Disease-Related Malnutrition and Sarcopenia Predict Worse Outcome in Medical Inpatients: A Cohort Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Type of Study and Ethical Aspects
2.2. Sample Size Calculation
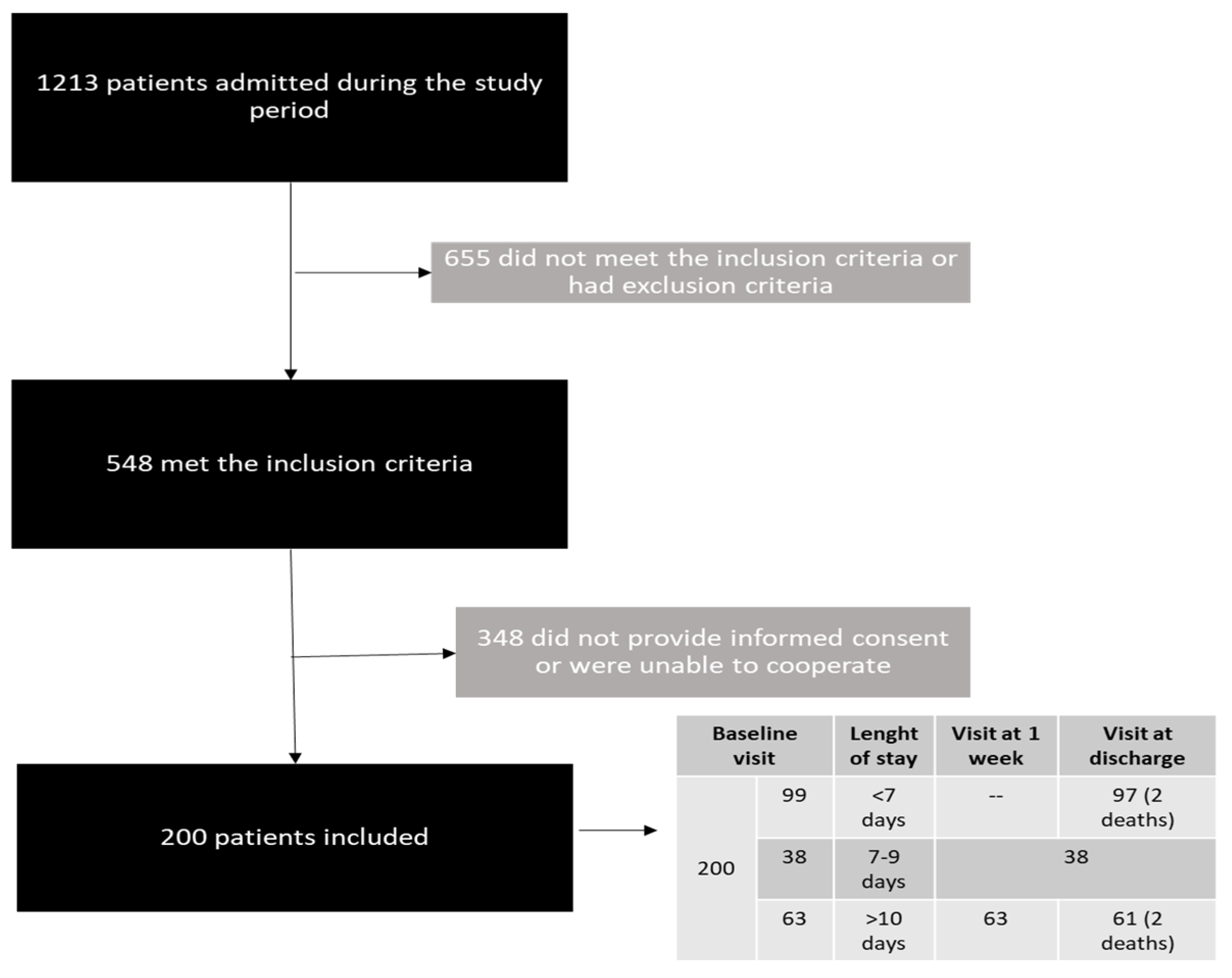
2.3. Inclusion and Exclusion Criteria
2.4. Collected and Analyzed Data
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Anthropometric and Muscle Mass/Function Data
3.3. Screening and Diagnosis of DRM
3.4. Screening and Diagnosis of Sarcopenia
3.5. Length of Stay, Quality of Life, Readmissions and Mortality and Associations with Low MM and HGS
3.6. Length of Stay, Quality of Life, Readmissions and Mortality and Associations with DRM or Sarcopenia
3.7. Changes in Muscle Mass and Function during Hospitalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2018, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Deutz, N.E.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The Underappreciated Role of Low Muscle Mass in the Management of Malnutrition. J. Am. Med. Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Camprubi-Robles, M.; Bear, D.; Cederholm, T.; Malafarina, V.; Welch, A.; Cruz-Jentoft, A. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2018, 38, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Caccialanza, R.; Cereda, E.; Klersy, C.; Bonardi, C.; Cappello, S.; Quarleri, L.; Turri, A.; Montagna, E.; Iacona, I.; Valentino, F.; et al. Phase Angle and Handgrip Strength Are Sensitive Early Markers of Energy Intake in Hypophagic, Non-Surgical Patients at Nutritional Risk, with Contraindications to Enteral Nutrition. Nutrients 2015, 7, 1828–1840. [Google Scholar] [CrossRef]
- Deutz, N.E.; Pereira, S.L.; Hays, N.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 Days of Bed Rest on Skeletal Muscle in Healthy Older Adults. JAMA 2007, 297, 1769–1774. [Google Scholar] [CrossRef]
- Kouw, I.W.; Groen, B.B.; Smeets, J.; Kramer, I.F.; van Kranenburg, J.M.; Nilwik, R.; Geurts, J.A.; Broeke, R.H.T.; Poeze, M.; van Loon, L.J.; et al. One Week of Hospitalization Following Elective Hip Surgery Induces Substantial Muscle Atrophy in Older Patients. J. Am. Med. Dir. Assoc. 2018, 20, 35–42. [Google Scholar] [CrossRef]
- Dirks, M.; Wall, B.T.; Van De Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.; Van Loon, L.J. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef] [Green Version]
- Cerri, A.P.; Bellelli, G.; Mazzone, A.; Pittella, F.; Landi, F.; Zambon, A.; Annoni, G. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clin. Nutr. 2015, 34, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; et al. The incidence of sarcopenia among hospitalized older patients: Results from the Glisten study. J. Cachex- Sarcopenia Muscle 2017, 8, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; Fantin, F.; Abete, P.; Bellelli, G.; Brombo, G.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; for the GLISTEN Group Investigators; et al. Association between hospitalization-related outcomes, dynapenia and body mass index: The Glisten Study. Eur. J. Clin. Nutr. 2018, 73, 743–750. [Google Scholar] [CrossRef]
- Gajete, L.M.; Pintor, B.; Fernández, P.; González, M.L.; Barajas, D.E.; Alejo, M.; Hernández, A.; García, S.; Cano, I.B.M.D. Impacto de la intervención dietética en la evolución de la ingesta proteica y la sarcopenia en el paciente hospitalizado. 59 congreso de la Sociedad Española de Endocrinología y Nutrición, Barcelona, 18-20 de octubre de 2017. Endocrinol. Diabetes Y Nutr. 2017, 64, 111. [Google Scholar]
- Hu, X.; Zhang, L.; Wang, H.; Hao, Q.; Dong, B.; Yang, M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci. Rep. 2017, 7, 3171. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Hu, X.; Wang, H.; Zhang, L.; Hao, Q.; Dong, B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: A prospective study. J. Cachex-Sarcopenia Muscle 2016, 8, 251–258. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Scafoglieri, A.; Clarys, J.P.; Bauer, J.M.; Verlaan, S.; Van Malderen, L.; Vantieghem, S.; Cederholm, T.; Sieber, C.C.; Mets, T.; Bautmans, I. Predicting appendicular lean and fat mass with bioelectrical impedance analysis in older adults with physical function decline–The PROVIDE study. Clin. Nutr. 2016, 36, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Shechtman, O.; Gestewitz, L.; Kimble, C. Reliability and Validity of the DynEx Dynamometer. J. Hand Ther. 2005, 18, 339–347. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, D.; Marco, E.; Dávalos-Yerovi, V.; López-Escobar, J.; Messaggi-Sartor, M.; Barrera, C.; Ronquillo-Moreno, N.; Vázquez-Ibar, O.; Calle, A.; Inzitari, M.; et al. Translation and Validation of the Spanish Version of the SARC-F Questionnaire to Assess Sarcopenia in Older People. J. Nutr. Health Aging 2019, 23, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.P.; Gonzalez, M.C.; Orlandi, S.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Heymsfield, S.B. COCONUT Study Group New Prediction Equations to Estimate Appendicular Skeletal Muscle Mass Using Calf Circumference: Results From NHANES 1999–2006. J. Parenter. Enter. Nutr. 2019, 43, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Schuetz, P.; Bounoure, L.; Austin, P.; Ballesteros-Pomar, M.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; Poulia, K.-A.; et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin. Nutr. 2017, 37, 336–353. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Hernández, J.; Planas Vila, M.; León-Sanz, M.; García de Lorenzo, A.; Celaya-Pérez, S.; García-Lorda, P.; Araujo, K.; Sarto Guerri, B. Prevalence and costs of malnutrition in hospitalized patients; the PREDyCES Study. Nutr. Hosp. 2012, 27, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Purcell, S.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.; Heymsfield, S.B. Implications of low muscle mass across the continuum of care: A narrative review. Ann. Med. 2018, 50, 675–693. [Google Scholar] [CrossRef] [Green Version]
- Jochem, C.; Leitzmann, M.; Volaklis, K.; Aune, D.; Strasser, B. Association Between Muscular Strength and Mortality in Clinical Populations: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2019, 20, 1213–1223. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Matheson, E.M.; Nelson, J.L.; Baggs, G.E.; Luo, M.; Deutz, N.E. Specialized oral nutritional supplement (ONS) improves handgrip strength in hospitalized, malnourished older patients with cardiovascular and pulmonary disease: A randomized clinical trial. Clin. Nutr. 2020, 40, 844–849. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Tribolet, P.; Baumgartner, A.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Hoess, C.; et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Pomar, M.; Calleja-Fernández, A.; González-Valdés, M.; la Maza, B.P.-D.; Taibo, R.V.; Vidal-Casariego, A.; Urioste-Fondo, A.; Torres-Torres, B.; de Luis, D.; Cano-Rodríguez, I. Repercusiones clínicas y económicas de la desnutrición relacionada con la enfermedad en un servicio de Medicina Interna: Estudio de cohortes prospectivas. Rev. Clin. Esp. 2016, 216, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Schuetz, P.; Sulo, S.; Walzer, S.; Vollmer, L.; Stanga, Z.; Gomes, F.; Rueda, R.; Mueller, B.; Partridge, J. Economic evaluation of individualized nutritional support in medical inpatients: Secondary analysis of the EFFORT trial. Clin. Nutr. 2020, 39, 3361–3368. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Meng, Q.; Jiang, Y.; Zhuang, Q.; Xi, Q.; Xu, J.; Zhao, J.; Sui, X.; Wu, G. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: A randomised clinical trial. Clin. Nutr. 2020, 40, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Camprubí-Robles, M.; Lopez-Pedrosa, J.M.; Pereira, S.L.; Rueda, R.; Ballesteros-Pomar, M.D.; Almeida, J.M.G.; Cruz-Jentoft, A.J. Role of Oral Nutritional Supplements Enriched with B-hydroxy-B-Methylbutyrate in Maintaining Muscle Function and Improving Clinical Outcomes in Various Clinical Settings. J. Nutr. Health Aging 2018, 22, 664–675. [Google Scholar] [CrossRef] [Green Version]

| Male (n = 100) | Female (n = 100) | p | |
|---|---|---|---|
| Age (years) | 76.9 (15.0) | 73.9 (30.0) | 0.044 |
| Charlson comorbidity index | 5.0 (3.0) | 3.5 (5.0) | 0.001 |
| Katz index | 0 (1) | 0 (2) | 0.518 |
| Weight (kg) | 72.2 (16.2) | 64.0 (18.7) | <0.001 |
| BMI (kg/m2) | 27.0 (5.8) | 25.4 (6.6) | 0.019 |
| % Weight loss | 2.8 (6.2) | 1.6 (5.7) | 0.254 |
| Arm circumference (cm) | 26.0 (4.3) | 25.0 (5.1) | 0.718 |
| Calf circumference (cm) | 32.0 (4.3) | 31.0 (5.0) | 0.373 |
| Hand grip strength (kg) | 21.4 (13.4) | 10.5 (8.1) | <0.001 |
| Resistance (Ω) | 478.0 (110.5) | 567.0 (94.5) | <0.001 |
| Reactance (Ω) | 44.0 (13.5) | 54.0 (15.0) | <0.001 |
| Phase angle (o) | 5.3 (1.3) | 5.3 (1.1) | 0.611 |
| ALM (kg) | 10.4 (2.1) | 11.4 (2.6) | 0.001 |
| ALMI (kg/m2) | 3.8 (0.7) | 4.6 (0.9) | <0.001 |
| HGS/ALMI (kg/kg/m2) | 5.4 (3.4) | 2.4 (2.1) | <0.001 |
| Blood glucose (mg/dL) | 108.0 (38.0) | 101.0 (32.0) | 0.002 |
| Albumin (g/dL) | 3.6 (0.7) | 3.7 (0.6) | 0.641 |
| C-reactive protein (mg/dL) | 56.9 (100.3) | 54.8 (127.0) | 0.879 |
| Normal | DRM-GLIM | Sarcopenia | DRM-GLIM + Sarcopenia | p | |
|---|---|---|---|---|---|
| N (%) | 100 (50) | 34 (17) | 45 (22.5) | 21 (10.5) | - |
| Age (years) | 69.4 (28.1) | 64.6 (18.5) | 81.5 (13.4) | 81.0 (11.1) | <0.001 |
| Sex (% men) | 53 | 55.9 | 48.9 | 28.6 | 0.194 |
| CCI | 3.0 (4.0) | 2.5 (5.0) | 5.0 (2.0) | 4.0 (4.0) | <0.001 |
| Katz index | 0 (1.0) | 0 (0) | 2.0 (2.0) | 2.5 (4.0) | <0.001 |
| Phase angle (o) | 5.7 (1.0) | 5.4 (1.4) | 4.7 (0.9) | 4.6 (0.8) | <0.001 |
| HGS (kg) | 19.0 (13.1) | 18.7 (10.5) | 9.2 (6.4) | 8.3 (4.4) | <0.001 |
| Length of stay(days) | 5.5 (5.0) | 7.0 (6.0) | 8.5 (7.0) | 8.0 (12.0) | 0.060 |
| Readmissions (%) | 27 | 26.5 | 40 | 47.6 | 0.151 |
| Deaths (%) | 0 | 5.9 | 13.3 | 23.8 | <0.001 |
| EuroQol total | 6.0 (2.0) | 6.0 (1.0) | 8.0 (2.0) | 8.0 (3.0) | <0.001 |
| VAS | 60.0 (30.0) | 50.0 (30.0) | 50.0 (18.0) | 40.0 (28.0) | <0.001 |
| Total QoL at Admission 1 | Quality of Life VAS 1 | Length of Stay 1 | Readmissions 2 | Deaths 2 | |
|---|---|---|---|---|---|
| ALM | NS | NS | NS | NS | NS |
| Calf circumference | NS | NS | NS | OR = 0.92 p = 0.024 | NS |
| Hand grip strength | Beta = −0.323. p = 0.001 | Beta = 0.360. p < 0.001 | NS | OR = 0.95 p = 0.026 | OR = 0.85 p = 0.014 |
| DRM-GLIM | NS | Beta = −0.146. p = 0.038 | NS | NS | OR = 4.36 p = 0.001 |
| Probable + confirmed sarcopenia | Beta = 0.534. p < 0.001 | Beta = −0.302. p < 0.001 | NS | OR = 2.25 p = 0.030 | OR = 8.16 p = 0.012 |
| DRM-GLIM + sarcopenia | Beta = 0.263. p < 0.001 | Beta = −0.179. p = 0.015 | NS | NS | OR = 5.27 p = 0.019 |
| Upon Admission * (n = 200) | Changes after One Week (n = 101) | Changes at Discharge (n = 196) | |
|---|---|---|---|
| Initial weight (kg) and changes in weight (%) | 68.5 (20.2) | −0.51 (5.0) p = 0.488 | −0.64 (6.0) p = 0.242 |
| Phase angle (o) | 5.3 (1.3) | 5.1 (1.3) p = 0.712 | 5.0 (1.2) p = 0.844 |
| Initial ALM and changes (kg) | 10.8 (2.4) | −0.091 (0.360) p = 0.236 | −0.118 (0.490) p = 0.039 |
| Hand grip strength and changes (kg) | 14.8 (12.9) | −0.5 (4.4) p = 0.824 | −0.65 (4.9) p = 0.364 |
| Calf circumference and changes (cm) | 31.0 (5.0) | 0.0 (3.0) p = 0.537 | 0.0 (3.0) p = 0.767 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballesteros-Pomar, M.D.; Gajete-Martín, L.M.; Pintor-de-la-Maza, B.; González-Arnáiz, E.; González-Roza, L.; García-Pérez, M.P.; González-Alonso, V.; García-González, M.A.; de Prado-Espinosa, R.; Cuevas, M.J.; et al. Disease-Related Malnutrition and Sarcopenia Predict Worse Outcome in Medical Inpatients: A Cohort Study. Nutrients 2021, 13, 2937. https://doi.org/10.3390/nu13092937
Ballesteros-Pomar MD, Gajete-Martín LM, Pintor-de-la-Maza B, González-Arnáiz E, González-Roza L, García-Pérez MP, González-Alonso V, García-González MA, de Prado-Espinosa R, Cuevas MJ, et al. Disease-Related Malnutrition and Sarcopenia Predict Worse Outcome in Medical Inpatients: A Cohort Study. Nutrients. 2021; 13(9):2937. https://doi.org/10.3390/nu13092937
Chicago/Turabian StyleBallesteros-Pomar, María D., Luisa Mercedes Gajete-Martín, Begoña Pintor-de-la-Maza, Elena González-Arnáiz, Lucía González-Roza, María Pilar García-Pérez, Verónica González-Alonso, María Ascensión García-González, Rocío de Prado-Espinosa, María José Cuevas, and et al. 2021. "Disease-Related Malnutrition and Sarcopenia Predict Worse Outcome in Medical Inpatients: A Cohort Study" Nutrients 13, no. 9: 2937. https://doi.org/10.3390/nu13092937






