Effect of Nutritional Supplementation on Oxidative Stress and Hormonal and Lipid Profiles in PCOS-Affected Females
Abstract
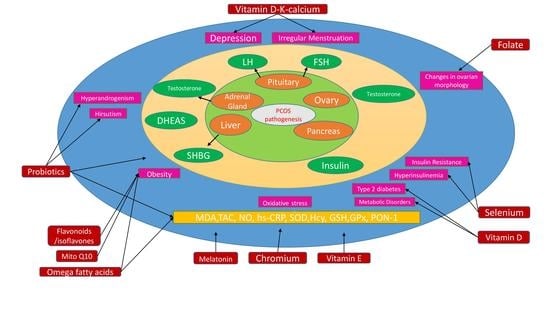
:1. Introduction
2. Oxidative Stress in PCOS
3. Markers of Oxidative Stress
3.1. Malondialdehyde
3.2. Nitric Oxide
3.3. Total Antioxidant Capacity
3.4. Reduced Glutathione (GSH)
4. Role of Supplementation in PCOS and Associated Comorbidities
4.1. Vitamin D
4.2. Flavonoids and Isoflavones
4.3. Selenium
4.4. Probiotics
4.5. Vitamin E, Folate and Omega-3 Fatty Acids
5. Inflammation in PCOS
6. Effective vs. Less Effective Supplementation
7. Effect on Gene Expression
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saadia, Z. Follicle stimulating hormone (LH: FSH) ratio in polycystic ovary syndrome (PCOS)—Obese vs. non- obese women. Med. Arch. 2020, 74, 289–293. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Dubey, P.; Cistola, D.P.; Reddy, S.Y. Association between obesity and cardiovascular outcomes: Updated evidence from meta-analysis studies. Curr. Cardiol. Rep. 2020, 22, 25. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Dubey, P.; Reddy, S.Y.; Alvarado, L.; Manuel, S.L.; Dwivedi, A.K. Prevalence of at-risk hyperandrogenism by age and race/ethnicity among females in the United States using NHANES III. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 189–197. [Google Scholar] [CrossRef]
- Kellow, N.J.; Savige, G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: A systematic review. Eur. J. Clin. Nutr. 2013, 67, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.I.; Naz, L.; Yasmeen, G. Obesity: An independent risk factor for systemic oxidative stress. Pak. J. Pharm. Sci. 2006, 19, 62–65. [Google Scholar]
- Macut, D.; Bjekic-Macut, J.; Savic-Radojevic, A. Dyslipidemia and oxidative stress in PCOS. Front. Horm. Res. 2013, 40, 51–63. [Google Scholar] [CrossRef]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Luo, W.Y.; Liao, H.; Wang, C.F.; Sun, Y. The effects of oxidative stress to PCOS. Sichuan Da Xue Xue Bao Yi Xue Ban 2008, 39, 421–423. [Google Scholar] [PubMed]
- Murri, M.; Luque-Ramirez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M. Oxidative stress and polycystic ovary syndrome: A brief review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Huang, Q.; Long, S.L.; Zhong, Q.; Mo, Z. Mitochondrial dysfunction in polycystic ovary syndrome. DNA Cell Biol. 2020, 39, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Abuja, P.M.; Albertini, R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef]
- Kuscu, N.K.; Var, A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2009, 88, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Dursun, P.; Demirtas, E.; Bayrak, A.; Yarali, H. Decreased serum paraoxonase 1 (PON1) activity: An additional risk factor for atherosclerotic heart disease in patients with PCOS? Hum. Reprod. 2006, 21, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Hassani, F.; Karami, M.; Nadoushan, M.R.J.; Yazdi, P.E. Nitric oxide-induced polycystic ovaries in the wistar rat. Int. J. Fertil. Steril. 2012, 6, 111–116. [Google Scholar]
- Oh, J.S.; Kim, H.; Vijayakumar, A.; Kwon, O.; Choi, Y.J.; Huh, K.B.; Chang, N. Association between dietary flavanones intake and lipid profiles according to the presence of metabolic syndrome in Korean women with type 2 diabetes mellitus. Nutr. Res. Pract. 2016, 10, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenkci, V.; Fenkci, S.; Yilmazer, M.; Serteser, M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Steril. 2003, 80, 123–127. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [Green Version]
- Sabuncu, T.; Vural, H.; Harma, M.; Harma, M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin. Biochem. 2001, 34, 407–413. [Google Scholar] [CrossRef]
- Dinger, Y.; Akcay, T.; Erdem, T.; Ilker Saygili, E.; Gundogdu, S. DNA damage, DNA susceptibility to oxidation and glutathione level in women with polycystic ovary syndrome. Scand. J. Clin. Lab. Investig. 2005, 65, 721–728. [Google Scholar] [CrossRef]
- Bahmani, F.; Karamali, M.; Shakeri, H.; Asemi, Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Clin. Endocrinol. 2014, 81, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, E.; Samimi, M.; Ebrahimi, F.A.; Foroozanfard, F.; Ahmadi, S.; Rahimi, M.; Jamilian, M.; Aghadavod, E.; Bahmani, F.; Taghizadeh, M.; et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol. Cell Endocrinol. 2017, 439, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Mirmasoumi, G.; Fazilati, M.; Foroozanfard, F.; Vahedpoor, Z.; Mahmoodi, S.; Taghizadeh, M.; Esfeh, N.K.; Mohseni, M.; Karbassizadeh, H.; Asemi, Z. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes 2018, 126, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Bahmani, F.; Foroozanfard, F.; Vahedpoor, Z.; Ghaderi, A.; Taghizadeh, M.; Karbassizadeh, H.; Asemi, Z. The effects of fish oil omega-3 fatty acid supplementation on mental health parameters and metabolic status of patients with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Psychosom. Obstet. Gynaecol. 2018, 12, 2000. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Q.; Pei, Y.H.; Ren, Q.L.; Chi, L.; Hu, R.K.; Tan, Y. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: A retrospective cohort study. BMC Womens Health 2020, 20, 69. [Google Scholar] [CrossRef] [Green Version]
- Masharani, U.; Gjerde, C.; Evans, J.L.; Youngren, J.F.; Goldfine, I.D. Effects of controlled-release alpha lipoic acid in lean, nondiabetic patients with polycystic ovary syndrome. J. Diabetes Sci. Technol. 2010, 4, 359–364. [Google Scholar] [CrossRef]
- Jamilian, H.; Jamilian, M.; Samimi, M.; Afshar Ebrahimi, F.; Rahimi, M.; Bahmani, F.; Aghababayan, S.; Kouhi, M.; Shahabbaspour, S.; Asemi, Z. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Gynecol. Endocrinol. 2017, 33, 442–447. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Krzyczkowska-Sendrakowska, M.; Nicol, F.; Wietecha-Posluszny, R.; Milewicz, T.; Kryczyk-Koziol, J.; Chaykivska, Z.; Jach, R. Selenium status parameters in patients with polycystic ovary syndrome. J. Trace Elem. Med. Biol. 2017, 44, 241–246. [Google Scholar] [CrossRef]
- Razavi, M.; Jamilian, M.; Kashan, Z.F.; Heidar, Z.; Mohseni, M.; Ghandi, Y.; Bagherian, T.; Asemi, Z. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm. Metab. Res. 2016, 48, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, F.M.; Hosseinzadeh-Attar, M.J.; Yekaninejad, M.S.; Rashidi, B. Effects of selenium supplementation on glucose homeostasis and free androgen index in women with polycystic ovary syndrome: A randomized, double blinded, placebo controlled clinical trial. J. Trace Elem. Med. Biol. 2016, 34, 56–61. [Google Scholar] [CrossRef]
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coskun, A.; Arikan, T.; Kilinc, M.; Arikan, D.C.; Ekerbicer, H.C. Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 183–186. [Google Scholar] [CrossRef]
- Jamilian, M.; Asemi, Z. The effects of soy isoflavones on metabolic status of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3386–3394. [Google Scholar] [CrossRef] [Green Version]
- Nasri, K.; Jamilian, M.; Rahmani, E.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on hormonal status, biomarkers of inflammation and oxidative stress in subjects with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. BMC Endocr. Disord. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Kashanian, M.; Alaeinasab, S.; Asemi, Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomised clinical trial. J. Hum. Nutr. Diet 2018, 31, 533–543. [Google Scholar] [CrossRef]
- Karamali, M.; Eghbalpour, S.; Rajabi, S.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Keneshlou, F.; Mirhashemi, S.M.; Chamani, M.; Hashem Gelougerdi, S.; et al. Effects of probiotic supplementation on hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Arch. Iran. Med. 2018, 21, 1–7. [Google Scholar] [PubMed]
- Razavi, M.; Jamilian, M.; Karamali, M.; Bahmani, F.; Aghadavod, E.; Asemi, Z. The effects of vitamin D-K-calcium co-supplementation on endocrine, inflammation, and oxidative stress biomarkers in vitamin D-deficient women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Horm. Metab. Res. 2016, 48, 446–451. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Rahmani, E.; Talebi, M.; Bahmani, F.; Asemi, Z. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients 2017, 9, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, K.; Akrami, S.; Rahimi, M.; Taghizadeh, M.; Behfar, M.; Mazandaranian, M.R.; Kheiry, A.; Memarzadeh, M.R.; Asemi, Z. The effects of vitamin D and evening primrose oil co-supplementation on lipid profiles and biomarkers of oxidative stress in vitamin D-deficient women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Endocr. Res. 2018, 43, 1–10. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Afshar Ebrahimi, F.; Aghadavod, E.; Talaee, R.; Jafarnejad, S.; Hashemi Dizaji, S.; Asemi, Z. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J. Affect. Disord. 2018, 238, 32–38. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Kyei, G.; Sobhani, A.; Nekonam, S.; Shabani, M.; Ebrahimi, F.; Qasemi, M.; Salahi, E.; Fardin, A. Assessing the effect of MitoQ10 and Vitamin D3 on ovarian oxidative stress, steroidogenesis and histomorphology in DHEA induced PCOS mouse model. Heliyon 2020, 6, e04279. [Google Scholar] [CrossRef] [PubMed]
- Lajtai, K.; Tarszabo, R.; Banyai, B.; Peterffy, B.; Gerszi, D.; Ruisanchez, E.; Sziva, R.E.; Korsos-Novak, A.; Benko, R.; Hadjadj, L.; et al. Effect of vitamin D status on vascular function of the aorta in a rat model of PCOS. Oxid. Med. Cell Longev. 2021, 2021, 8865979. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Mirhosseini, N.; Kavossian, E.; Aghadavod, E.; Bahmani, F.; Ostadmohammadi, V.; Kia, M.; Eftekhar, T.; Ayati, E.; et al. Effects of melatonin supplementation on hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. Front. Endocrinol. 2019, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. 2020, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Jamilian, M.; Karamali, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Hum. Fertil. 2017, 20, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lagowska, K.; Bajerska, J.; Jamka, M. The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2018, 10, 1637. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Ostadmohammadi, V.; Lankarani, K.B.; Tabrizi, R.; Kolahdooz, F.; Heydari, S.T.; Kavari, S.H.; Mirhosseini, N.; Mafi, A.; Dastorani, M.; et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res. 2018, 50, 271–279. [Google Scholar] [CrossRef]
- Jafari-Sfidvajani, S.; Ahangari, R.; Hozoori, M.; Mozaffari-Khosravi, H.; Fallahzadeh, H.; Nadjarzadeh, A. The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. J. Endocrinol. Invest 2018, 41, 597–607. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.; Snackey, C.; Louwers, Y.; Lips, P.; Lambalk, C.B.; Laven, J.S.; Simsek, S. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome: A systematic review. Eur. J. Endocrinol. 2013, 169, 853–865. [Google Scholar] [CrossRef] [Green Version]
- Yildizhan, R.; Kurdoglu, M.; Adali, E.; Kolusari, A.; Yildizhan, B.; Sahin, H.G.; Kamaci, M. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2009, 280, 559–563. [Google Scholar] [CrossRef]
- Joham, A.E.; Teede, H.J.; Cassar, S.; Stepto, N.K.; Strauss, B.J.; Harrison, C.L.; Boyle, J.; de Courten, B. Vitamin D in polycystic ovary syndrome: Relationship to obesity and insulin resistance. Mol. Nutr. Food Res. 2016, 60, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.F.; Li, B.X.; Zhang, Q. Vitamin D improves levels of hormonal, oxidative stress and inflammatory parameters in polycystic ovary syndrome: A meta-analysis study. Ann. Palliat. Med. 2021, 10, 169–183. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, P.; Xue, K.; Duan, X.; Cao, J.; Luan, T.; Li, Q.; Gu, L. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: A meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 487–496. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Romualdi, D.; Costantini, B.; Campagna, G.; Lanzone, A.; Guido, M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil. Steril. 2008, 90, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Mirone, M.; Giannetta, E.; Isidori, A.M. Selenium and reproductive function. A systematic review. J. Endocrinol. Investig. 2013, 36, 28–36. [Google Scholar]
- Hajizadeh-Sharafabad, F.; Moludi, J.; Tutunchi, H.; Taheri, E.; Izadi, A.; Maleki, V. Selenium and polycystic ovary syndrome: Current knowledge and future directions: A systematic review. Horm. Metab. Res. 2019, 51, 279–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabani, A.; Noshadian, M.; Jamilian, M.; Chamani, M.; Mohammadi, S.; Asemi, Z. The effects of a novel combination of selenium and probiotic on weight loss, glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome. J. Funct. Foods 2018, 46, 329–334. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in human medicine. Gut 1991, 32, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J. Nutr. 2010, 140, 483–488. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrution 2007, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Shoaei, T.; Heidari-Beni, M.; Tehrani, H.G.; Feizi, A.; Esmaillzadeh, A.; Askari, G. Effects of probiotic supplementation on pancreatic beta-cell function and C-reactive protein in women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Int. J. Prev. Med. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between polycystic ovary syndrome and gut microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef] [Green Version]
- Cicek, N.; Eryilmaz, O.G.; Sarikaya, E.; Gulerman, C.; Genc, Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J. Assist. Reprod. Genet. 2012, 29, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Duleba, A.J.; Dokras, A. Is PCOS an inflammatory process? Fertil. Steril. 2012, 97, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Montanino Oliva, M.; Buonomo, G.; Calcagno, M.; Unfer, V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J. Ovarian Res. 2018, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Dai, Y. Study on chronic low-grade inflammation and influential factors of polycystic ovary syndrome. Med. Princ. Pract. 2009, 18, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.C.; Lyall, H.; Petrie, J.R.; Gould, G.W.; Connell, J.M.; Sattar, N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, D.; Andersen, M.; Richelsen, B.; Bruun, J.M. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin. Endocrinol. 2009, 71, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Botella-Carretero, J.I.; Villuendas, G.; Sancho, J.; San Millán, J.L. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: Relationship to insulin resistance and to obesity. J. Clin. Endocrinol. Metab. 2004, 89, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Herlihy, A.C.; Kelly, R.E.; Hogan, J.L.; O’Connor, N.; Farah, N.; Turner, M.J. Polycystic ovary syndrome and the peripheral blood white cell count. J. Obstet. Gynaecol. 2011, 31, 242–244. [Google Scholar] [CrossRef]
- De Jager, S.C.; Kraaijeveld, A.O.; Grauss, R.W.; de Jager, W.; Liem, S.S.; van der Hoeven, B.L.; Prakken, B.J.; Putter, H.; van Berkel, T.J.; Atsma, D.E.; et al. CCL3 (MIP-1 alpha) levels are elevated during acute coronary syndromes and show strong prognostic power for future ischemic events. J. Mol. Cell. Cardiol. 2008, 45, 446–452. [Google Scholar] [CrossRef]
- Orio, F., Jr.; Palomba, S.; Cascella, T.; De Simone, B.; Di Biase, S.; Russo, T.; Labella, D.; Zullo, F.; Lombardi, G.; Colao, A. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 4588–4593. [Google Scholar] [CrossRef] [Green Version]
- Tarkun, I.; Arslan, B.C.; Cantürk, Z.; Türemen, E.; Sahin, T.; Duman, C. Endothelial dysfunction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low-grade chronic inflammation. J. Clin. Endocrinol. Metab. 2004, 89, 5592–5596. [Google Scholar] [CrossRef]
- Zadeh Modarres, S.; Heidar, Z.; Foroozanfard, F.; Rahmati, Z.; Aghadavod, E.; Asemi, Z. The effects of selenium supplementation on gene expression related to insulin and lipid in infertile polycystic ovary syndrome women candidate for in vitro fertilization: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2018, 183, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Shahnazi, V.; Zaree, M.; Nouri, M.; Mehrzad-Sadaghiani, M.; Fayezi, S.; Darabi, M.; Khani, S.; Darabi, M. Influence of ω-3 fatty acid eicosapentaenoic acid on IGF-1 and COX-2 gene expression in granulosa cells of PCOS women. Iran. J. Reprod. Med. 2015, 13, 71–78. [Google Scholar] [PubMed]
| Supplement Composition | Year | Country | Animal/Human Studies | Number of Patients/Animals | Age Group | Type of Study |
|---|---|---|---|---|---|---|
| Folate supplementation [22] | 2014 | Iran | Human | 69 | 18–40 | Randomized, double-blind, placebo-controlled |
| Omega-3 fatty acids and vitamin E [23] | 2017 | Iran | Human | 68 | 18–40 | Randomized double-blind, placebo-controlled |
| Flaxseed oil omega-3 fatty acids [24] | 2017 | Iran | Human | 60 | 18–40 | Randomized double-blind, placebo-controlled |
| Fish oil omega-3 fatty acid [25] | 2018 | Iran | Human | 60 | 18–40 | Randomized double-blind, placebo-controlled trial |
| Vitamin E [26] | 2020 | China | Human | 321 | Retrospective cohort clinical trial | |
| Alpha lipoic acid [27] | 2010 | USA | Human | 6 | 23–34 | NR |
| Carnitine supplementation [28] | 2017 | Iran | Human | 60 | 18–40 | Randomized, double-blind, placebo-controlled trial |
| Selenium supplementation [29] | 2017 | Poland | Human | 59 | 14–18 | NR |
| Selenium supplementation [30] | 2015 | Iran | Human | 64 | 18–40 | Randomized double-blind, placebo-controlled |
| Selenium supplementation [31] | 2016 | Iran | Human | 53 | 18–42 | Randomized, double-blind and placebo-controlled trial |
| Probiotic and selenium co-supplementation [32] | 2018 | Iran | Human | 60 | 18–40 | Randomized, double-blinded, placebo-controlled clinical trial |
| Selenium supplementation [33] | 2015 | Iran | Human | 70 | 18–40 | Randomized, double-blind, placebo-controlled trial |
| Soy isoflavones [34] | 2018 | Iran | Human | 70 | 18–40 | Randomized, double-blind, placebo-controlled trial |
| Synbiotic supplementation [35] | 2018 | Iran | Human | 60 | Randomized, double-blind, placebo-controlled trial | |
| Dietary soy [36] | 2018 | Iran | Human | 60 | 18–40 | Randomized, double-blind, placebo-controlled trial |
| Probiotic supplementation [37] | 2018 | Iran | Human | 60 | 18–40 | Randomized, double-blind, placebo-controlled trial |
| Vitamin D-K-calcium co-Supplementation [38] | 2016 | Iran | Human | 60 | 18–40 | Randomized, double-blind, placebo-controlled trial |
| Vitamin D [39] | 2017 | Iran | Human | 60 | 20–40 | Case-control study |
| Vitamin D and evening primrose oil [40] | 2017 | Iran | Human | 60 | 18–40 | Randomized double-blind, placebo-controlled trial |
| Vitamin D and omega-3 co-supplementation [41] | 2018 | Iran | Human | 60 | 18–40 | Randomized double-blind, placebo-controlled trial |
| Vitamin D and probiotic co-supplementation [42] | 2019 | Iran | Human | 60 | 18–40 | Randomized double-blind, placebo-controlled trial |
| MitoQ10 and vitamin D3 [43] | 2020 | Iran | Mouse | 48 | NR | |
| Vitamin D [44] | 2021 | Iran | Mouse | 40 | NR |
| Supplement | Dosage | Hormonal Changes | Changes in Lipid Profile | Changes in Oxidative Stress Markers | Genes Affected |
|---|---|---|---|---|---|
| Folate [22] | 5 mg/d for 8 weeks | ↓ Plasma Hcy, MDA, serum hs-CRP; ↑ plasma TAC and total GSH levels | |||
| Omega-3 fatty acids and vitamin E [25] | Co-supplementation for 12 weeks | ↓ Serum TGA, VLDL, LDL- and total-/HDL cholesterol in PCOS subjects | ↑ Plasma TAC levels, ↓ malondialdehyde levels | Gene expression of Lp(a) and Ox-LDL | |
| Flaxseed oil omega-3 fatty acids [24] | Supplementation for 12 weeks | ↑ mFG scores | Beneficial effects on insulin metabolism, serum triglycerides, VLDL-cholesterol and hs-CRP levels | ||
| Fish oil omega-3 fatty acid [25] | Supplementation for 12 weeks | Beneficial effects on mental health parameters, total testosterone, hirsutism | Effective on insulin markers | ↓ Inflammatory markers and oxidative stress | |
| Vitamin E [26] | 100 mg/d | No significant differences of ovulation rate, clinical pregnancy rate and ongoing pregnancy rate | ↓ Oxidative stress | ||
| Alpha lipoic acid [27] | 600 mg twice daily for 16 weeks | No improvement in serum oxidative stress markers | |||
| Carnitine and chromium co-supplementation [28] | 1000 mg/d carnitine plus 200 mg/d chromium as chromium picolinate for 12 weeks | ↓ Total testosterone and hirsutism | ↓ Malondialdehyde (MDA) levels and higher total antioxidant capacity (TAC) | ↑ Gene expression of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-a) | |
| Melatonin supplementation [45] | 5 mg melatonin supplements | ↓ Hirsutism, serum total testosterone | ↓ Malondialdehyde (MDA) levels, ↑ plasma total antioxidant capacity (TAC) levels and total glutathione (GSH) | ↓ Gene expression of IL-1, (TNF-α) | |
| Curcumin supplementation [46] | 1500 mg/d Curcumin for 12 weeks | ↑ Serum activity of GPx | ↑ SIRT1 gene expression | ||
| Selenium supplementation [30] | 200 μg selenium per day for 8 weeks | ↑ Pregnancy rate, ↓ Alopecia and acne, ↓ Serum (DHEAS), hirsutism (modified Ferriman–Gallwey scores) | |||
| Probiotic and selenium co- supplementation [32] | 8 × 109 CFU/d probiotic 200 μg/d | ↓Total testosterone, hirsutism | ↓ MDA levels, ↑ TAC and GSH levels | ||
| Selenium supplementation [29] | 200 mcg/d for 8 weeks | ↓ Serum triglycerides and VLDL-C concentrations | ↓ Serum insulin levels, homeostasis model of assessment-insulin resistance (HOMA-IR), (HOMA-B); ↑quantitative insulin sensitivity check index (QUICKI) | ||
| Soy isoflavones [34] | 50 mg/d soy isoflavones for 12 weeks | ↓ Circulating serum levels of insulin and HOMA-IR, ↑quantitative insulin sensitivity check index, ↓ free androgen index and serum triglycerides, ↑ plasma total glutathione,↓malondialdehyde | ↓ Free androgen index and serum triglycerides | ↓ Circulating serum levels of insulin and HOMA-IR, ↑ Quantitative insulin sensitivity check index, ↑ Plasma total glutathione ↓Malondialdehyde levels | |
| Synbiotic supplementation [35] | Synbiotic supplements containing Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | ↑ (SHBG), plasma NO, ↓ modified Ferriman–Gallwey (mFG) scores and serum hs-CRP | |||
| Dietary soy [36] | 0.8 g protein kg−1 body weight (35% animal proteins, 35% soy protein and 30% vegetable proteins) | ↓ Total testosterone, ↓ triglycerides | ↓Triglycerides, body mass index (BMI), fasting plasma glucose, insulin and insulin resistance; ↑ quantitative insulin sensitivity check index | ↓ MDA, ↑ NO and GSH | |
| Probiotic supplementation [47] | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2 × 109 CFU/g each) for 12 weeks | ↑ Serum SHBG and plasma TAC; ↓ serum total testosterone, mFG scores, serum hs-CRP and plasma MDA | ↑ Plasma TAC, ↓ plasma MDA | ||
| Vitamin D-K-calcium co-supplementation [38] | 200 IU vitamin D, 90 μg vitamin K plus, 500 mg calcium supplements for 8 weeks | ↓ Serum-free testosterone, ↓ luteinizing hormone | ↑ Plasma TAC, ↓ plasma MDA | ||
| Vitamin D [48] | 4000 IU vitamin D or 1000 IU of vitamin D for 12 weeks | ↓ Total testosterone, free androgen index (FAI), hirsutism; ↑ mean change in SHBG | ↑ Total antioxidant capacity (TAC) | ||
| Vitamin D [49] | 50,000 IU Vitamin D | ↓ FPG, insulin, HOMA-IR, estimated B cell function; ↑ quantitative insulin sensitivity check index | ↓ Plasma malondialdehyde (MDA) levels | ||
| Vitamin D and [40] | 1000 IU vitamin D3 plus 1000 mg Vitamin E | ↓ Triglycerides, very low-density lipoprotein (VLDL) cholesterol levels | ↑ Serum 25(OH) D and plasma GSH, ↓ MDA | ||
| Vitamin D and omega-3 co-supplementation [41] | 50,000 IU vitamin D every 2 weeks plus 2000 mg/d omega-3 fatty acid from fish oil | ↓ Serum total testosterone levels; ↑ Beck depression inventory, general health questionnaire scores and depression anxiety and stress scale scores | ↓ Serum hs-CRP and MDA, ↑ TAC compared with the placebo | ↓ Gene expression of (IL-1) and (VEGF) | |
| Vitamin D and probiotic co-supplementation [42] | 50,000 IU vitamin D and 8 × 109 CFU/d probiotic every 2 weeks | ↓ Total testosterone | ↓ MDA, ↑ TAC and GSH | ||
| MitoQ10 and Vitamin D3 [43] | ↓ Estradiol, progesterone, FSH, LH, LH/FSH | ↓ MDA and SOD | ↓ mRNAs of 3β-HSD, Cyp19a1, Cyp11a1, StAR, Keap1, HO-1 and Nrf2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, P.; Reddy, S.; Boyd, S.; Bracamontes, C.; Sanchez, S.; Chattopadhyay, M.; Dwivedi, A. Effect of Nutritional Supplementation on Oxidative Stress and Hormonal and Lipid Profiles in PCOS-Affected Females. Nutrients 2021, 13, 2938. https://doi.org/10.3390/nu13092938
Dubey P, Reddy S, Boyd S, Bracamontes C, Sanchez S, Chattopadhyay M, Dwivedi A. Effect of Nutritional Supplementation on Oxidative Stress and Hormonal and Lipid Profiles in PCOS-Affected Females. Nutrients. 2021; 13(9):2938. https://doi.org/10.3390/nu13092938
Chicago/Turabian StyleDubey, Pallavi, Sireesha Reddy, Sarah Boyd, Christina Bracamontes, Sheralyn Sanchez, Munmun Chattopadhyay, and Alok Dwivedi. 2021. "Effect of Nutritional Supplementation on Oxidative Stress and Hormonal and Lipid Profiles in PCOS-Affected Females" Nutrients 13, no. 9: 2938. https://doi.org/10.3390/nu13092938
APA StyleDubey, P., Reddy, S., Boyd, S., Bracamontes, C., Sanchez, S., Chattopadhyay, M., & Dwivedi, A. (2021). Effect of Nutritional Supplementation on Oxidative Stress and Hormonal and Lipid Profiles in PCOS-Affected Females. Nutrients, 13(9), 2938. https://doi.org/10.3390/nu13092938