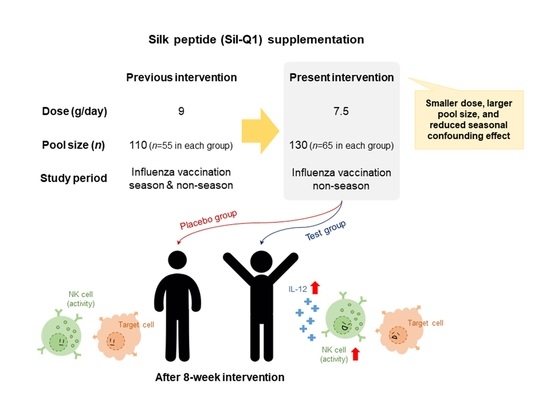
Supplementation with a Natural Source of Amino Acids, Sil-Q1 (Silk Peptide), Enhances Natural Killer Cell Activity: A Redesigned Clinical Trial with a Reduced Supplementation Dose and Minimized Seasonal Effects in a Larger Population
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Ethical Review
2.2. Study Subjects and Recruitment Criteria
2.3. Group Size Determination
2.4. Study Interventions and Materials
2.5. Safety of SQ and Dose Calculation
2.6. Anthropometric Parameters, Physical Activity, and Dietary Intake
2.7. Blood Collection, Hematology, Biochemical Parameters, and Urinalysis
2.8. Primary and Secondary Endpoints
2.9. Analysis Group and Statistical Method
3. Results
3.1. Baseline Information on Subjects
3.2. Dietary Intake and Physical Activity
3.3. Sil-Q1 Efficacy Test: NK Cell Cytotoxicity and Cytokines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wayne, S.J.; Rhyne, R.L.; Garry, P.J.; Goodwin, J.S. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J. Gerontol. 1990, 45, M45–M48. [Google Scholar] [CrossRef]
- Alvarez-Rodríguez, L.; López-Hoyos, M.; Muñoz-Cacho, P.; Martínez-Taboada, V.M. Aging is associated with circulating cytokine dysregulation. Cell Immunol. 2012, 273, 124–132. [Google Scholar] [CrossRef]
- Pae, M.; Meydani, S.N.; Wu, D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012, 3, 91–129. [Google Scholar] [PubMed]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional foods and the immune system: A review. Eur. J. Clin. Nutr. 2002, 56, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Jang, S.H.; Oh, M.S.; Baek, H.I.; Ha, K.C.; Lee, J.Y.; Jang, Y.S. Oral administration of silk peptide enhances the maturation and cytolytic activity of natural killer cells. Immune Netw. 2018, 18, e37. [Google Scholar] [CrossRef]
- Jang, S.H.; Oh, M.S.; Baek, H.I.; Ha, K.C.; Lee, J.Y.; Jang, Y.S. Silk peptide treatment potentiates natural killer cell activity in vitro and induces natural killer cell maturation and activation in mouse splenocytes. Pharm. Biol. 2019, 57, 369–379. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, H.S.; Lee, H.J.; Kim, J.M.; Lee, K.W.; Suh, H.J. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profiles. Nutr. Res. 2010, 30, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Ryu, J.M.; Seo, I.K.; Lee, K.M.; Yeon, S.; Kang, S.; Hwang, S.Y.; Kim, Y.B. Effects of red ginseng powder and silk peptide on hypercholesterolemia and atherosclerosis in rabbits. Lab. Anim. Res. 2008, 24, 67–75. [Google Scholar]
- Matarese, G.; Procaccini, C.; De Rosa, V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol. Rev. 2012, 249, 116–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Sacristán, J.A. Exploratory trials, confirmatory observations: A new reasoning model in the era of patient-centered medicine. BMC Med. Res. Methodol. 2011, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- Frank, M.G.; Hendricks, S.E.; Burke, W.J.; Johnson, D.R. Clinical response augments NK cell activity independent of treatment modality: A randomized double-blind placebo controlled antidepressant trial. Psychol. Med. 2004, 34, 491–498. [Google Scholar] [CrossRef]
- ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J. Postgrad. Med. 2001, 47, 199–203. [Google Scholar]
- Hwang, J.T.; Cho, J.M.; Jeong, I.H.; Lee, J.; Ha, K.C.; Baek, H.I.; Yang, H.J.; Kim, M.J.; Lee, J.H. The effect of silk peptide on immune system, A randomized, double-blind, placebo-controlled clinical trial. J. Funct. Foods 2019, 55, 275–284. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, S.K.; Lee, C.K.; Lee, S.G.; Lee, H.S.; Cho, K.J. Establishment of reference value using Korean adult medical checkup data and interpretation of test results. J. Lab. Med. Qual. Assur. 2006, 28, 229–237. [Google Scholar]
- Orces, C.H. Association between leisure-time aerobic physical activity and vitamin D concentrations among US older adults: The NHANES 2007–2012. Aging Clin. Exp. Res. 2019, 31, 685–693. [Google Scholar] [CrossRef]
- Singh, A.; Purohit, B. Evaluation of Global Physical Activity Questionnaire (GPAQ) among healthy and obese health professionals in central India. Balt. J. Health Phys. Act. 2011, 3, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Shin, D.Y.; Lee, J.H. Immune activation of Bio-Germanium in a randomized, double-blind, placebo-controlled clinical trial with 130 human subjects: Therapeutic opportunities from new insights. PLoS ONE 2020, 15, e0240358. [Google Scholar] [CrossRef] [PubMed]
- Zelig, R.; Rigassio Radler, D. Understanding the properties of common dietary supplements: Clinical implications for healthcare practitioners. Nutr. Clin. Pract. 2012, 27, 767–776. [Google Scholar] [CrossRef]
- Welbourne, T.C. Increased plasma bicarbonate and growth hormone after an oral glutamine load. Am. J. Clin. Nutr. 1995, 61, 1058–1061. [Google Scholar] [CrossRef]
- Melis, G.C.; ter Wengel, N.; Boelens, P.G.; van Leeuwen, P.A. Glutamine: Recent developments in research on the clinical significance of glutamine. Curr. Opin. Clin. Nutr. Metab. Care. 2004, 7, 59–70. [Google Scholar] [CrossRef]
- Grimble, R.F. The effects of sulfur amino acid intake on immune function in humans. J. Nutr. 2006, 136, 1660S–1665S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Martins, E., Jr.; Santos, R.V.; Caperuto, E.C.; Rogeri, P.; Costa Rosa, L.F. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 2002, 18, 376–379. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, R.B.; Sackett, D.L.; Richardson, W.S.; Rosenberg, W.; Langley, G.R. Evidence-based medicine: How to practice & teach EBM. CMAJ 1997, 157, 788. [Google Scholar]
- Meldrum, M.L. A brief history of the randomized controlled trial. From oranges and lemons to the gold standard. Hematol. Oncol. Clin. North Am. 2000, 14, 745–760. [Google Scholar] [CrossRef]
- Cartwright, N. Are RCTs the Gold Standard? BioSocieties 2007, 2, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Ono, S. Exploratory analysis of the factors associated with success rates of confirmatory randomized controlled trials in cancer drug development. Clin. Transl. Sci. 2021, 14, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Manwani, B.; Leng, S.X. Frailty, inflammation, and immunity. Aging Dis. 2011, 2, 466–473. [Google Scholar] [PubMed]
- Leng, S.X.; Hung, W.; Cappola, A.R.; Yu, Q.; Xue, Q.L.; Fried, L.P. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Leng, S.X.; Xue, Q.L.; Tian, J.; Huang, Y.; Yeh, S.H.; Fried, L.P. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: Results from the Women’s Health and Aging Studies I. Exp. Gerontol. 2009, 44, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Metter, E.J.; Cherubini, A.; Maggio, M.; Sen, R.; Najjar, S.S.; Windham, G.B.; Ble, A.; Senin, U.; Ferrucci, L. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J. Am. Coll. Cardiol. 2007, 49, 1841–1850. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; Tarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Krause, M.; Newsholme, P. Amino acid supplementation and impact on immune function in the context of exercise. J. Int. Soc. Sports Nutr. 2014, 11, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grohmann, U.; Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010, 236, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef]
- Froh, M.; Thurman, R.G.; Wheeler, M.D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G856–G863. [Google Scholar] [CrossRef] [Green Version]
- Duval, D.; Demangel, C.; Munier-Jolain, K.; Miossec, S.; Geahel, I. Factors controlling cell proliferation and antibody production in mouse hybridoma cells: I. Influence of the amino acid supply. Biotechnol. Bioeng. 1991, 38, 561–570. [Google Scholar] [CrossRef]
- Simon, R.R. Glutamine and Zinc Methionine Supplementation to Dairy Calves. Master’s Thesis, Texas A&M Univeristy, College Station, TX, USA, 1999. [Google Scholar]
- Ikejima, K.; Iimuro, Y.; Forman, D.T.; Thurman, R.G. A diet containing glycine improves survival in endotoxin shock in the rat. Am. J. Physiol. 1996, 271, G97–G103. [Google Scholar] [CrossRef]
- Perry, R.J.; Wang, Y.; Cline, G.W.; Rabin-Court, A.; Song, J.D.; Dufour, S.; Zhang, X.M.; Petersen, K.F.; Shulman, G.I. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell 2018, 172, 234–248.e17. [Google Scholar] [CrossRef]
- Newsholme, P.; Newsholme, E.A. Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem. J. 1989, 261, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Spencer, T.E.; Bazer, F.W.; Wu, G. Developmental changes of amino acids in ovine fetal fluids. Biol. Reprod. 2003, 68, 1813–1820. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A.J.; Dubbelhuis, P.F. Amino acid signaling and the integration of metabolism. Biochem. Biophys. Res. Commun. 2004, 313, 397–403. [Google Scholar] [CrossRef]
- Grau, T.; Bonet, A.; Miñambres, E.; Piñeiro, L.; Irles, J.A.; Robles, A.; Acosta, J.; Herrero, I.; Palacios, V.; Lopez, J.; et al. Metabolism, Nutrition Working Group, SEMICYUC, Spain. The effect of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit. Care Med. 2011, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stehle, P.; Zander, J.; Mertes, N.; Albers, S.; Puchstein, C.; Lawin, P.; Fürst, P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet 1989, 1, 231–233. [Google Scholar] [CrossRef]
- Kim, S.; Mateo, R.; Yin, Y.; Wu, G. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Anim. Biosci. 2007, 20, 295–306. [Google Scholar] [CrossRef]
- Trapani, J.A. Target cell apoptosis induced by cytotoxic T cells and natural killer cells involves synergy between the pore-forming protein, perforin, and the serine protease, granzyme B. Aust. N. Z. J. Med. 1995, 25, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Rousalova, I.; Krepela, E. Granzyme B-induced apoptosis in cancer cells and its regulation (review). Int. J. Oncol. 2010, 37, 1361–1378. [Google Scholar] [PubMed] [Green Version]
- Martín-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Cooper, M.A.; Nuovo, G.J.; Cella, M.; Facchetti, F.; Colonna, M.; Caligiuri, M.A. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood 2003, 101, 3052–3057. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- De Groote, D.; Zangerle, P.F.; Gevaert, Y.; Fassotte, M.F.; Beguin, Y.; Noizat-Pirenne, F.; Pirenne, J.; Gathy, R.; Lopez, M.; Dehart, I. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992, 4, 239–248. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Rasmussen, C.J.; Lancaster, S.L.; Magu, B.; Smith, P.; Melton, C.; Greenwood, M.; Almada, A.L.; Earnest, C.P.; Kreider, R.B. The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. J. Strength Cond. Res. 2006, 20, 643–653. [Google Scholar] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veluchamy, J.P.; Kok, N.; van der Vliet, H.J.; Verheul, H.M.W.; de Gruijl, T.D.; Spanholtz, J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: Recent innovations and future developments. Front. Immunol. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U. Homeostatic roles of naturally occurring antibodies: An overview. J. Autoimmun. 2007, 29, 287–294. [Google Scholar] [CrossRef]

| Type of Test | Species | Dose | Test Result | |
|---|---|---|---|---|
| Single administration | Rodent | SD Rat (Male (M) 15, Female (F) 15) | 0, 2000, 5000 mg/kg/body weight (bw) |
|
| Dose-range finding (4 weeks) | Rodent | SD Rat (M 10, F 10) | 0, 500, 1000, 2000 mg/kg/bw |
|
| Repeated administration (13 weeks) | Rodent | SD Rat (M 40, F 40) | 0, 500, 1000, 2000 mg/kg/bw |
|
| Genotoxicity | Ames test |
| 0~5000 μg/plate | Does not cause reverting mutations |
| Chromosomal abnormality test | CHL cells | 0~5000 μg/mL | Does not cause chromosomal abnormalities on CHL cells | |
| Micronucleus test | ICR mouse bone marrow cells | 1250, 2500, 5000 mg/kg | Does not induce micronuclei in bone marrow cells of ICR mice | |
| SQ Group (n = 61) | Placebo Group (n = 57) | Total (n = 118) | p-Value a | |
|---|---|---|---|---|
| Male/Female (n, %) | 2 (3.28)/59 (96.72) | 5 (8.77)/52 (91.23) | 7 (5.93)/111 (94.07) | 0.261 b |
| Age (years) | 55.90 ± 4.68 | 57.04 ± 4.65 | 56.45 ± 4.68 | 0.190 |
| Height (cm) | 157.51 ± 5.41 | 158.07 ± 5.53 | 157.78 ± 5.45 | 0.578 |
| Weight (kg) | 58.03 ± 7.26 | 59.85 ± 8.27 | 58.91 ± 7.78 | 0.207 |
| BMI (kg/m2) | 23.36 ± 2.47 | 23.85 ± 2.57 | 23.60 ± 2.52 | 0.294 |
| Waist circumference (cm) | 85.98 ± 6.55 | 87.60 ± 6.80 | 86.76 ± 6.69 | 0.191 |
| Hip circumference (cm) | 95.07 ± 4.33 | 96.31 ± 5.02 | 95.67 ± 4.70 | 0.153 |
| Fat mass (%) | 32.38 ± 4.53 | 33.04 ± 4.60 | 32.69 ± 4.56 | 0.433 |
| Currently drinking (n, %) | 6 (9.84) | 7 (12.28) | 13 (11.02) | 0.672 c |
| Currently smoking (n, %) | 0 (0.00) | 2 (3.51) | 2 (1.69) | 0.231 b |
| Prescription compliance (%) | 96.05 ± 4.55 | 94.50 ± 5.43 | 95.30 ± 5.03 | 0.095 |
| SQ Group (n = 61) | Placebo Group (n = 57) | p-Value b | |||||
|---|---|---|---|---|---|---|---|
| T0 | T8 | p-Value a | T0 | T8 | p-Value a | ||
| Dietary intake | |||||||
| Calorie (kcal) | 1871.49 ± 114.89 | 1854.92 ± 117.46 | 0.081 | 1889.91 ± 121.31 | 1852.97 ± 105.72 | 0.001 | |
| ∆ | −16.58 ± 72.82 | −36.94 ± 75.12 | 0.138 | ||||
| Carbohydrate (g) | 287.50 ± 18.41 | 285.31 ± 18.05 | 0.173 | 291.06 ± 19.12 | 284.84 ± 16.01 | 0.001 | |
| ∆ | −2.20 ± 12.46 | −6.23 ± 12.30 | 0.080 | ||||
| Protein (g) | 73.72 ± 4.77 | 73.78 ± 4.92 | 0.903 | 74.56 ± 5.03 | 73.38 ± 4.52 | 0.033 | |
| ∆ | 0.06 ± 3.86 | −1.18 ± 4.08 | 0.091 | ||||
| Lipid (g) | 47.09 ± 3.53 | 46.34 ± 3.84 | 0.083 | 47.18 ± 3.88 | 46.44 ± 3.60 | 0.155 | |
| ∆ | −0.75 ± 3.33 | −0.74 ± 3.88 | 0.988 | ||||
| Fiber (g) | 11.18 ± 1.94 | 11.36 ± 2.21 | 0.605 | 11.49 ± 1.96 | 11.29 ± 2.25 | 0.614 | |
| ∆ | 0.17 ± 2.57 | −0.19 ± 2.90 | 0.469 | ||||
| Physical activity | |||||||
| MET (min/week) | 2942.95 ± 3536.50 | 3097.70 ± 3583.62 | 0.623 | 3370.88 ± 4008.54 | 3943.86 ± 5940.32 | 0.497 | |
| ∆ | 154.75 ± 2443.23 | 572.98 ± 6327.03 | 0.642 | ||||
| SQ Group (n = 61) | Placebo Group (n = 57) | p-Value b | |||||
|---|---|---|---|---|---|---|---|
| T0 | T8 | p-Value a | T0 | T8 | p-Value a | ||
| NK cell cytotoxicity E:T ratio (%) | |||||||
| 10:1 | 41.83 ± 12.75 | 49.60 ± 18.23 | 0.002 | 45.87 ± 17.57 | 45.24 ± 17.41 | 0.817 | |
| ∆ | 7.77 ± 18.83 | –0.63 ± 20.52 | 0.022 | ||||
| 5:1 | 27.43 ± 9.60 | 32.41 ± 12.08 | 0.004 | 32.28 ± 14.82 * | 28.87 ± 11.14 | 0.144 | |
| ∆ | 4.98 ± 12.88 | –3.41 ± 17.33 | 0.001 c | ||||
| 2.5:1 | 17.85 ± 7.11 | 20.65 ± 7.58 | 0.023 | 20.79 ± 10.87 | 18.55 ± 7.29 | 0.188 | |
| ∆ | 2.81 ± 9.39 | –2.24 ± 12.70 | 0.016 | ||||
| 1.25:1 | 11.36 ± 5.35 | 13.41 ± 5.96 | 0.030 | 13.01 ± 7.44 | 12.37 ± 5.42 | 0.587 | |
| ∆ | 2.05 ± 7.21 | –0.64 ± 8.80 | 0.071 | ||||
| Cytokine | |||||||
| IL-12 (pg/mL) | 12.13 ± 18.31 | 20.82 ± 40.97 | 0.041 | 13.69 ± 33.03 | 11.49 ± 27.95 | 0.329 | |
| ∆ | 8.70 ± 32.44 | –2.20 ± 16.89 | 0.023 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.M.; Yoo, D.; Lee, J.-Y.; Oh, M.-S.; Ha, K.-C.; Baek, H.-I.; Lee, S.-M.; Lee, J.H.; Yoo, H.J. Supplementation with a Natural Source of Amino Acids, Sil-Q1 (Silk Peptide), Enhances Natural Killer Cell Activity: A Redesigned Clinical Trial with a Reduced Supplementation Dose and Minimized Seasonal Effects in a Larger Population. Nutrients 2021, 13, 2930. https://doi.org/10.3390/nu13092930
Cho JM, Yoo D, Lee J-Y, Oh M-S, Ha K-C, Baek H-I, Lee S-M, Lee JH, Yoo HJ. Supplementation with a Natural Source of Amino Acids, Sil-Q1 (Silk Peptide), Enhances Natural Killer Cell Activity: A Redesigned Clinical Trial with a Reduced Supplementation Dose and Minimized Seasonal Effects in a Larger Population. Nutrients. 2021; 13(9):2930. https://doi.org/10.3390/nu13092930
Chicago/Turabian StyleCho, Jung Min, Dokyeong Yoo, Jeong-Yong Lee, Mi-Sun Oh, Ki-Chan Ha, Hyang-Im Baek, Seung-Min Lee, Jong Ho Lee, and Hye Jin Yoo. 2021. "Supplementation with a Natural Source of Amino Acids, Sil-Q1 (Silk Peptide), Enhances Natural Killer Cell Activity: A Redesigned Clinical Trial with a Reduced Supplementation Dose and Minimized Seasonal Effects in a Larger Population" Nutrients 13, no. 9: 2930. https://doi.org/10.3390/nu13092930
APA StyleCho, J. M., Yoo, D., Lee, J.-Y., Oh, M.-S., Ha, K.-C., Baek, H.-I., Lee, S.-M., Lee, J. H., & Yoo, H. J. (2021). Supplementation with a Natural Source of Amino Acids, Sil-Q1 (Silk Peptide), Enhances Natural Killer Cell Activity: A Redesigned Clinical Trial with a Reduced Supplementation Dose and Minimized Seasonal Effects in a Larger Population. Nutrients, 13(9), 2930. https://doi.org/10.3390/nu13092930