0.00001 | 0.976 | −0.023 | 0.001 | 0.836 | 0.216 | 0.047 | 0.044 | ||||
| Cystatin-C (µg/mL) | 0.144 | 0.021 | 0.165 | −0.007 | 0.00005 | 0.948 | 0.057 | 0.003 | 0.594 | 0.012 | 0.0001 | 0.914 | ||||
| Dependent | Blood Glucose (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) | TGs a (mmol/L) | HBA1C (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| IL-6 (pg/mL) | 0.054 | 0.688 | −0.090 | 0.580 | −0.020 | 0.877 | −0.171 | 0.245 | 0.168 | 0.281 | |
| Insulin (µlU/mL) | 0.489 | <0.0001 * | −0.029 | 0.841 | −0.278 | 0.016 * | 0.335 | 0.011 * | 0.199 | 0.148 | |
| TNF-α (pg/mL) | −0.308 | 0.016 * | 0.244 | 0.134 | −0.078 | 0.553 | 0.028 | 0.838 | -0.261 | 0.075 | |
| Adiponectin (µg/mL) | −0.064 | 0.559 | 0.128 | 0.337 | 0.389 | 0.001 * | 0.045 | 0.704 | -0.130 | 0.302 | |
| Leptin (ng/mL) | −0.158 | 0.177 | 0.068 | 0.637 | 0.057 | 0.620 | 0.060 | 0.637 | -0.201 | 0.139 | |
| PAI-1 (ng/mL) | 0.014 | 0.899 | 0.172 | 0.214 | 0.007 | 0.952 | 0.302 | 0.018 * | 0.124 | 0.350 | |
| Resistin (ng/mL) | 0.283 | 0.012 | −0.118 | 0.391 | −0.002 | 0.987 | −0.056 | 0.643 | -0.016 | 0.904 | |
| CRP (µg/mL) | 0.155 | 0.213 | −0.057 | 0.710 | 0.056 | 0.652 | 0.021 | 0.880 | 0.066 | 0.649 | |
| Ferritin (ng/mL) | 0.024 | 0.819 | −0.005 | 0.967 | −0.122 | 0.238 | −0.259 | 0.025 * | 0.007 | 0.952 | |
| Cystatin-C (µg/mL) | −0.018 | 0.862 | 0.148 | 0.239 | 0.073 | 0.474 | 0.121 | 0.281 | 0.050 | 0.671 | |
| Adjusted-r2 | |||||||||||
| p-value | p < 0.0001 * | p = 0.396 | p < 0.0001 * | p = 0.018 * | p = 0.299 | ||||||
| Dependent | WC (cm) | Weight (kg) | BMI (kg/m2) | Body Fat (%) | |||||||
| Predictors | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |||
| IL-6 (pg/mL) | 0.145 | 0.236 | 0.011 | 0.923 | −0.034 | 0.763 | −0.050 | 0.667 | |||
| Insulin (µlU/mL) | 0.454 | <0.0001 * | 0.485 | <0.0001 * | 0.361 | <0.0001 * | 0.118 | 0.247 | |||
| TNF-α (pg/mL) | −0.207 | 0.071 | −0.173 | 0.118 | −0.222 | 0.035 * | −0.179 | 0.093 | |||
| Adiponectin (µg/mL) | −0.238 | 0.017 * | −0.125 | 0.189 | −0.012 | 0.898 | 0.161 | 0.094 | |||
| Leptin (ng/mL) | 0.109 | 0.305 | 0.227 | 0.029 * | 0.459 | <0.0001 * | 0.557 | <0.0001 * | |||
| PAI-1 (ng/mL) | −0.055 | 0.593 | −0.037 | 0.711 | −0.057 | 0.551 | 0.042 | 0.671 | |||
| Resistin (ng/mL) | 0.066 | 0.515 | 0.029 | 0.770 | 0.139 | 0.135 | 0.176 | 0.065 | |||
| CRP (µg/mL) | 0.051 | 0.654 | 0.153 | 0.165 | 0.272 | 0.010 * | 0.261 | 0.016* | |||
| Ferritin (ng/mL) | 0.196 | 0.041 * | 0.203 | 0.030 * | 0.070 | 0.423 | −0.060 | 0.497 | |||
| Cystatin-C (µg/mL) | −0.025 | 0.785 | −0.009 | 0.919 | 0.041 | 0.631 | 0.124 | 0.162 | |||
| Adjusted-r2 | |||||||||||
| p-value | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | |||||||
| Dependent | MAP (mmHG) | PWV (m/s) | AIP | HOMA1-IR a | |||||||
| Predictors | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |||
| IL-6 (pg/mL) | 0.314 | 0.043 * | 0.279 | 0.082 | −0.172 | 0.214 | 0.063 | 0.241 | |||
| Insulin (µlU/mL) | 0.239 | 0.078 | 0.208 | 0.138 | 0.409 | 0.001 * | 0.896 | <0.0001 * | |||
| TNF-α (pg/mL) | −0.175 | 0.223 | −0.092 | 0.532 | 0.086 | 0.524 | −0.158 | 0.002 * | |||
| Adiponectin (µg/mL) | −0.013 | 0.914 | −0.030 | 0.813 | −0.143 | 0.207 | −0.111 | 0.013 * | |||
| Leptin (ng/mL) | 0.054 | 0.686 | 0.045 | 0.747 | 0.038 | 0.754 | −0.017 | 0.712 | |||
| PAI-1 (ng/mL) | −0.121 | 0.354 | 0.026 | 0.844 | 0.230 | 0.051 | 0.096 | 0.036 * | |||
| Resistin (ng/mL) | −0.015 | 0.904 | 0.002 | 0.988 | −0.063 | 0.588 | 0.080 | 0.071 | |||
| CRP (µg/mL) | −0.109 | 0.445 | −0.119 | 0.419 | 0.001 | 0.991 | −0.039 | 0.433 | |||
| Ferritin (ng/mL) | 0.035 | 0.772 | −0.048 | 0.701 | −0.177 | 0.099 | −0.008 | 0.855 | |||
| Cystatin-C (µg/mL) | 0.123 | 0.295 | −0.003 | 0.978 | 0.074 | 0.484 | 0.030 | 0.465 | |||
| Adjusted-r2 p-value | p = 0.138 | p = 0.450 | p < 0.0001 * | p < 0.0001 * | |||||||
| MetS | At Least Two Risk Factors | |||
|---|---|---|---|---|
| Unadjusted OR (95%CI) | Adjusted OR (95%CI) | Unadjusted OR (95%CI) | Adjusted OR (95%CI) | |
| IL-6 (pg/mL) | 1.32 (1.06–1.64) | 1.21 (0.91–1.62) | 1.34 (1.03–1.74) | 1.35 (0.92–1.97) |
| Insulin (µlU/mL) | 1.24 (1.12–1.37) | 1.25 (1.09–1.43) | 1.33 (1.16–1.53) | 1.87 (1.24–2.83) |
| TNF-α (pg/mL) | 1.37 (1.02–1.84) | 1.22 (0.84–1.77) | 1.14 (0.89–1.48) | 1.38 (0.84–2.27) |
| Adiponectin (µg/mL) | 0.90 (0.79–1.02) | 0.91 (0.79–1.06) | 0.94 (0.86–1.02) | 0.89 (0.75–1.06) |
| Leptin (ng/mL) | 1.04 (1.00–1.09) | 1.002 (0.94–1.07) | 1.08 (1.00–1.16) | 1.03 (0.93–1.14) |
| PAI-1 (ng/mL) | 1.06 (1.00–1.13) | 1.05 (0.97–1.13) | 1.04 (0.99–1.10) | 1.04 (0.95–1.13) |
| Resistin (ng/mL) | 1.27 (1.04–1.54) | 1.20 (0.95–1.51) | 1.37 (1.06–1.78) | 1.44 (0.96–2.15) |
| CRP (µg/mL) | 1.29 (1.09–1.54) | 1.18 (0.96–1.46) | 1.19 (0.97–1.46) | 1.03 (0.76–1.41) |
| Ferritin (ng/mL) | 1.003 (1–1.007) | 1.003 (1–1.009) | 1 (1.00–1.004) | 1.00 (1–1.006) |
| Cystatin-C (µg/mL) | 1.09 (0.73–1.61) | 0.99 (0.57–1.74) | 0.91 (0.62–1.36) | 0.81 (0.45–1.44) |
| MetS | At Least Two Risk Factors | |||
|---|---|---|---|---|
| AUC (95%CI) a | AUC b | AUC (95%CI) a | AUC b | |
| IL-6 (pg/mL) | 0.629 (0.500–0.758) | 0.644 | 0.613 (0.499–0.727) | 0.629 |
| Insulin (µlU/mL) | 0.773 (0.653–0.893) | 0.783 | 0.775 (0.683–0.866) | 0.785 |
| TNF-α (pg/mL) | 0.615 (0.482–0.749) | 0.635 | 0.518 (0.400–0.636) | 0.563 |
| Adiponectin (µg/mL) | 0.620 (0.490 −0.749) | 0.640 | 0.555 (0.435–0.675) | 0.619 |
| Leptin (ng/mL) | 0.590 (0.447–0.734) | 0.681 | 0.663 (0.552–0.775) | 0.711 |
| PAI-1 (ng/mL) | 0.637 (0.520–0.755) | 0.643 | 0.576 (0.455–0.698) | 0.606 |
| Resistin (ng/mL) | 0.544 (0.394–0.694) | 0.555 | 0.559 (0.444–0.673) | 0.598 |
| CRP (µg/mL) | 0.593 (0.448–0.737) | 0.620 | 0.614 (0.501–0.728) | 0.678 |
| Ferritin (ng/mL) | 0.514 (0.382–0.646) | 0.611 | 0.332 (0.219–0.446) | 0.464 |
| Cystatin-C (µg/mL) | 0.447 (0.317–0.576) | 0.744 | 0.429 (0.311–0.546) | 0.377 |
| 5-Fold Cross-Validation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MetS | At Least Two Risk Factors | |||||||||
| TPR | TNR | PPV | NPV | BA | TPR | TNR | PPV | NPV | BA | |
| IL-6 (pg/mL) | 16% | 97.2% | 66.7% | 76.7% | 56.6% | 73.7% | 25.6% | 59.1% | 40% | 49.7% |
| Insulin (µlU/mL) | 44% | 94.4% | 73.3% | 82.7% | 69.2% | 71.9% | 61.5% | 73.2% | 60% | 66.7% |
| TNF-α (pg/mL) | 0% | 97.2% | 0% | 73.4% | 48.6% | 80.7% | 5.1% | 55.4% | 15.4% | 42.9% |
| Adiponectin (µg/mL) | 0% | 100% | 0% | 73.4% | 50% | 88.9% | 25% | 61.5% | 62.5% | 56.9% |
| Leptin (ng/mL) | 4% | 97.2% | 33.3% | 74.2% | 50.6% | 98.2% | 7.7% | 60.9% | 75% | 53% |
| PAI-1 (ng/mL) | 0% | 98.6% | 0% | 73.7% | 49.3% | 87.7% | 15.4% | 60.2% | 46.1% | 51.5% |
| Resistin (ng/mL) | 16% | 98.6% | 80% | 76.9% | 57.3% | 73.7% | 25.6% | 59.1% | 40% | 49.7% |
| CRP (µg/mL) | 28% | 97.2% | 77.8% | 79.3% | 62.6% | 94.6% | 10% | 59.5% | 57.1% | 52.3% |
| Ferritin (ng/mL) | 0% | 97% | 0% | 72.7 | 48.5% | 94.3% | 2.7% | 58.1% | 25% | 48.5% |
| Cystatin-C (µg/mL) | 4% | 98.6% | 50% | 74.5% | 51.3% | 100% | 7.5% | 60.2% | 100% | 53.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassannejad, R.; Sharrouf, H.; Haghighatdoost, F.; Kirk, B.; Amirabdollahian, F. Diagnostic Power of Circulatory Metabolic Biomarkers as Metabolic Syndrome Risk Predictors in Community-Dwelling Older Adults in Northwest of England (A Feasibility Study). Nutrients 2021, 13, 2275. https://doi.org/10.3390/nu13072275
Hassannejad R, Sharrouf H, Haghighatdoost F, Kirk B, Amirabdollahian F. Diagnostic Power of Circulatory Metabolic Biomarkers as Metabolic Syndrome Risk Predictors in Community-Dwelling Older Adults in Northwest of England (A Feasibility Study). Nutrients. 2021; 13(7):2275. https://doi.org/10.3390/nu13072275
Chicago/Turabian StyleHassannejad, Razieh, Hamsa Sharrouf, Fahimeh Haghighatdoost, Ben Kirk, and Farzad Amirabdollahian. 2021. "Diagnostic Power of Circulatory Metabolic Biomarkers as Metabolic Syndrome Risk Predictors in Community-Dwelling Older Adults in Northwest of England (A Feasibility Study)" Nutrients 13, no. 7: 2275. https://doi.org/10.3390/nu13072275
APA StyleHassannejad, R., Sharrouf, H., Haghighatdoost, F., Kirk, B., & Amirabdollahian, F. (2021). Diagnostic Power of Circulatory Metabolic Biomarkers as Metabolic Syndrome Risk Predictors in Community-Dwelling Older Adults in Northwest of England (A Feasibility Study). Nutrients, 13(7), 2275. https://doi.org/10.3390/nu13072275







