Use of Different Food Classification Systems to Assess the Association between Ultra-Processed Food Consumption and Cardiometabolic Health in an Elderly Population with Metabolic Syndrome (PREDIMED-Plus Cohort)
Abstract
:1. Introduction
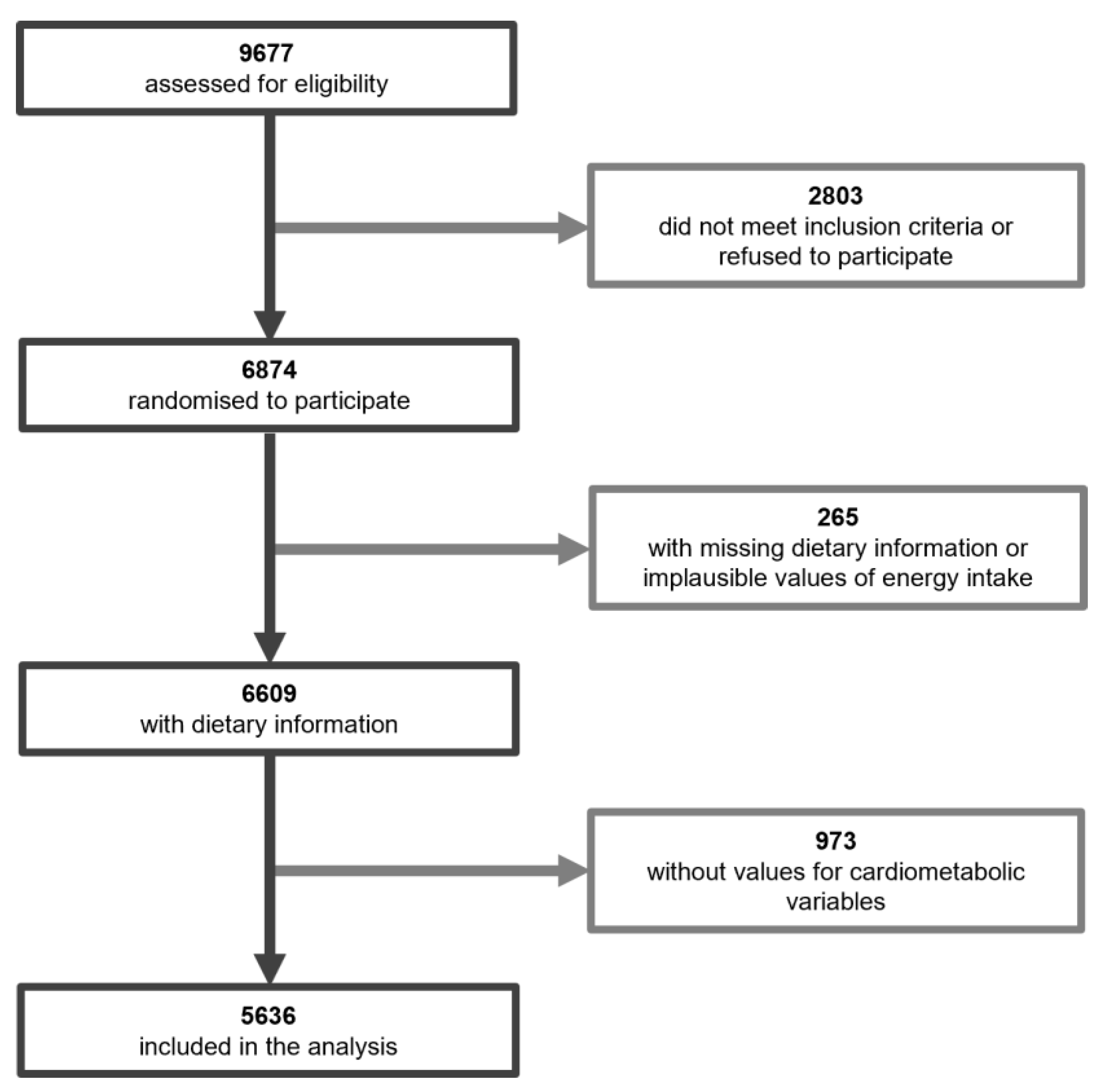
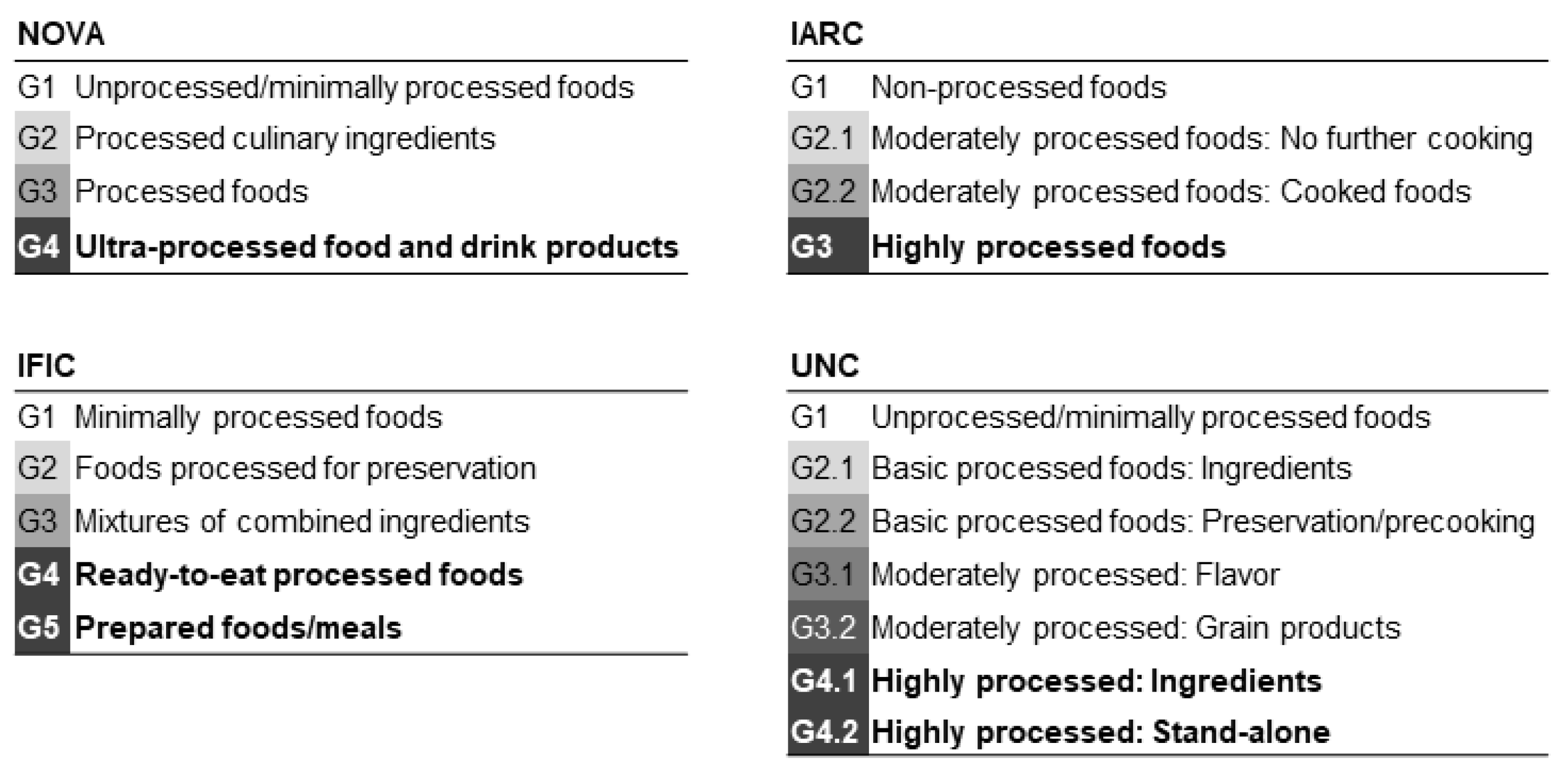
2. Materials and Methods
3. Results
3.1. UPF Consumption in the PREDIMED-Plus Cohort
3.2. Nutritional Profile of UPF Consumption Quintiles According to the Classification System
3.3. Association between Cardiometabolic Health Markers and UPF Consumption with Different Classification Systems
3.4. Concordance and Subject Agreement between Classification Systems in Quintiles of UPF Consumption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fardet, A. Food health potential is primarily due to its matrix structure, then nutrient composition: A new paradigm for food classification according to technological processes applied. J. Nutr. Health Food Eng. 2014, 1, 208–209. [Google Scholar] [CrossRef] [Green Version]
- Fardet, A. Characterization of the Degree of Food Processing in Relation with Its Health Potential and Effects. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 85, pp. 79–129. [Google Scholar]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009, 12, 729–731. [Google Scholar] [CrossRef] [Green Version]
- Moubarac, J.-C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef]
- Kelly, B.; Jacoby, E. Public Health Nutrition special issue on ultra-processed foods. Public Health Nutr. 2018, 21, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraldi, L.G.; Martinez Steele, E.; Canella, D.S.; Monteiro, C.A. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: Evidence from a nationally representative cross-sectional study. BMJ Open 2018, 8, e020574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cediel, G.; Reyes, M.; Da Costa Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A.; Corvalán, C.; Uauy, R. Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr. 2018, 21, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louzada, M.L.d.C.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev. Saude Publica 2015, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.; Martinez, L.; Allès, B.; Touvier, M.; Hercberg, S.; Méjean, C.; Kesse-Guyot, E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr. 2018, 21, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubarac, J.-C.C.; Batal, M.; Martins, A.P.B.; Claro, R.; Levy, R.B.; Cannon, G.; Monteiro, C. Processed and ultra-processed food products: Consumption trends in Canada from 1938 to 2011. Can. J. Diet. Pract. Res. 2014, 75, 15–21. [Google Scholar] [CrossRef]
- Slimani, N.; Deharveng, G.; Southgate, D.A.T.; Biessy, C.; Chajès, V.; van Bakel, M.M.E.; Boutron-Ruault, M.C.; McTaggart, A.; Grioni, S.; Verkaik-Kloosterman, J.; et al. Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European Prospective Investigation into Cancer and Nutrition study. Eur. J. Clin. Nutr. 2009, 63, S206–S225. [Google Scholar] [CrossRef]
- Rauber, F.; Louzada, M.L.d.C.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Canella, D.S.; Levy, R.B.; Martins, A.P.B.; Claro, R.M.; Moubarac, J.-C.C.; Baraldi, L.G.; Cannon, G.; Monteiro, C.A. Ultra-Processed Food Products and Obesity in Brazilian Households (2008–2009). PLoS ONE 2014, 9, e92752. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, R.d.D.; Lopes, A.C.S.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017, 30, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, R.d.D.; Pimenta, A.M.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.S.; Bes-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Bertazzi Levy, R.; Laura Louzada, M.C.; Constante Jaime, P. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association between Ultraprocessed Food Consumption and Risk of Mortality among Middle-aged Adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smaira, F.I.; Mazzolani, B.C.; Peçanha, T.; dos Santos, K.M.; Rezende, D.A.N.; Araujo, M.E.; Bonfiglioli, K.; Scagliusi, F.B.; Benatti, F.B.; de Sá Pinto, A.L.; et al. Ultra-processed food consumption associates with higher cardiovascular risk in rheumatoid arthritis. Clin. Rheumatol. 2020, 39, 1423–1428. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef]
- Schnabel, L.; Buscail, C.; Sabate, J.M.; Bouchoucha, M.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Monteiro, C.A.; Hercberg, S.; Benamouzig, R.; et al. Association Between Ultra-Processed Food Consumption and Functional Gastrointestinal Disorders: Results From the French NutriNet-Santé Cohort. Am. J. Gastroenterol. 2018, 113, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; Campagnolo, P.D.B.; Hoffman, D.J.; Vitolo, M.R. Consumption of ultra-processed food products and its effects on children’s lipid profiles: A longitudinal study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Louzada, M.L.d.C.; Baraldi, L.G.; Steele, E.M.; Martins, A.P.B.; Canella, D.S.; Moubarac, J.C.; Levy, R.B.; Cannon, G.; Afshin, A.; Imamura, F.; et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev. Med. (Baltim). 2015, 81, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Juul, F.; Hemmingsson, E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr. 2015, 18, 3096–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.M.; Giatti, L.; de Figueiredo, R.C.; Molina, M.D.C.B.; de Oliveira Cardoso, L.; Duncan, B.B.; Barreto, S.M. Consumption of ultra-processed food and obesity: Cross sectional results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort (2008–2010). Proc. Int. Astron. Union 2018, 21, 2271–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, C.A.; Moubarac, J.C.; Levy, R.B.; Canella, D.S.; Da Costa Louzada, M.L.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Rojo, R.; Sandoval-Insausti, H.; López-Garcia, E.; Graciani, A.; Ordovás, J.M.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Consumption of Ultra-Processed Foods and Mortality: A National Prospective Cohort in Spain. Mayo Clin. Proc. 2019, 94, 2178–2188. [Google Scholar] [CrossRef] [Green Version]
- Rico-Campà, A.; Martínez-González, M.A.; Alvarez-Alvarez, I.; de Deus Mendonça, R.; de La Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hu, E.A.; Rebholz, C.M. Ultra-processed food intake and mortality in the USA: Results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019, 22, 1777–1785. [Google Scholar] [CrossRef]
- Augustin, M.A.; Riley, M.; Stockmann, R.; Bennett, L.; Kahl, A.; Lockett, T.; Osmond, M.; Sanguansri, P.; Stonehouse, W.; Zajac, I.; et al. Role of food processing in food and nutrition security. Trends Food Sci. Technol. 2016, 56, 115–125. [Google Scholar] [CrossRef]
- European Food Information Council Food processing: The Advantages of Processed Foods. 2010. Available online: https://www.eufic.org/en/food-production/article/the-greatest-thing-since-sliced-bread-a-review-of-the-benefits-of-processed (accessed on 20 May 2020).
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. Increasing consumption of ultra-processed foods and likely impact on human health: Evidence from Brazil. Public Health Nutr. 2011, 14, 5–13. [Google Scholar] [CrossRef]
- Luiten, C.M.; Steenhuis, I.H.M.; Eyles, H.; Mhurchu, C.N.; Waterlander, W.E. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets. Public Health Nutr. 2016, 19, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, E.M.; Monteiro, C.A. Association between Dietary Share of Ultra-Processed Foods and Urinary Concentrations of Phytoestrogens in the US. Nutrients 2017, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodie, R.; Stuckler, D.; Monteiro, C.; Sheron, N.; Neal, B.; Thamarangsi, T.; Lincoln, P.; Casswell, S. Profits and pandemics: Prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 2013, 381, 670–679. [Google Scholar] [CrossRef]
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Morales, F.J.; Mesías, M.; Delgado-Andrade, C. Association between Heat-Induced Chemical Markers and Ultra-Processed Foods: A Case Study on Breakfast Cereals. Nutrients 2020, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide levels and dietary exposure from foods in the United States, an update based on 2011–2015 data. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1475–1490. [Google Scholar] [CrossRef]
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-processed foods in human health: A critical appraisal. Am. J. Clin. Nutr. 2017, 106, 717–724. [Google Scholar] [CrossRef] [Green Version]
- AESAN. Revista del Comité Científico de la AESAN No31; AESAN: Madrid, Spain, 2020; Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/publicaciones/revistas_comite_cientifico/comite_cientifico_31.pdf (accessed on 20 May 2020).
- Crino, M.; Barakat, T.; Trevena, H.; Neal, B. Systematic Review and Comparison of Classification Frameworks Describing the Degree of Food Processing. Nutr. Food Technol. 2017, 3, 138. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright (Food classification. Public health). World Nutr. 2016, 7, 28–38. [Google Scholar]
- Chajès, V.; Biessy, C.; Byrnes, G.; Deharveng, G.; Saadatian-Elahi, M.; Jenab, M.; Peeters, P.H.M.; Ocké, M.; Bueno-De-Mesquita, H.B.; Johansson, I.; et al. Ecological-Level associations between highly processed food intakes and plasma phospholipid elaidic acid concentrations: Results from a cross-sectional study within the European prospective investigation into Cancer and nutrition (EPIC). Nutr. Cancer 2011, 63, 1235–1250. [Google Scholar] [CrossRef]
- Eicher-Miller, H.A.; Fulgoni Iii, V.L.; Keast, D.R. Processed Food Contributions to Energy and Nutrient Intake Differ among US Children by Race/Ethnicity. Nutrients 2015, 7, 10076–10088. [Google Scholar] [CrossRef] [Green Version]
- Eicher-Miller, H.A.; Fulgoni, V.L.; Keast, D.R. Contributions of Processed Foods to Dietary Intake in the US from 2003–2008: A Report of the Food and Nutrition Science Solutions Joint Task Force of the Academy of Nutrition and Dietetics, American Society for Nutrition, Institute of Food Technologists. J. Nutr. 2012, 142, 2065S–2072S. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Mendez, M.A.; Ng, S.W.; Popkin, B.M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am. J. Clin. Nutr. 2015, 101, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Steele, E.M.; Baraldi, L.G.; Laura Da Costa Louzada, M.; Moubarac, J.-C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, e009892. [Google Scholar] [CrossRef] [Green Version]
- Silva Meneguelli, T.; Viana Hinkelmann, J.; Hermsdorff, H.H.M.; Zulet, M.Á.; Martínez, J.A.; Bressan, J. Food consumption by degree of processing and cardiometabolic risk: A systematic review. Int. J. Food Sci. Nutr. 2020, 71, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Alfredo Martínez, J.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.A.N.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente-Arrillaga, C.; Vá Zquez Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2009, 13, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos (Spanish Food Composition Tables), 19th ed.; Piramide: New York, NY, USA, 2018; ISBN 978-84-368-2391-2. [Google Scholar]
- Mataix Verdú, J.; García Diz, L.; Mañas Almendros, M.; Martinez de Vitoria, E.; Llopis González, J. Tablas de Composición de Alimentos (Spanish Food Composition Tables), 4th ed.; Editorial Universidad de Granada: Granada, Spain, 2009. [Google Scholar]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Dolores Zomeño, M.; Vioque, J.; Alfredo Martínez, J.; et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 20 May 2020).
- RStudio Team RStudio: Integrated Development Environment for R. Available online: http://www.rstudio.com/ (accessed on 20 May 2020).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for descriptive statistics. R package version 0.99.37. Available online: https://cran.r-project.org/package=DescTools (accessed on 20 May 2020).
- Revelle, W. psych: Procedures for Personality and Psychological Research. R package version 2.0.7. Available online: https://cran.r-project.org/package=psych (accessed on 20 May 2020).
- Yoshida, K.; Bartel, A. Tableone: Create “Table 1” to Describe Baseline Characteristics with or without Propensity Score Weights. R package version 0.12.0. Available online: https://cran.r-project.org/package=tableone (accessed on 20 May 2020).
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.2-1. Available online: https://cran.r-project.org/package=emmeans (accessed on 20 May 2020).
- Gamer, M.; Lemon, J.; Fellows, I.; Singh, P. Irr: Various Coefficients of Interrater Reliability and Agreement. R package version 0.84. Available online: https://cran.r-project.org/package=irr (accessed on 20 May 2020).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Moubarac, J.-C.; Paula, A.; Martins, B.; Moreira Claro, R.; Levy, R.B.; Cannon, G.; Monteiro, C.A. Consumption of ultra-processed foods and likely impact on human health. Evidence from Canada. Public Health Nutr. 2012, 16, 2240–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleiweiss-Sande, R.; Chui, K.; Evans, E.W.; Goldberg, J.; Amin, S.; Sacheck, J. Robustness of food processing classification systems. Nutrients 2019, 11, 1344. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, B.; Villamor, E.; Mora-Plazas, M.; Marin, C.; Monteiro, C.A.; Baylin, A. Processed and ultra-processed foods are associated with lower-quality nutrient profiles in children from Colombia. Public Health Nutr. 2017, 21, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Montero-Salazar, H.; Donat-Vargas, C.; Moreno-Franco, B.; Sandoval-Insausti, H.; Civeira, F.; Guallar-Castillón, P. High consumption of ultra-processed food may double the risk of subclinical coronary atherosclerosis : The Aragon Workers’ Health Study (AWHS). BMC Med. 2020, 18, 235. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Relationship between added sugars consumption and chronic disease risk factors: Current understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 20 May 2020).
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morenga, L.T.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/publications/i/item/9789241504836 (accessed on 20 May 2020).
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardet, A.; Rock, E.; Bassama, J.; Bohuon, P.; Prabhasankar, P.; Monteiro, C.; Moubarac, J.C.; Achir, N. Current food classifications in epidemiological studies do not enable solid nutritional recommendations for preventing diet-related chronic diseases: The impact of food processing. Adv. Nutr. 2015, 6, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; Steele, E.M.; Louzada, M.L.d.C.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008–2016). PLoS ONE 2020, 15, e0232676. [Google Scholar] [CrossRef]
- Beslay, M.; Srour, B.; Méjean, C.; Allès, B.; Fiolet, T.; Debras, C.; Chazelas, E.; Deschasaux, M.; Wendeu-Foyet, M.G.; Hercberg, S.; et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: A prospective analysis of the French NutriNet-Santé cohort. PLOS Med. 2020, 17, e1003256. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotto, D.; Zied, E. The Standard American Diet and Its Relationship to the Health Status of Americans. Nutr. Clin. Pract. 2010, 25, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.M.; Reedy, J.; Krebs-Smith, S.M. American Diet Quality: Where It Is, Where It Is Heading, and What It Could Be. J. Acad. Nutr. Diet. 2016, 116, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Hwalla, N.; Itani, L.; Karam, S.; Sibai, A.M.; Nasreddine, L. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13–19 years): A cross-sectional study. Br. J. Nutr. 2015, 114, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Preservation and Processing of Foods and the Risk of Cancer; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Ganesan, K.; Xu, B. Deep frying cooking oils promote the high risk of metastases in the breast-A critical review. Food Chem. Toxicol. 2020, 144, 111648. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Recommendations and Public Health and Policy Implications; World Cancer Research Fund International: London, UK, 2018; Available online: https://www.wcrf.org/wp-content/uploads/2021/01/Recommendations.pdf (accessed on 20 May 2020).
- Sullivan, B.L.; Brown, J.; Williams, P.G.; Meyer, B.J. Dietary validation of a new Australian food-frequency questionnaire that estimates long-chain n-3 polyunsaturated fatty acids. Br. J. Nutr. 2008, 99, 660–666. [Google Scholar] [CrossRef]
- Ishihara, J.; Todoriki, H.; Inoue, M.; Tsugane, S.; Sasaki, S.; Kobayashi, M.; Sobue, T.; Yamamoto, S.; Akabane, M.; Litoi, Y.; et al. Validity of a self-administered food-frequency questionnaire in the estimation of amino acid intake. Br. J. Nutr. 2009, 101, 1393–1399. [Google Scholar] [CrossRef]

| NOVA | IARC | IFIC | UNC | |
|---|---|---|---|---|
| Energy intake (kcal/day) | ▲ | ▲ | ▲ | ▲ |
| Protein (g/day) | ▲ | ▲ | ▲ | ▲ |
| Protein (% of energy intake) | ▼ | ▼ | ▼ | ▼ |
| Total fat (g/day) | ▲ | ▲ | ▲ | ▲ |
| Total fat (% of energy intake) | ▲ | ▼ | NS | ▼ |
| Saturated fat (g/day) | ▲ | ▲ | ▲ | ▲ |
| Saturated fat (% of energy intake) | ▲ | ▲ | ▲ | ▲ |
| Monounsaturated fat (g/day) | ▲ | ▲ | ▲ | ▲ |
| Monounsaturated fat (% of energy intake) | NS | ▼ | ▼ | ▼ |
| Polyunsaturated (g/day) | ▲ | ▲ | ▲ | ▲ |
| Polyunsaturated (% of energy intake) | NS | ▼ | ▼ | ▼ |
| Carbohydrate (g/day) | ▲ | ▲ | ▲ | ▲ |
| Carbohydrate (% of energy intake) | NS | NS | ▼ | ▼ |
| Fiber (g/day) | ▼ | ▼ | ▼ | ▼ |
| Simple sugars (g/day) | ▲ | ▲ | ▲ | ▲ |
| Sodium (mg/day) | ▲ | ▲ | ▲ | ▲ |
| Glycemic Index | ▼ | ▲ | ▼ | ▲ |
| Glycemic Load | ▲ | ▲ | ▲ | ▲ |
| Omega 3 (g/day) | ▼ | ▼ | ▼ | ▼ |
| NOVA | IARC | IFIC | UNC | |
|---|---|---|---|---|
| Weight (kg) | + | + | + | + |
| BMI (kg/m2) | + | NS | NS | NS |
| Waist circumference (cm) | + | + | + | + |
| Glucose (mg/dL) | NS | NS | NS | + |
| HbA1c (% mmol/mol) | NS | + | NS | NS |
| Triglycerides (mg/dL) | NS | NS | NS | NS |
| Total cholesterol (mg/dL) | NS | + | + | + |
| LDL cholesterol (mg/dL) | NS | NS | NS | NS |
| HDL cholesterol (mg/dL) | − | + | + | + |
| Creatinine (mg/dL) | + | NS | NS | NS |
| Systolic BP (mmHg) | NS | NS | NS | + |
| Diastolic BP (mmHg) | NS | NS | NS | + |
| NOVA | IARC | IFIC | UNC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | β | CI | p | β | CI | p | β | CI | p | β | CI | p | |
| Weight (kg) | 1 | 0.49 | (0.27, 0.71) | <0.001 | 0.23 | (0.11, 0.36) | <0.001 | 0.25 | (0.11, 0.38) | <0.001 | 0.20 | (0.06, 0.33) | 0.004 |
| 2 | 0.34 | (0.12, 0.56) | 0.002 | 0.13 | (0, 0.26) | 0.056 | 0.17 | (0.03, 0.31) | 0.017 | 0.12 | (−0.02, 0.25) | 0.097 | |
| 3 | 0.35 | (0.13, 0.57) | 0.002 | 0.14 | (0.01, 0.27) | 0.033 | 0.20 | (0.06, 0.34) | 0.005 | 0.14 | (0, 0.28) | 0.048 | |
| BMI (kg/m2) | 1 | 0.14 | (0.07, 0.21) | 0.001 | 0.04 | (0, 0.08) | 0.049 | 0.03 | (−0.01, 0.08) | 0.117 | 0.04 | (0, 0.08) | 0.062 |
| 2 | 0.11 | (0.04, 0.18) | 0.001 | 0.01 | (−0.03, 0.05) | 0.501 | 0.03 | (−0.01, 0.07) | 0.172 | 0.03 | (−0.01, 0.07) | 0.158 | |
| 3 | 0.11 | (0.05, 0.18) | 0.001 | 0.02 | (−0.02, 0.06) | 0.397 | 0.04 | (0, 0.08) | 0.067 | 0.04 | (−0.01, 0.08) | 0.089 | |
| Waist circumference (cm) | 1 | 0.34 | (0.16, 0.52) | 0.001 | 0.23 | (0.12, 0.33) | <0.001 | 0.15 | (0.04, 0.26) | 0.008 | 0.20 | (0.09, 0.31) | <0.001 |
| 2 | 0.25 | (0.07, 0.42) | 0.006 | 0.14 | (0.04, 0.25) | 0.008 | 0.11 | (0, 0.22) | 0.048 | 0.16 | (0.05, 0.27) | 0.005 | |
| 3 | 0.26 | (0.08, 0.43) | 0.004 | 0.15 | (0.04, 0.25) | 0.005 | 0.15 | (0.04, 0.26) | 0.008 | 0.18 | (0.07, 0.29) | 0.001 | |
| Glucose (mg/dL) | 1 | −0.43 | (−0.99, 0.12) | 0.123 | 0.29 | (−0.02, 0.61) | 0.07 | −0.28 | (−0.62, 0.07) | 0.115 | 0.06 | (−0.28, 0.39) | 0.745 |
| 2 | −0.42 | (−0.98, 0.14) | 0.145 | 0.33 | (0, 0.65) | 0.053 | −0.23 | (−0.59, 0.12) | 0.198 | 0.12 | (−0.23, 0.47) | 0.497 | |
| 3 | −0.28 | (−0.72, 0.2) | 0.254 | 0.26 | (−0.02, 0.54) | 0.066 | 0.16 | (−0.14, 0.46) | 0.292 | 0.32 | (0.02, 0.62) | 0.034 | |
| HbA1c (% mmol/mol) | 1 | 0.00 | (−0.02, 0.02) | 0.907 | 0.01 | (0, 0.02) | 0.045 | −0.02 | (−0.03, −0.01) | <0.001 | −0.01 | (−0.02, 0) | 0.02 |
| 2 | 0.00 | (−0.01,0.02) | 0.77 | 0.01 | (0, 0.02) | 0.026 | −0.02 | (−0.03, −0.01) | 0.002 | −0.01 | (−0.02, 0) | 0.048 | |
| 3 | 0.01 | (−0.01, 0.02) | 0.34 | 0.01 | (0, 0.02) | 0.036 | −0.01 | (−0.01, 0) | 0.31 | −0.01 | (−0.01, 0) | 0.277 | |
| Triglycerides (mg/dL) | 1 | 1.05 | (−0.01, 2.11) | 0.053 | 0.53 | (−0.08, 1.14) | 0.089 | 0.14 | (−0.53, 0.8) | 0.691 | 0.48 | (−0.16, 1.12) | 0.479 |
| 2 | 0.87 | (−0.21, 1.95) | 0.114 | 0.39 | (−0.25, 1.02) | 0.23 | 0.03 | (−0.66, 0.71) | 0.937 | 0.36 | (−0.32, 1.03) | 0.299 | |
| 3 | 0.87 | (−0.2, 1.95) | 0.112 | 0.36 | (−0.28, 0.99) | 0.273 | 0.03 | (−0.65, 0.71) | 0.931 | 0.35 | (−0.32, 1.02) | 0.311 | |
| Total cholesterol (mg/dL) | 1 | −0.33 | (−1.04, 0.38) | 0.364 | 0.32 | (−0.09, 0.73) | 0.122 | 1.12 | (0.68, 1.56) | <0.001 | 0.83 | (0.4, 1.26) | <0.001 |
| 2 | −0.47 | (−1.19, 0.25) | 0.202 | 0.27 | (−0.15, 0.7) | 0.212 | 1.02 | (0.57, 1.48) | <0.001 | 0.77 | (0.32, 1.21) | 0.001 | |
| 3 | −0.45 | (−1.11, 0.21) | 0.181 | 0.46 | (0.07, 0.84) | 0.021 | 0.88 | (0.46, 1.29) | <0.001 | 0.79 | (0.38, 1.2) | <0.001 | |
| LDL cholesterol (mg/dL) | 1 | −0.19 | (−0.82, 0.45) | 0.561 | −0.04 | (−0.41, 0.33) | 0.826 | 0.41 | (0.01, 0.81) | 0.043 | 0.11 | (−0.27, 0.5) | 0.565 |
| 2 | −0.29 | (−0.94, 0.36) | 0.383 | −0.09 | (−0.47, 0.29) | 0.63 | 0.31 | (−0.1, 0.72) | 0.136 | 0.02 | (−0.38, 0.43) | 0.908 | |
| 3 | −0.25 | (−0.83, 0.32) | 0.391 | 0.10 | (−0.24, 0.43) | 0.578 | 0.20 | (−0.16, 0.57) | 0.282 | 0.07 | (−0.29, 0.43) | 0.702 | |
| HDL cholesterol (mg/dL) | 1 | −0.35 | (−0.56, −0.13) | 0.001 | 0.26 | (0.14, 0.38) | <0.001 | 0.68 | (0.55, 0.81) | <0.001 | 0.62 | (0.05, 0.75) | <0.001 |
| 2 | −0.35 | (−0.57, −0.14) | 0.001 | 0.29 | (0.16, 0.41) | <0.001 | 0.71 | (0.57, 0.84) | <0.001 | 0.67 | (0.54, 0.81) | <0.001 | |
| 3 | −0.37 | (−0.58, −0.15) | <0.001 | 0.29 | (0.16, 0.42) | <0.001 | 0.67 | (0.54, 0.81) | <0.001 | 0.65 | (0.52, 0.78) | <0.001 | |
| Creatinine (mg/dL) | 1 | 0.01 | (0, 0.01) | <0.001 | 0.00 | (0, 0) | 0.683 | 0.00 | (0, 0) | 0.691 | 0.00 | (0, 0) | 0.976 |
| 2 | 0.01 | (0, 0.01) | <0.001 | 0.00 | (0, 0) | 0.424 | 0.00 | (0, 0) | 0.715 | 0.00 | (0, 0) | 0.641 | |
| 3 | 0.01 | (0, 0.01) | <0.001 | 0.00 | (0, 0) | 0.435 | 0.00 | (0, 0) | 0.694 | 0.00 | (0, 0) | 0.7 | |
| Systolic BP (mmHg) | 1 | −0.21 | (−0.53, 0.12) | 0.218 | −0.07 | (−0.26, 0.12) | 0.454 | 0.03 | (−0.17, 0.23) | 0.773 | 0.21 | (0.02, 0.41) | 0.213 |
| 2 | −0.17 | (−0.5, 0.16) | 0.313 | −0.08 | (−0.28, 0.11) | 0.419 | 0.05 | (−0.16, 0.26) | 0.663 | 0.24 | (0.03, 0.44) | 0.045 | |
| 3 | −0.17 | (−0.5, 0.16) | 0.321 | −0.08 | (−0.27, 0.12) | 0.45 | 0.08 | (−0.13, 0.29) | 0.482 | 0.25 | (0.04, 0.46) | 0.018 | |
| Diastolic BP (mmHg) | 1 | 0.10 | (−0.08, 0.28) | 0.274 | 0.01 | (−0.1, 0.11) | 0.913 | 0.10 | (−0.02, 0.21) | 0.092 | 0.14 | (0.03, 0.25) | 0.013 |
| 2 | 0.08 | (−0.1, 0.27) | 0.378 | −0.02 | (−0.13, 0.08) | 0.661 | 0.07 | (0.04, 0.19) | 0.21 | 0.12 | (0, 0.23) | 0.045 | |
| 3 | 0.08 | (−0.1, 0.26) | 0.383 | −0.01 | (−0.12, 0.1) | 0.821 | 0.07 | (−0.05, 0.18) | 0.252 | 0.12 | (0, 0.23) | 0.042 | |
| ICC3 | Overall | Q1 | Q5 | ||
|---|---|---|---|---|---|
| NOVA | IARC | 0.32 | 28.0 | 7.2 | 7.8 |
| NOVA | IFIC | 0.45 | 32.3 | 8.8 | 8.9 |
| NOVA | UNC | 0.38 | 30.0 | 8.3 | 7.4 |
| IARC | IFIC | 0.61 | 38.4 | 9.8 | 12.3 |
| IARC | UNC | 0.59 | 38.4 | 9.4 | 12.1 |
| IFIC | UNC | 0.74 | 48.6 | 10.8 | 15.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Perez, C.; San-Cristobal, R.; Guallar-Castillon, P.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Castañer, O.; Martinez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Use of Different Food Classification Systems to Assess the Association between Ultra-Processed Food Consumption and Cardiometabolic Health in an Elderly Population with Metabolic Syndrome (PREDIMED-Plus Cohort). Nutrients 2021, 13, 2471. https://doi.org/10.3390/nu13072471
Martinez-Perez C, San-Cristobal R, Guallar-Castillon P, Martínez-González MÁ, Salas-Salvadó J, Corella D, Castañer O, Martinez JA, Alonso-Gómez ÁM, Wärnberg J, et al. Use of Different Food Classification Systems to Assess the Association between Ultra-Processed Food Consumption and Cardiometabolic Health in an Elderly Population with Metabolic Syndrome (PREDIMED-Plus Cohort). Nutrients. 2021; 13(7):2471. https://doi.org/10.3390/nu13072471
Chicago/Turabian StyleMartinez-Perez, Celia, Rodrigo San-Cristobal, Pilar Guallar-Castillon, Miguel Ángel Martínez-González, Jordi Salas-Salvadó, Dolores Corella, Olga Castañer, Jose Alfredo Martinez, Ángel M. Alonso-Gómez, Julia Wärnberg, and et al. 2021. "Use of Different Food Classification Systems to Assess the Association between Ultra-Processed Food Consumption and Cardiometabolic Health in an Elderly Population with Metabolic Syndrome (PREDIMED-Plus Cohort)" Nutrients 13, no. 7: 2471. https://doi.org/10.3390/nu13072471
APA StyleMartinez-Perez, C., San-Cristobal, R., Guallar-Castillon, P., Martínez-González, M. Á., Salas-Salvadó, J., Corella, D., Castañer, O., Martinez, J. A., Alonso-Gómez, Á. M., Wärnberg, J., Vioque, J., Romaguera, D., López-Miranda, J., Estruch, R., Tinahones, F. J., Lapetra, J., Serra-Majem, L., Bueno-Cavanillas, A., Tur, J. A., ... Daimiel, L. (2021). Use of Different Food Classification Systems to Assess the Association between Ultra-Processed Food Consumption and Cardiometabolic Health in an Elderly Population with Metabolic Syndrome (PREDIMED-Plus Cohort). Nutrients, 13(7), 2471. https://doi.org/10.3390/nu13072471




















