Timing of Creatine Supplementation around Exercise: A Real Concern?
Abstract
:1. Introduction
2. Creatine Supplementation
Factors Modifying the Effect of Creatine Supplementation on Muscle Creatine Content
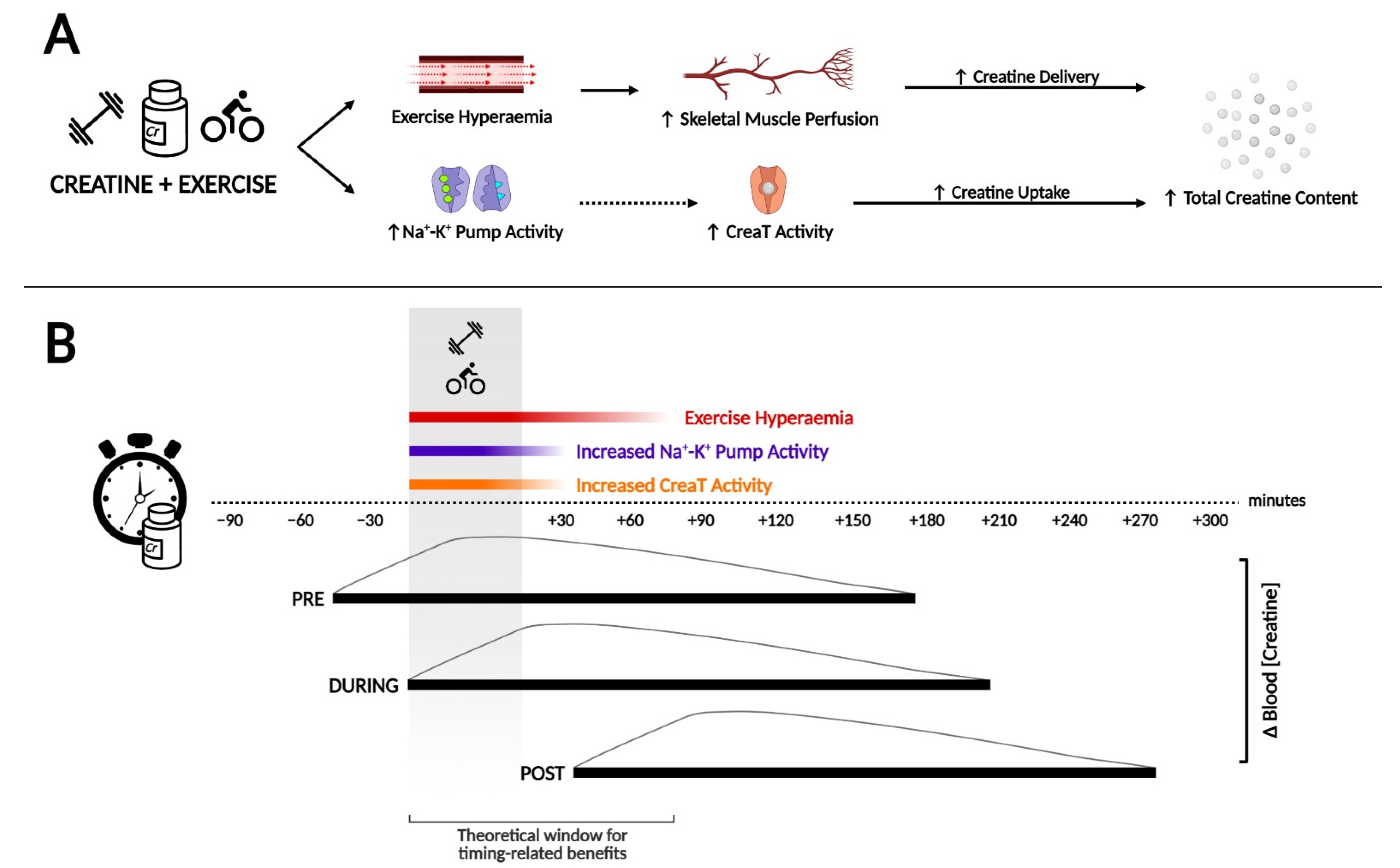
3. How Creatine Timing around Exercise May Influence Subsequent Loading
3.1. Creatine Concentration in the Bloodstream and Training Duration
3.2. Na+-K+ Pump Activity and Exercise
4. Creatine Supplementation Pre-, During- or Post-Workout: The Evidence
5. Future Directions for Research
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Kreider, R.B.; Jung, Y.P. Creatine supplementation in exercise, sport, and medicine. J. Exerc. Nutr. Biochem. 2011, 15, 53–69. [Google Scholar] [CrossRef]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition position stand: Creatine supplementation and exercise. J. Int. Soc. Sports Nutr. 2007, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. (Lond.) 1992, 83, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Green, A.L.; Simpson, E.J.; Littlewood, J.J.; Macdonald, I.A.; Greenhaff, P.L. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol. Scand. 1996, 158, 195–202. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Casey, A.; Short, A.H.; Harris, R.; Soderlund, K.; Hultman, E. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clin. Sci. (Lond.) 1993, 84, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Solis, M.Y.; Artioli, G.G.; Otaduy, M.C.G.; Leite, C.D.C.; Arruda, W.; Veiga, R.R.; Gualano, B. Effect of age, diet, and tissue type on PCr response to creatine supplementation. J. Appl. Physiol. (1985) 2017, 123, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef]
- Antonio, J.; Ciccone, V. The effects of pre versus post workout supplementation of creatine monohydrate on body composition and strength. J. Int. Soc. Sports Nutr. 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Melton, C.; Rasmussen, C.J.; Greenwood, M.; Lancaster, S.; Cantler, E.C.; Milnor, P.; Almada, A.L. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol. Cell. Biochem. 2003, 244, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Padilla, S. Creatine supplementation as an ergogenic aid for sports performance in highly trained athletes: A critical review. Int. J. Sports Med. 1997, 18, 491–496. [Google Scholar] [CrossRef]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281 Pt 1, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Sahlin, K. Muscle Energetics During Explosive Activities and Potential Effects of Nutrition and Training. Sports Med. 2014, 44, S167–S173. [Google Scholar] [CrossRef] [Green Version]
- Green, A.L.; Hultman, E.; Macdonald, I.A.; Sewell, D.A.; Greenhaff, P.L. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am. J. Physiol. 1996, 271, E821–E826. [Google Scholar] [CrossRef]
- Greenwood, M.; Kreider, R.; Earnest, C.; Rasmussen, C.; Almada, A. Differences in creatine retention among three nutritional formulations of oral creatine supplements. J. Exerc. Physiol. Online 2003, 6, 37–43. [Google Scholar]
- Robinson, T.M.; Sewell, D.A.; Hultman, E.; Greenhaff, P.L. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J. Appl. Physiol. (1985) 1999, 87, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Candow, D.G.; Chilibeck, P.D. Timing of creatine or protein supplementation and resistance training in the elderly. Appl. Physiol. Nutr. Metab. 2008, 33, 184–190. [Google Scholar] [CrossRef]
- Candow, D.G.; Zello, G.A.; Ling, B.; Farthing, J.P.; Chilibeck, P.D.; McLeod, K.; Harris, J.; Johnson, S. Comparison of creatine supplementation before versus after supervised resistance training in healthy older adults. Res. Sports Med. 2014, 22, 61–74. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wilkins, B.W. Exercise hyperaemia: Is anything obligatory but the hyperaemia? J. Physiol. 2007, 583, 855–860. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.A.; Fox, J.; Peirce, N.; Jones, S.W.; Casey, A.; Greenhaff, P.L. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids 2016, 48, 1831–1842. [Google Scholar] [CrossRef]
- Persky, A.M.; Brazeau, G.A.; Hochhaus, G. Pharmacokinetics of the dietary supplement creatine. Clin. Pharmacokinet. 2003, 42, 557–574. [Google Scholar] [CrossRef]
- Jäger, R.; Harris, R.C.; Purpura, M.; Francaux, M. Comparison of new forms of creatine in raising plasma creatine levels. J. Int. Soc. Sports Nutr. 2007, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawson, E.S.; Clarkson, P.M.; Price, T.B.; Miles, M.P. Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol. Scand. 2002, 174, 57–65. [Google Scholar] [CrossRef]
- Steenge, G.R.; Simpson, E.J.; Greenhaff, P.L. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J. Appl. Physiol. (1985) 2000, 89, 1165–1171. [Google Scholar] [CrossRef]
- Schedel, J.M.; Tanaka, H.; Kiyonaga, A.; Shindo, M.; Schutz, Y. Acute creatine ingestion in human: Consequences on serum creatine and creatinine concentrations. Life Sci. 1999, 65, 2463–2470. [Google Scholar] [CrossRef]
- Hackett, D.A.; Johnson, N.A.; Chow, C.M. Training practices and ergogenic aids used by male bodybuilders. J. Strength Cond. Res. 2013, 27, 1609–1617. [Google Scholar] [CrossRef]
- Bangsbo, J.; Hellsten, Y. Muscle blood flow and oxygen uptake in recovery from exercise. Acta Physiol. Scand. 1998, 162, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Korthuis, R.J. Chapter 4, Exercise Hyperemia and Regulation of Tissue Oxygenation During Muscular Activity. In Skeletal Muscle Circulation; Korthuis, R.J., Ed.; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011. [Google Scholar]
- Christie, D.L. Functional insights into the creatine transporter. Sub Cell. Biochem. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Daly, M.M.; Seifter, S. Uptake of creatine by cultured cells. Arch. Biochem. Biophys. 1980, 203, 317–324. [Google Scholar] [CrossRef]
- Odoom, J.E.; Kemp, G.J.; Radda, G.K. The regulation of total creatine content in a myoblast cell line. Mol. Cell. Biochem. 1996, 158, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G. Timing of Creatine Supplementation and Resistance Training: A Brief Review. J. Exerc. Nutr. 2018, 1, 1–6. [Google Scholar]
- Green, H.J.; Chin, E.R.; Ball-Burnett, M.; Ranney, D. Increases in human skeletal muscle Na(+)-K(+)-ATPase concentration with short-term training. Am. J. Physiol. 1993, 264, C1538–C1541. [Google Scholar] [CrossRef] [PubMed]
- Aughey, R.J.; Murphy, K.T.; Clark, S.A.; Garnham, A.P.; Snow, R.J.; Cameron-Smith, D.; Hawley, J.A.; McKenna, M.J. Muscle Na+-K+-ATPase activity and isoform adaptations to intense interval exercise and training in well-trained athletes. J. Appl. Physiol. (1985) 2007, 103, 39–47. [Google Scholar] [CrossRef]
- Holloszy, J.O. Exercise-induced increase in muscle insulin sensitivity. J. Appl. Physiol. (1985) 2005, 99, 338–343. [Google Scholar] [CrossRef]
- Cribb, P.J.; Hayes, A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med. Sci. Sports Exerc. 2006, 38, 1918–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipton, K.D.; Rasmussen, B.B.; Miller, S.L.; Wolf, S.E.; Owens-Stovall, S.K.; Petrini, B.E.; Wolfe, R.R. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E197–E206. [Google Scholar] [CrossRef] [Green Version]
- Stecker, R.A.; Harty, P.S.; Jagim, A.R.; Candow, D.G.; Kerksick, C.M. Timing of ergogenic aids and micronutrients on muscle and exercise performance. J. Int. Soc. Sports Nutr. 2019, 16, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainani, K.L. The Problem with “Magnitude-based Inference”. Med. Sci. Sports Exerc. 2018, 50, 2166–2176. [Google Scholar] [CrossRef]
- Sainani, K.L.; Lohse, K.R.; Jones, P.R.; Vickers, A. Magnitude-based Inference is not Bayesian and is not a valid method of inference. Scand. J. Med. Sci. Sports 2019, 29, 1428–1436. [Google Scholar] [CrossRef]
- Chappell, A.J.; Simper, T.; Helms, E. Nutritional strategies of British professional and amateur natural bodybuilders during competition preparation. J. Int. Soc. Sports Nutr. 2019, 16, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Alves, R.C.; Prestes, J.; Enes, A. Training Programs Designed for Muscle Hypertrophy in Bodybuilders: A Narrative Review. Sports 2020, 8, 149. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Franchi, M.V.; Longo, S.; Mallinson, J.; Quinlan, J.I.; Taylor, T.; Greenhaff, P.L.; Narici, M.V. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand. J. Med. Sci. Sports 2018, 28, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Raglin, J.; Szabo, A. Understanding placebo and nocebo effects in the context of sport: A psychological perspective. Eur. J. Sport Sci. 2020, 20, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Soares-Caldeira, L.F.; Ritti-Dias, R.M.; Okuno, N.M.; Cyrino, E.S.; Gurjão, A.L.; Ploutz-Snyder, L.L. Familiarization indexes in sessions of 1-RM tests in adult women. J. Strength Cond. Res. 2009, 23, 2039–2045. [Google Scholar] [CrossRef]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Preen, D.; Dawson, B.; Goodman, C.; Lawrence, S.; Beilby, J.; Ching, S. Pre-exercise oral creatine ingestion does not improve prolonged intermittent sprint exercise in humans. J. Sports Med. Phys. Fit. 2002, 42, 320–329. [Google Scholar]
- Mills, S.; Candow, D.G.; Forbes, S.C.; Neary, J.P. Effects of Creatine Supplementation during Resistance Training Sessions in Physically Active Young Adults. Nutrients 2020, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Mouser, J.G.; Counts, B.R.; Buckner, S.L.; Loenneke, J.P. Training to Fatigue: The Answer for Standardization When Assessing Muscle Hypertrophy? Sports Med. 2017, 47, 1021–1027. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Roberts, B.M.; Vigotsky, A.D.; Schoenfeld, B.J.; Roberts, M.D. A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So does the Measurement. Front. Physiol. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Population | Intervention | Outcomes |
|---|---|---|---|
| Antonio and Ciccone, [11] | 19 recreational male bodybuilders. | 4 weeks of 5 g·day−1 Cr: Group 1: Cr pre-exercise Group 2: Cr post-exercise. RT consisted of 5 d·wk−1 sessions. | ↔ ΔBM, ΔFFM, ΔFM and Δ1-RM BP. Possibly (FFM, FM) and likely (1-RM BP) beneficial for Cr post vs. Cr pre. |
| Candow et al. [22] | 9 men and 13 women, non-RT healthy older adults. | 12 weeks of 0.1 g·kg−1 Cr and 0.1 g·kg−1 PL: Group 1: Cr before + PL after Group 2: PL before + Cr after. Cr ingested only on training days: 3 d·wk−1 RT session. | ↔ ΔFFM ↔ ΔLimb muscle thickness ↔ Δ1-RM BP ↔ Δ1-RM LP |
| Candow et al. [12] | 22 women and 17 men, non-RT healthy older adults. | 32 weeks of 0.1 g·kg−1 Cr and/or 0.1 g·kg−1 PL: Group 1: Cr pre + PL post Group 2: Cr post + PL pre Group 3: Placebo control. Cr ingested only on training days: 3 d·wk−1 RT session. | ΔLBM: ↑ Cr post PL; ↔ Cr pre vs. Cr post and PL. ↔ Cr groups for 1-RM BP and LP ↑ Strength for both Cr groups compared to PL. |
| Mills et al. [55] | 22 Physically active men and women. | 6 weeks of Cr or PL post each set (intra-workout). Group 1: 0.0055 g·kg−1 Cr post each set Group 2: 0.0055 g·kg−1 Pl post each set. Cr was ingested only on training days: 5 d·wk−1 RT session. | ↑ 1-RM BP and LP for Cr vs. PL Cr pre to post-intervention: ↑ 1-RM ↑ Muscle endurance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, F.; Longobardi, I.; Perim, P.; Duarte, B.; Ferreira, P.; Gualano, B.; Roschel, H.; Saunders, B. Timing of Creatine Supplementation around Exercise: A Real Concern? Nutrients 2021, 13, 2844. https://doi.org/10.3390/nu13082844
Ribeiro F, Longobardi I, Perim P, Duarte B, Ferreira P, Gualano B, Roschel H, Saunders B. Timing of Creatine Supplementation around Exercise: A Real Concern? Nutrients. 2021; 13(8):2844. https://doi.org/10.3390/nu13082844
Chicago/Turabian StyleRibeiro, Felipe, Igor Longobardi, Pedro Perim, Breno Duarte, Pedro Ferreira, Bruno Gualano, Hamilton Roschel, and Bryan Saunders. 2021. "Timing of Creatine Supplementation around Exercise: A Real Concern?" Nutrients 13, no. 8: 2844. https://doi.org/10.3390/nu13082844
APA StyleRibeiro, F., Longobardi, I., Perim, P., Duarte, B., Ferreira, P., Gualano, B., Roschel, H., & Saunders, B. (2021). Timing of Creatine Supplementation around Exercise: A Real Concern? Nutrients, 13(8), 2844. https://doi.org/10.3390/nu13082844








