Association of Patient-Reported Outcomes and Nutrition with Body Composition in Women with Gynecologic Cancer Undergoing Post-Operative Pelvic Radiotherapy: An Observational Study
Abstract
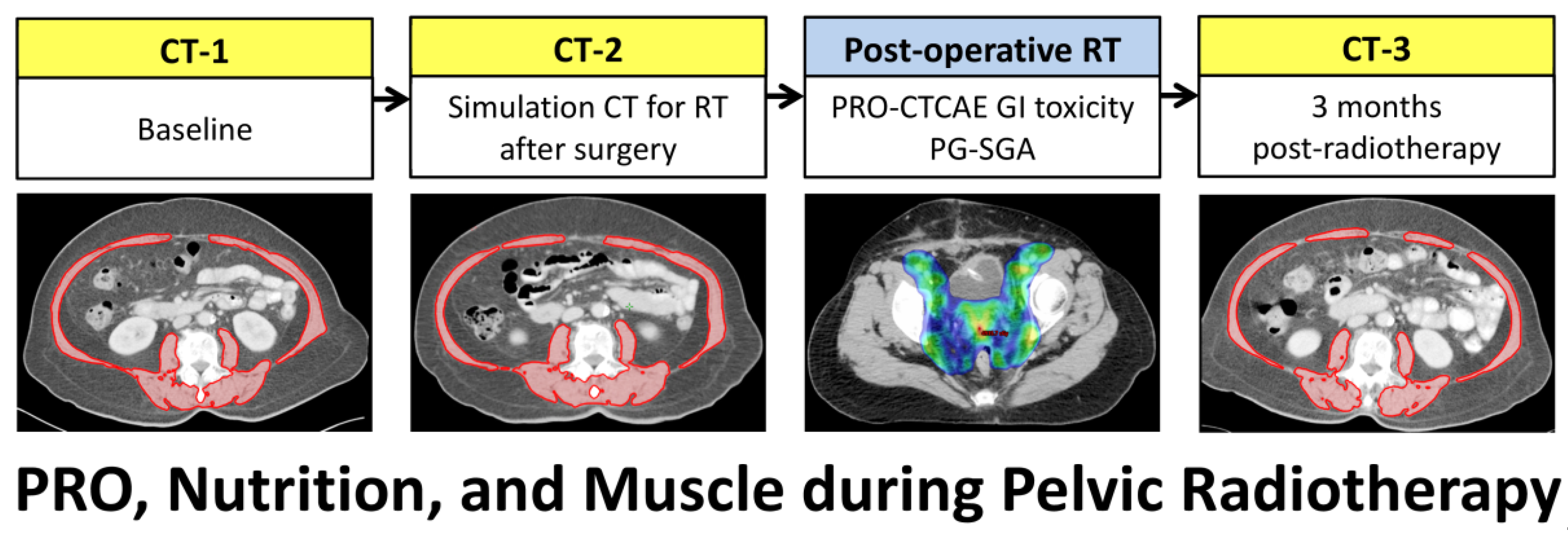
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatments
2.3. Toxicity Assessment
2.4. Nutritional Assessment
2.5. Body Composition on CT Scans
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. PRO-CTCAE and Physician-Reported CTCAE
3.3. PG-SGA Score at the Beginning and End of Radiotherapy
3.4. Body Composition Changes after Surgery and Post-Operative Radiotherapy
3.5. Body Composition Changes by PRO-CTCAE or Physician-Reported CTCAE
3.6. Body Composition Changes by PG-SGA
3.7. Predictor of Weight, Muscle, or Adipose Tissue Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Peters, W.A., 3rd; Liu, P.Y.; Barrett, R.J., 2nd; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W., Jr.; Thompson, S.; et al. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef]
- Gil, K.M.; Pugh, S.L.; Klopp, A.H.; Yeung, A.R.; Wenzel, L.; Westin, S.N.; Gaffney, D.K.; Small, W., Jr.; Thompson, S.; Doncals, D.E.; et al. Expanded validation of the EPIC bowel and urinary domains for use in women with gynecologic cancer undergoing postoperative radiotherapy. Gynecol. Oncol. 2019, 154, 183–188. [Google Scholar] [CrossRef]
- Yeung, A.R.; Pugh, S.L.; Klopp, A.H.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gaffney, D.K.; Small, W., Jr.; Thompson, S.; Doncals, D.E.; et al. Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study. J. Clin. Oncol. 2020, 38, 1685–1692. [Google Scholar] [CrossRef]
- Lee, J.; Lin, J.B.; Wu, M.H.; Chang, C.L.; Jan, Y.T.; Sun, F.J.; Chen, Y.J. Association of bowel radiation dose-volume with skeletal muscle loss during pelvic intensity-modulated radiotherapy in cervical cancer. Support Care Cancer 2021, 29, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Castro-Eguiluz, D.; Luvian-Morales, J.; Jimenez-Lima, R.; Aguilar-Ponce, J.L.; Isla-Ortiz, D.; Cetina, L. Deterioration of nutritional status of patients with locally advanced cervical cancer during treatment with concomitant chemoradiotherapy. J. Hum. Nutr. Diet. 2019, 32, 480–491. [Google Scholar] [CrossRef]
- Lee, J.; Chang, C.L.; Lin, J.B.; Wu, M.H.; Sun, F.J.; Jan, Y.T.; Hsu, S.M.; Chen, Y.J. Skeletal Muscle Loss Is an Imaging Biomarker of Outcome after Definitive Chemoradiotherapy for Locally Advanced Cervical Cancer. Clin. Cancer Res. 2018, 24, 5028–5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedlake, L.J. Nutritional strategies to prevent gastrointestinal toxicity during pelvic radiotherapy. Proc. Nutr. Soc. 2018, 77, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Allanson, E.R.; Peng, Y.; Choi, A.; Hayes, S.; Janda, M.; Obermair, A. A systematic review and meta-analysis of sarcopenia as a prognostic factor in gynecological malignancy. Int. J. Gynecol. Cancer 2020, 30, 1791–1797. [Google Scholar] [CrossRef]
- Kiyotoki, T.; Nakamura, K.; Haraga, J.; Omichi, C.; Ida, N.; Saijo, M.; Nishida, T.; Kusumoto, T.; Masuyama, H. Sarcopenia Is an Important Prognostic Factor in Patients With Cervical Cancer Undergoing Concurrent Chemoradiotherapy. Int. J. Gynecol. Cancer 2018, 28, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lin, J.B.; Wu, M.H.; Jan, Y.T.; Chang, C.L.; Huang, C.Y.; Sun, F.J.; Chen, Y.J. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 814–826. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Xiao, C.; Polomano, R.; Bruner, D.W. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs. 2013, 36, E1–E16. [Google Scholar] [CrossRef]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef] [PubMed]
- Falchook, A.D.; Green, R.; Knowles, M.E.; Amdur, R.J.; Mendenhall, W.; Hayes, D.N.; Grilley-Olson, J.E.; Weiss, J.; Reeve, B.B.; Mitchell, S.A.; et al. Comparison of Patient- and Practitioner-Reported Toxic Effects Associated With Chemoradiotherapy for Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.H.; Chera, B.S.; Deal, A.M.; Wang, Y.; Muss, H.B.; Vander Walde, N.A. Clinician-observed and patient-reported toxicities and their association with poor tolerance to therapy in older patients with head and neck or lung cancer treated with curative radiotherapy. J. Geriatr. Oncol. 2019, 10, 42–47. [Google Scholar] [CrossRef]
- van Seventer, E.E.; Fintelmann, F.J.; Roeland, E.J.; Nipp, R.D. Leveraging the Potential Synergy Between Patient-Reported Outcomes and Body Composition Analysis in Patients with Cancer. Oncologist 2020, 25, 271–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M.; et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef]
- Atallah, S.; Barbera, L.; Folwell, M.; Howell, D.; Liu, Z.; Croke, J. Feasibility of implementing a cervix cancer-specific patient-reported outcome measure in routine ambulatory clinics. Supportive Care Cancer 2021, 29, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.M.; Swain, T.A.; Mayhew, D.L.; Cardan, R.A.; Baker, C.B.; Harris, D.M.; Yang, E.S.; Fiveash, J.B. CT Measures of Bone Mineral Density and Muscle Mass Can Be Used to Predict Noncancer Death in Men with Prostate Cancer. Radiology 2017, 282, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Sun, F.J.; Lee, J. Prognostic value of muscle measurement using the standardized phase of computed tomography in patients with advanced ovarian cancer. Nutrition 2020, 72, 110642. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, S.H.; Chen, J.C.; Leu, Y.S.; Liu, C.J.; Chen, Y.J. Progressive muscle loss is an independent predictor for survival in locally advanced oral cavity cancer: A longitudinal study. Radiother. Oncol. 2021, 158, 83–89. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Lee, L.J.; Eswara, J.R.; Horowitz, N.S.; Konstantinopoulos, P.A.; Mirabeau-Beale, K.L.; Rose, B.S.; von Keudell, A.G.; Wo, J.Y. Complications of pelvic radiation in patients treated for gynecologic malignancies. Cancer 2014, 120, 3870–3883. [Google Scholar] [CrossRef]
- Klassen, P.; Baracos, V.; Gramlich, L.; Nelson, G.; Mazurak, V.; Martin, L. Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway. Nutrients 2020, 12, 3745. [Google Scholar] [CrossRef]
- Zambrano, D.N.; Xiao, J.; Prado, C.M.; Gonzalez, M.C. Patient-Generated Subjective Global Assessment and Computed Tomography in the assessment of malnutrition and sarcopenia in patients with cirrhosis: Is there any association? Clin. Nutr. 2020, 39, 1535–1540. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.M.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yang, Y.C.; Chen, T.C.; Chen, J.R.; Chen, Y.J.; Wu, M.H.; Jan, Y.T.; Chang, C.L.; Lee, J. Muscle loss during primary debulking surgery and chemotherapy predicts poor survival in advanced-stage ovarian cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 534–546. [Google Scholar] [CrossRef] [Green Version]
- Naumann, P.; Eberlein, J.; Farnia, B.; Liermann, J.; Hackert, T.; Debus, J.; Combs, S.E. Cachectic Body Composition and Inflammatory Markers Portend a Poor Prognosis in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers 2019, 11, 1655. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, S.H.; Dai, K.Y.; Huang, Y.M.; Li, C.J.; Chen, J.C.; Leu, Y.S.; Liu, C.J.; Chen, Y.J. Sarcopenia and Systemic Inflammation Synergistically Impact Survival in Oral Cavity Cancer. Laryngoscope 2021, 131, E1530–E1538. [Google Scholar] [CrossRef] [PubMed]
- Heijkoop, S.T.; Nout, R.A.; Quint, S.; Mens, J.W.M.; Heijmen, B.J.M.; Hoogeman, M.S. Dynamics of patient reported quality of life and symptoms in the acute phase of online adaptive external beam radiation therapy for locally advanced cervical cancer. Gynecol. Oncol. 2017, 147, 439–449. [Google Scholar] [CrossRef]
- Basch, E.; Jia, X.; Heller, G.; Barz, A.; Sit, L.; Fruscione, M.; Appawu, M.; Iasonos, A.; Atkinson, T.; Goldfarb, S.; et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J. Natl. Cancer Inst. 2009, 101, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Quinten, C.; Maringwa, J.; Gotay, C.C.; Martinelli, F.; Coens, C.; Reeve, B.B.; Flechtner, H.; Greimel, E.; King, M.; Osoba, D.; et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J. Natl. Cancer Inst. 2011, 103, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Anker, S.D.; Coats, A.J.S.; Laviano, A.; von Haehling, S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: Avoiding the wildfire. J. Cachexia Sarcopenia Muscle 2021, 12, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.W.; Tanksley, J.; Chino, J.; Willett, C.G.; Dewhirst, M.W. Long-term Consequences of Pelvic Irradiation: Toxicities, Challenges, and Therapeutic Opportunities with Pharmacologic Mitigators. Clin. Cancer Res. 2020, 26, 3079–3090. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Caan, B.J.; Meyerhardt, J.A.; Weltzien, E.; Xiao, J.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Castillo, A.; Kwan, M.L.; Prado, C.M. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: A population-based cohort study (C-SCANS). J. Cachexia Sarcopenia Muscle 2018, 9, 664–672. [Google Scholar] [CrossRef]



| Characteristics | Overall (n = 210) |
|---|---|
| Age (years) | 56 (50–62) |
| Disease cite | |
| Endometrium | 142 (67.6) |
| Cervix | 68 (32.4) |
| Surgery type | |
| Open | 164 (78.1) |
| Minimally invasive | 46 (21.9) |
| Radiation dose | |
| 45 Gy | 95 (45.2) |
| 50.4 Gy | 115 (54.8) |
| Brachytherapy | |
| Yes | 142 (67.6) |
| No | 68 (32.4) |
| Chemotherapy | |
| Yes | 84 (40.0) |
| No | 126 (60.0) |
| NLR | |
| ≤3 | 134 (63.8) |
| >3 | 76 (36.2) |
| Bowel radiation dose-volume a V45 (mL) | 158.9 (122.9–190.3) |
| Variable | PRO-CTCAE Score | Physician-Reported CTCAE Grade | ||||
|---|---|---|---|---|---|---|
| 0–2 (n = 162) | 3–4 (n = 48) | p-Value | 0–2 (n = 194) | 3–4 (n = 16) | p-Value | |
| BMI change, n (%) | ||||||
| Gain or loss <5% | 148 (91.4) | 30 (62.5) | <0.001 | 166 (85.6) | 12 (75.0) | 0.28 |
| Loss ≥5% | 14 (8.6) | 18 (37.5) | 28 (14.4) | 4 (25.0) | ||
| SMI change, n (%) | ||||||
| Gain or loss <5% | 150 (92.6) | 20 (41.7) | <0.001 | 158 (81.4) | 12 (75.0) | 0.51 |
| Loss ≥5% | 12 (7.4) | 28 (58.3) | 36 (18.6) | 4 (25.0) | ||
| TATI change, n (%) | ||||||
| Gain or loss <5% | 130 (80.2) | 14 (29.2) | <0.001 | 133 (68.6) | 11 (68.8) | 0.99 |
| Loss ≥5% | 32 (19.8) | 34 (70.8) | 61 (31.4) | 5 (31.3) | ||
| Variable | PG-SGA at the Beginning of Radiotherapy | PG-SGA at the End of Radiotherapy | ||||
|---|---|---|---|---|---|---|
| ≤3 (n = 181) | ≥4 (n = 29) | p-Value | ≤3 (n = 134) | ≥4 (n = 76) | p-Value | |
| BMI change, n (%) | ||||||
| Gain or loss <5% | 153 (84.5) | 25 (86.4) | 1.00 | 133 (99.3) | 45 (59.2) | <0.001 |
| Loss ≥5% | 28 (15.5) | 4 (13.8) | 1 (0.7) | 31 (40.8) | ||
| SMI change, n (%) | ||||||
| Gain or loss <5% | 149 (82.3) | 21 (72.4) | 0.21 | 133 (99.3) | 37 (48.7) | <0.001 |
| Loss ≥5% | 32 (17.7) | 8 (27.6) | 1 (0.7) | 39 (51.3) | ||
| TATI change, n (%) | ||||||
| Gain or loss <5% | 125 (69.1) | 19 (65.5) | 0.67 | 116 (86.6) | 28 (36.8) | <0.001 |
| Loss ≥5% | 56 (30.9) | 10 (34.5) | 18 (13.4) | 48 (63.2) | ||
| Variable | Weight Loss ≥5% | Muscle Loss ≥5% | Adipose Tissue Loss ≥5% | |||
|---|---|---|---|---|---|---|
| OR (95% CI) a,b | p-Value | OR (95% CI) a,b | p-Value | OR (95% CI) a,b | p-Value | |
| PG-SGA score at the end of RT | ||||||
| 0–3 | Reference | Reference | Reference | |||
| ≥4 | 74.07 (9.34–587.3) | <0.001 | 72.96 (9.45–563.18) | <0.001 | 8.01 (3.78–16.98) | <0.001 |
| Any PRO-CTCAE score | ||||||
| 0–2 | Reference | Reference | Reference | |||
| ≥3 | 3.63(1.43–9.17) | 0.007 | 8.81 (3.26–20.04) | <0.001 | 6.67(2.94–15.12) | <0.001 |
| PRO-CTCAE, diarrhea frequency | ||||||
| 0–2 | Reference | Reference | Reference | |||
| ≥3 | 4.73 (1.85–12.13) | 0.001 | 7.65 (3.08–19.00) | <0.001 | 7.62 (3.23–18.00) | <0.001 |
| PRO-CTCAE, abdominal pain severity | ||||||
| 0–2 | Reference | Reference | Reference | |||
| ≥3 | 1.13 (0.33–3.85) | 0.84 | 3.97 (1.23–12.85) | 0.02 | 1.98 (0.69–5.67) | 0.20 |
| PRO-CTCAE, abdominal pain interference | ||||||
| 0–2 | Reference | Reference | Reference | |||
| ≥3 | 5.47 (1.88–15.93) | 0.002 | 19.47 (5.25–72.28) | <0.001 | 10.12 (2.72–37.59) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Chen, T.-C.; Jan, Y.-T.; Li, C.-J.; Chen, Y.-J.; Wu, M.-H. Association of Patient-Reported Outcomes and Nutrition with Body Composition in Women with Gynecologic Cancer Undergoing Post-Operative Pelvic Radiotherapy: An Observational Study. Nutrients 2021, 13, 2629. https://doi.org/10.3390/nu13082629
Lee J, Chen T-C, Jan Y-T, Li C-J, Chen Y-J, Wu M-H. Association of Patient-Reported Outcomes and Nutrition with Body Composition in Women with Gynecologic Cancer Undergoing Post-Operative Pelvic Radiotherapy: An Observational Study. Nutrients. 2021; 13(8):2629. https://doi.org/10.3390/nu13082629
Chicago/Turabian StyleLee, Jie, Tze-Chien Chen, Ya-Ting Jan, Chi-Jung Li, Yu-Jen Chen, and Meng-Hao Wu. 2021. "Association of Patient-Reported Outcomes and Nutrition with Body Composition in Women with Gynecologic Cancer Undergoing Post-Operative Pelvic Radiotherapy: An Observational Study" Nutrients 13, no. 8: 2629. https://doi.org/10.3390/nu13082629
APA StyleLee, J., Chen, T.-C., Jan, Y.-T., Li, C.-J., Chen, Y.-J., & Wu, M.-H. (2021). Association of Patient-Reported Outcomes and Nutrition with Body Composition in Women with Gynecologic Cancer Undergoing Post-Operative Pelvic Radiotherapy: An Observational Study. Nutrients, 13(8), 2629. https://doi.org/10.3390/nu13082629







