Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
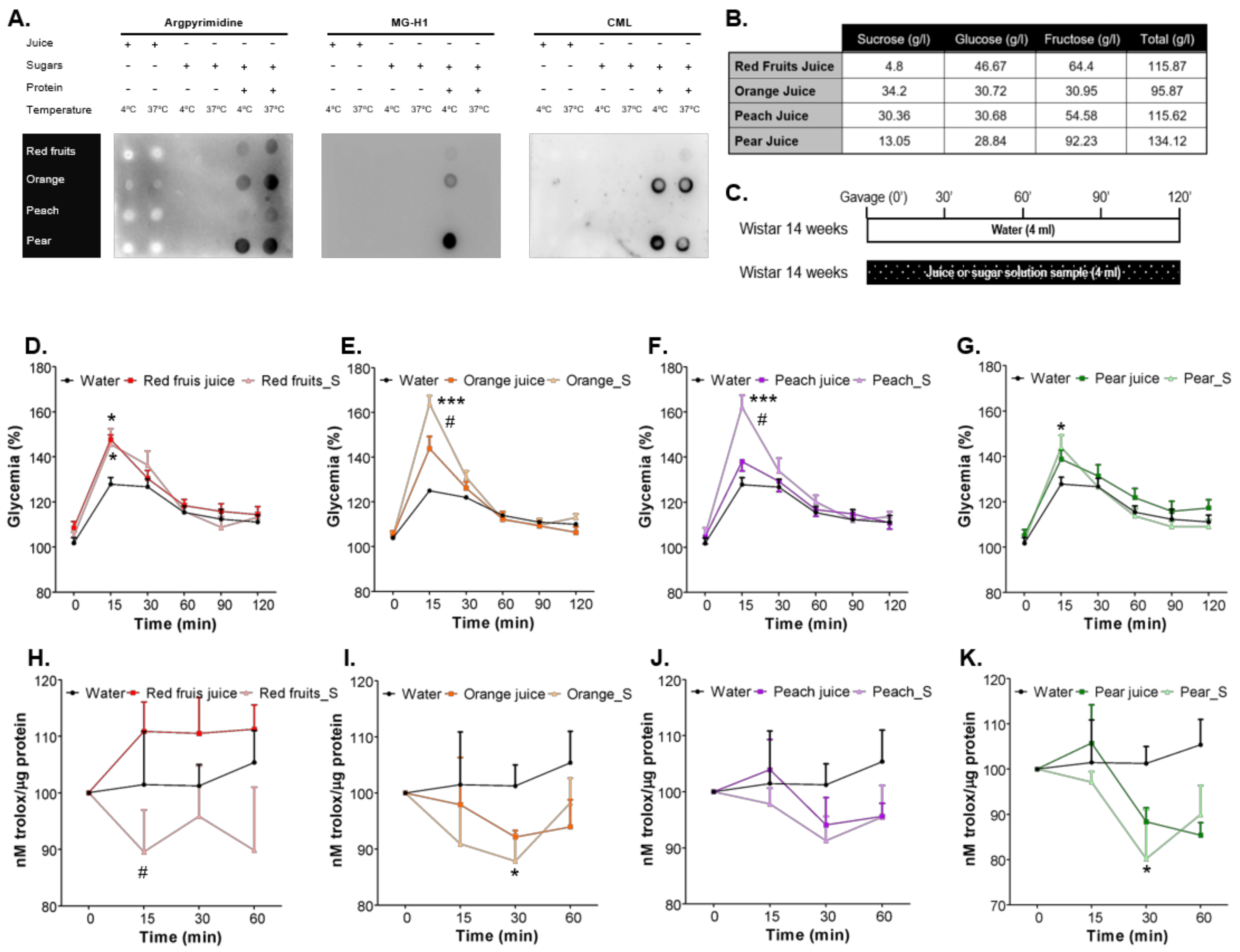
2.2. Fruit Juice and Sugars Samples
2.3. In Vitro Determination of AGEs Formation from Fruit Juices and Sugars’ Solutions
2.4. Determination of Post-Prandial Glycaemia and Total Antioxidant Capacity
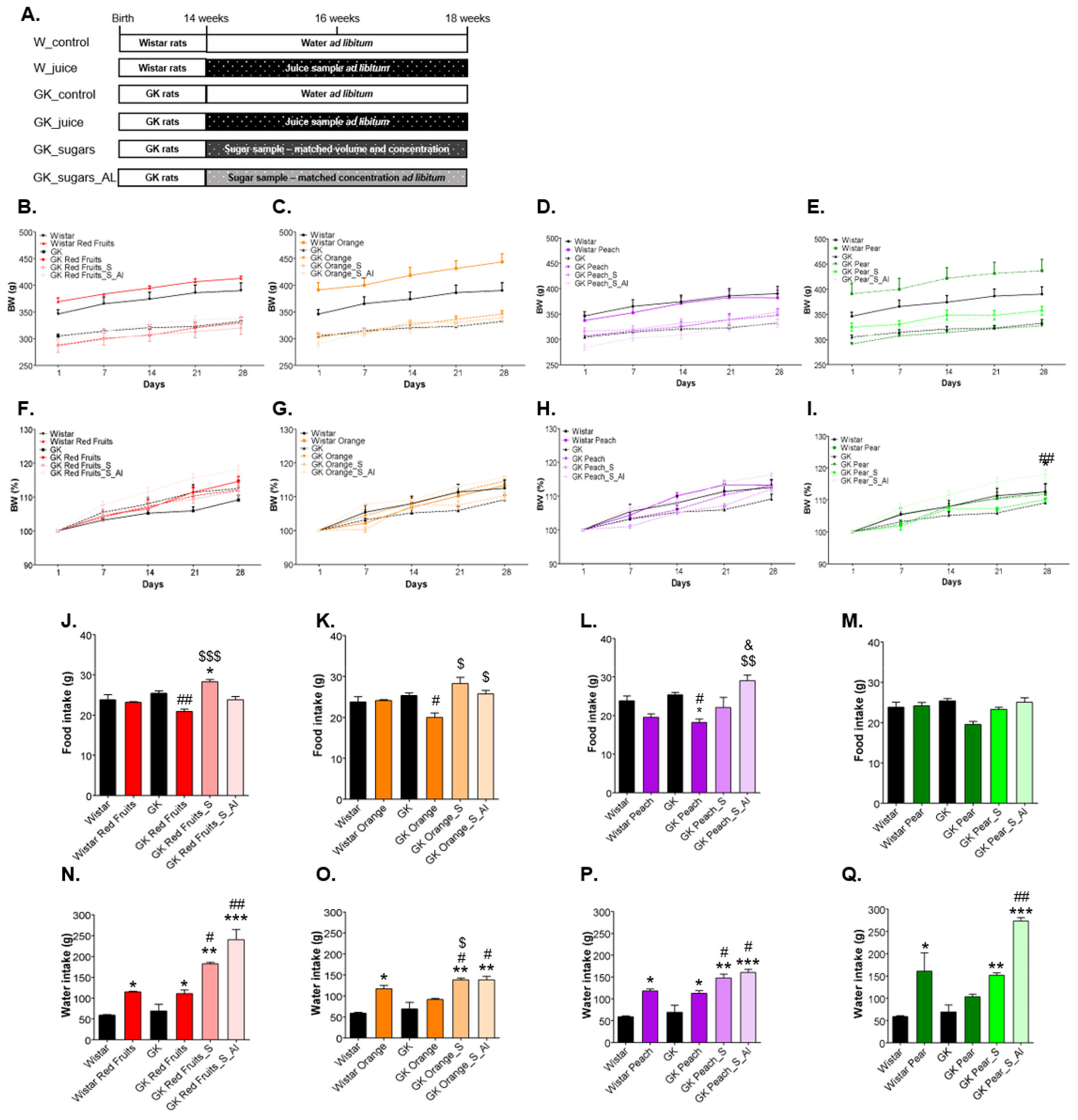
2.5. Animal Maintenance and Treatment
2.6. In Vivo Data and Sample Collection
2.7. Western Blot and Dot Blot
2.8. Dihydroethidium (DHE) Staining
2.9. Statistical Analysis
3. Results
3.1. Sugars Naturally Present in Fruit Juices Are Less Prone to AGEs Formation Than Added Sugars in Sugary Solutions and Cause a Lower Increase of Postprandial Glycaemia
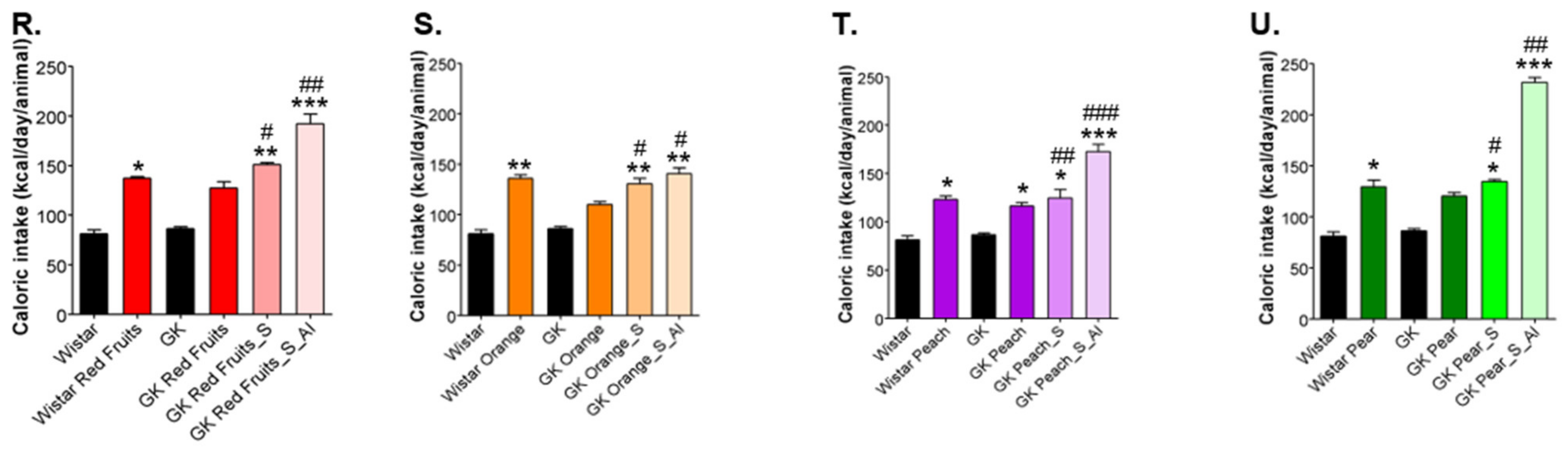
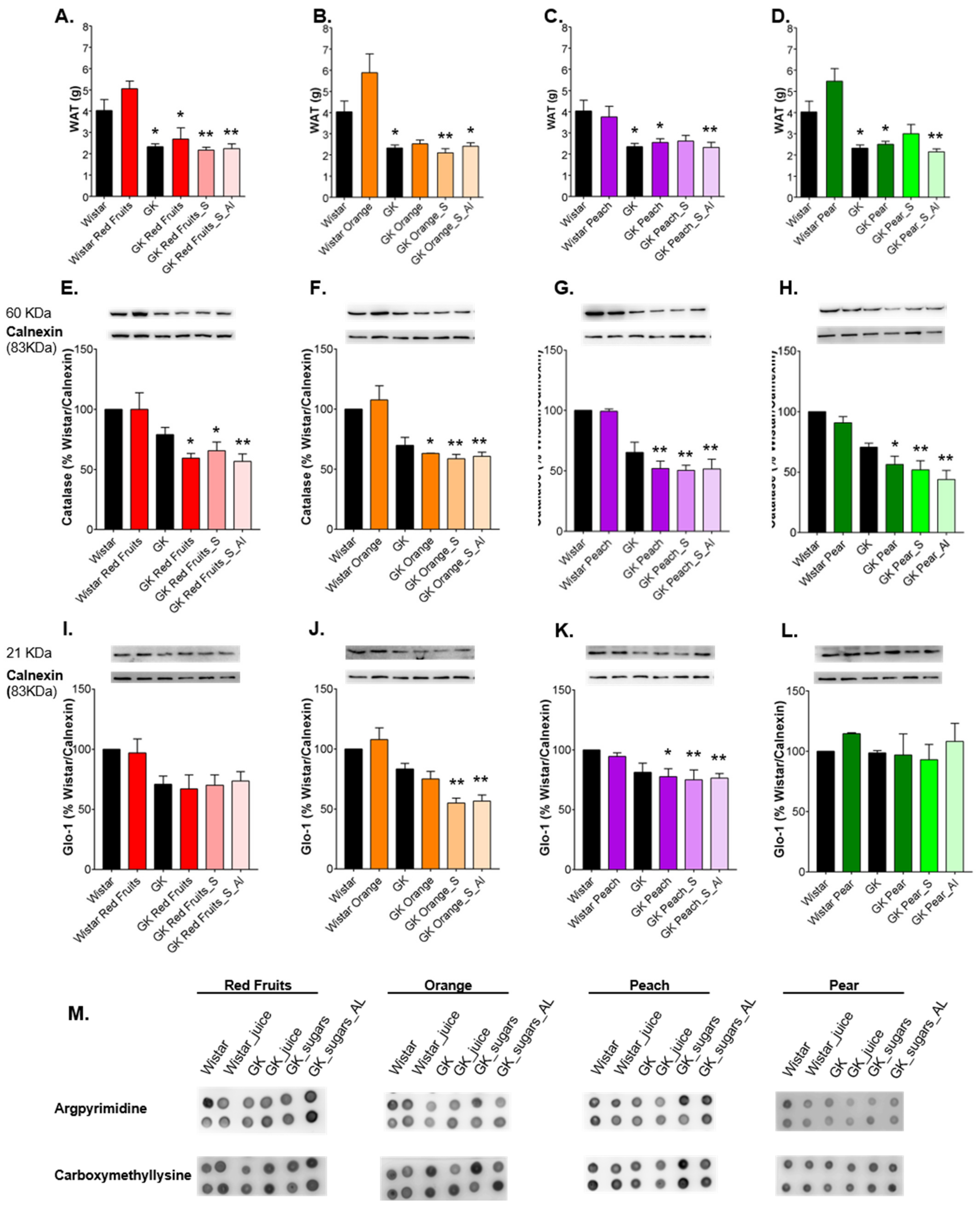
3.2. Added Sugars Do Not Cause Satiety and Lead to Higher Body Weight, Caloric Intake and Impaired Glycemic Profile When Available Ad Libitum
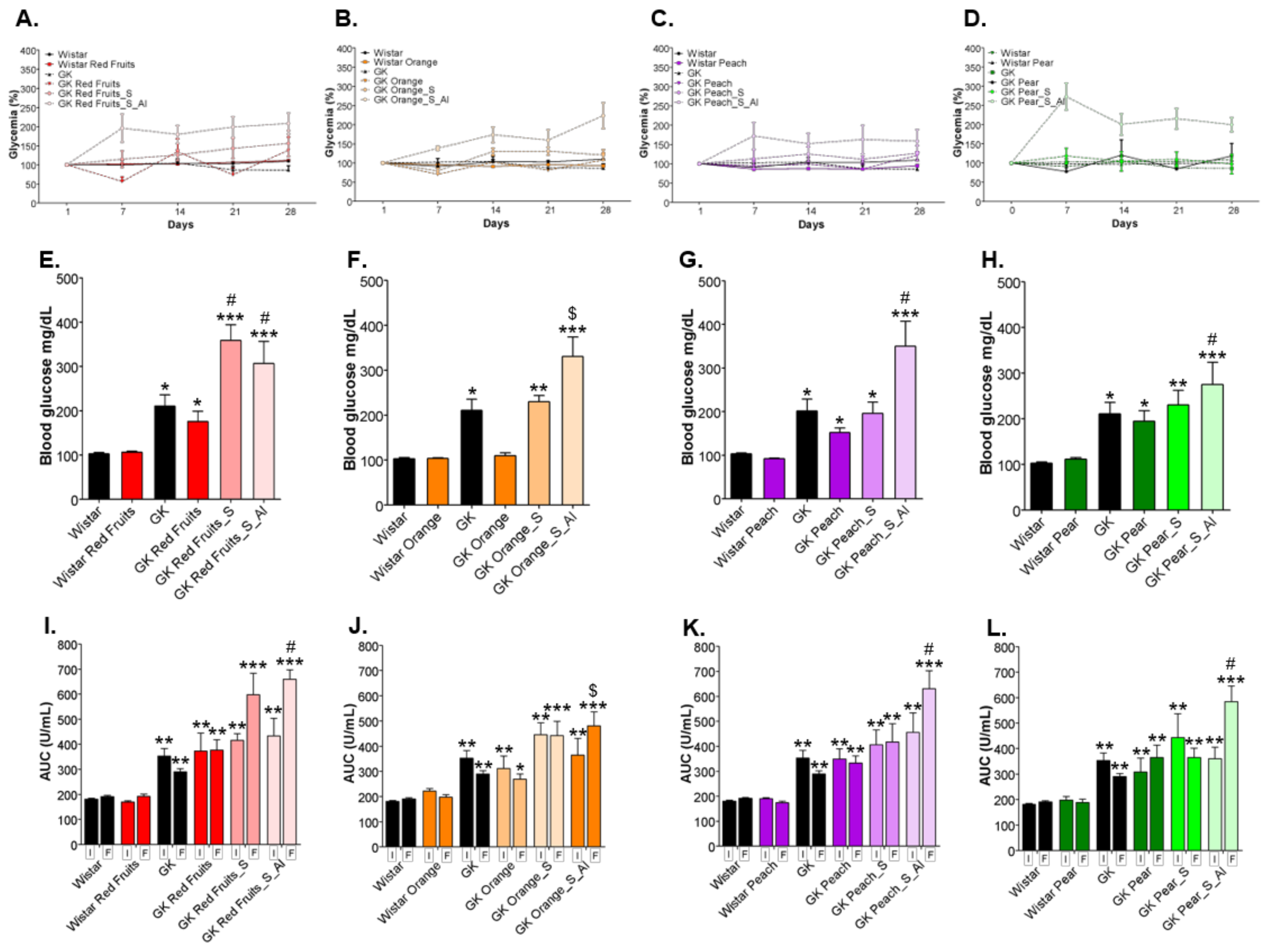
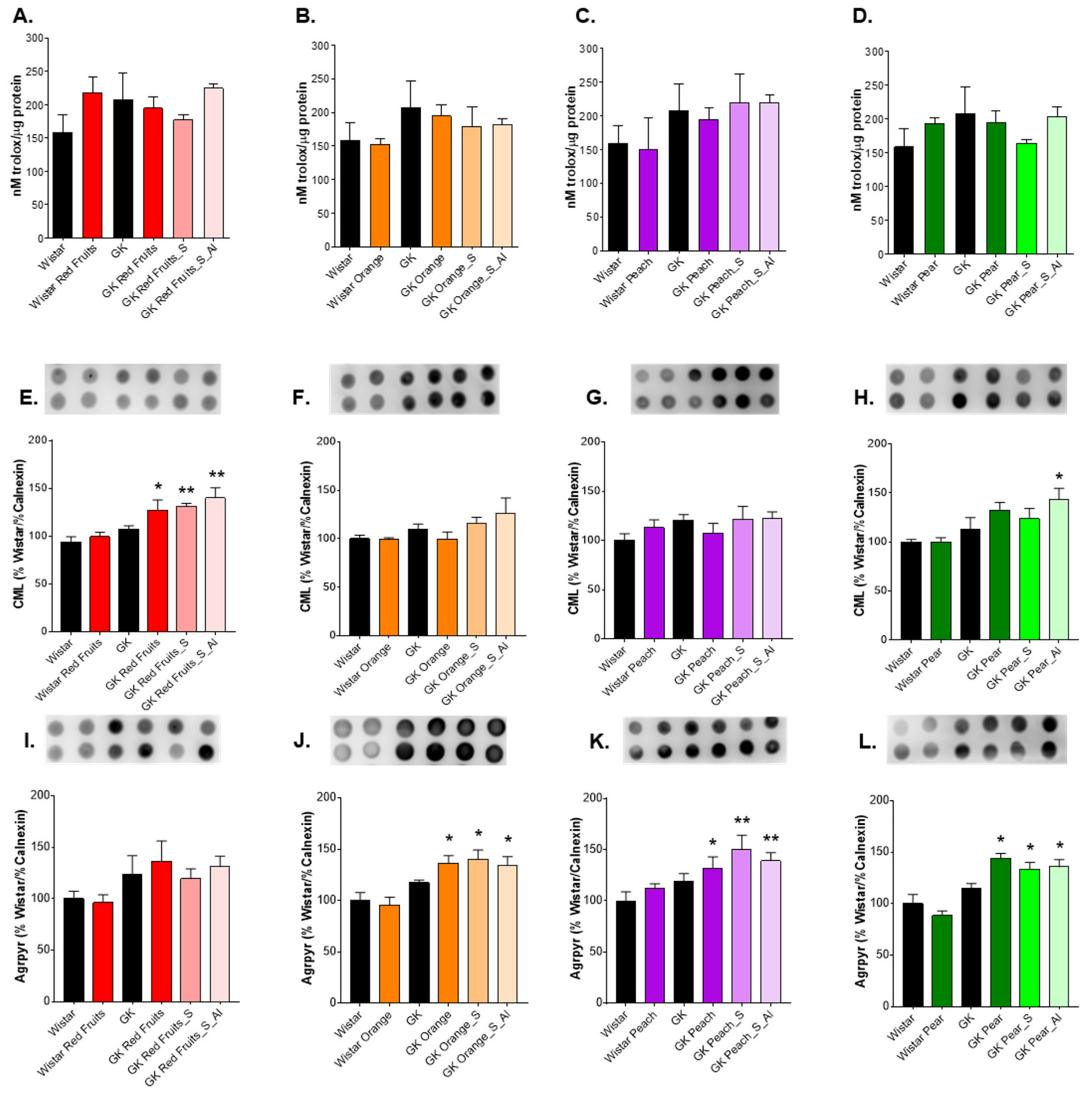
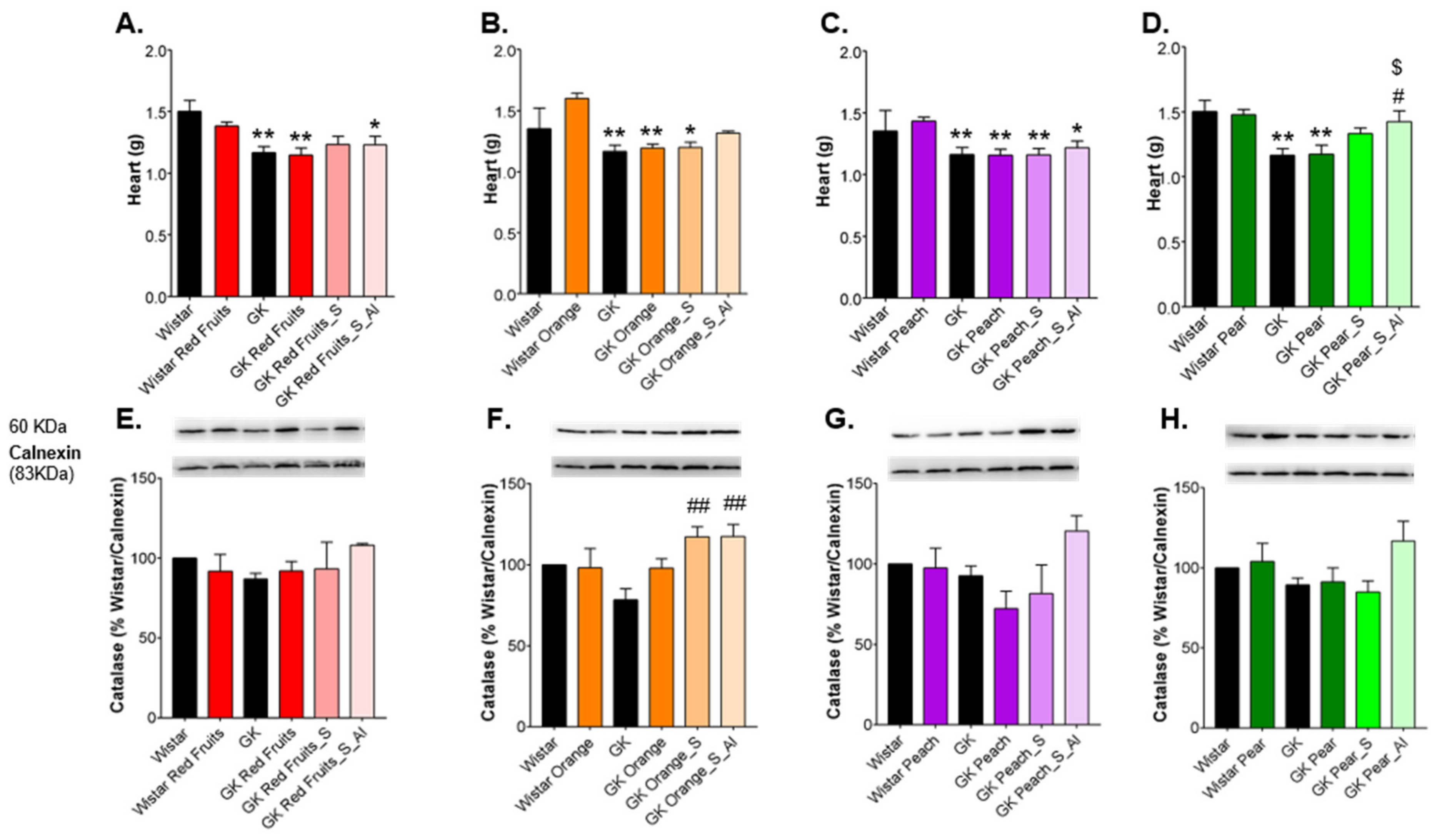
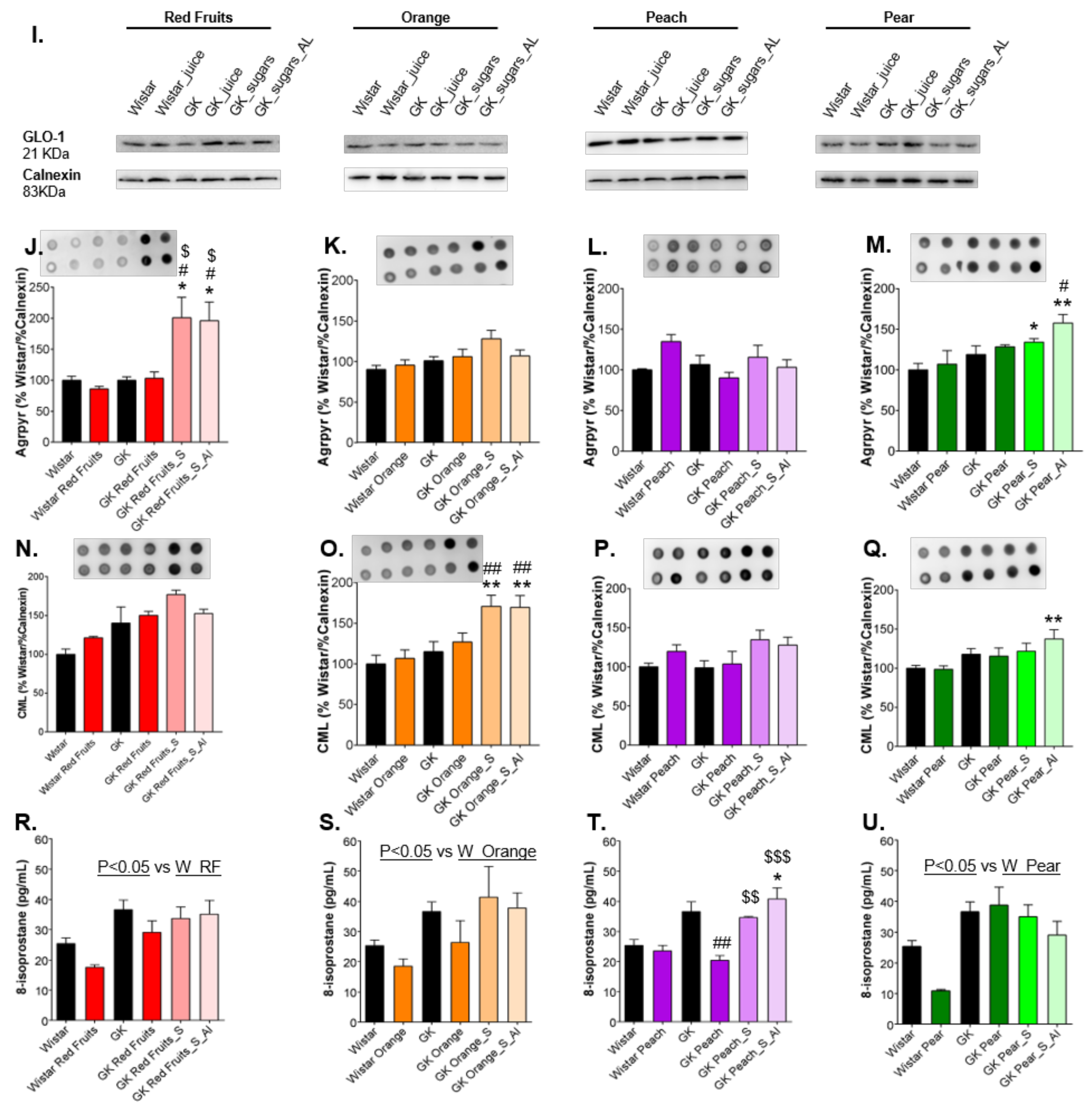
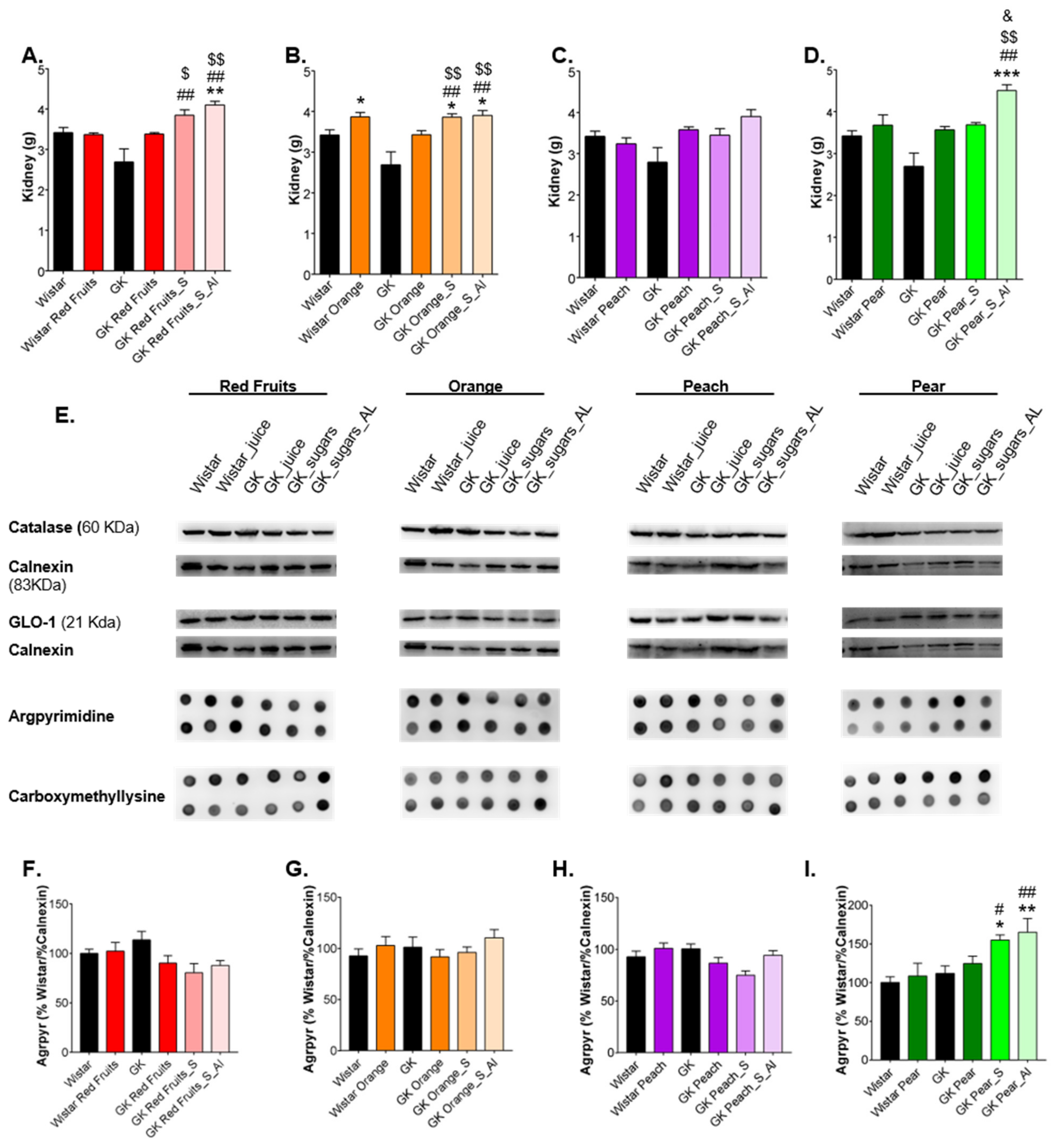
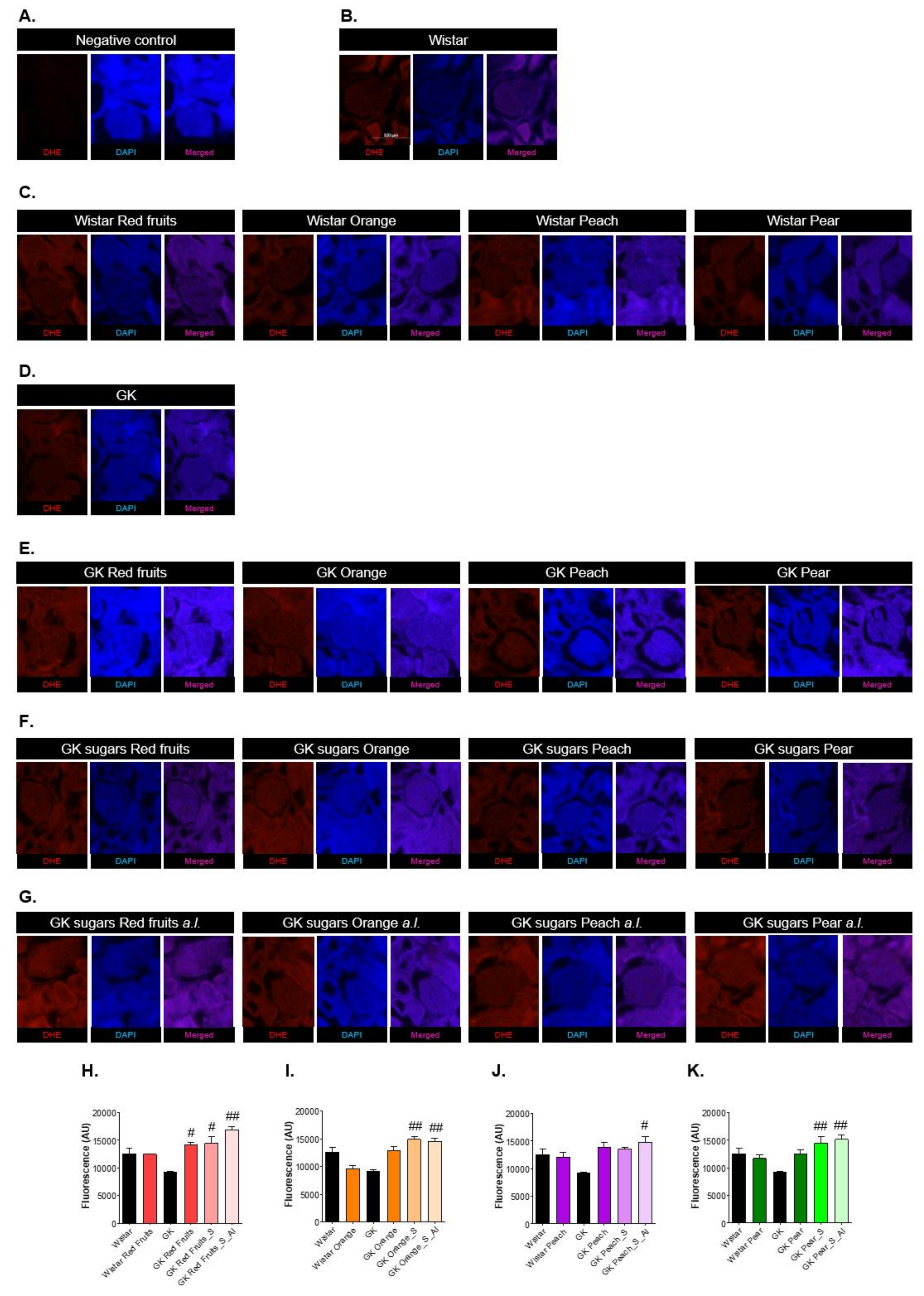
3.3. Consumption of Added Sugars Further Impair Glycation and Oxidative Stress Markers in Liver, Adipose Tissue, Heart and Kidney
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deforche, B.; van Dyck, D.; Deliens, T.; de Bourdeaudhuij, I. Changes in Weight, Physical Activity, Sedentary Behaviour and Dietary Intake during the Transition to Higher Education: A Prospective Study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, G.L.; Jomori, M.M.; Fernandes, A.C.; da Costa Proença, R.P. Consumo alimentar de estudantes universitários. Rev. Nutr. 2017, 30, 847–865. [Google Scholar] [CrossRef] [Green Version]
- Vorster, H.H.; Kruger, A.; Wentzel-Viljoen, E.; Kruger, H.S.; Margetts, B.M. Added Sugar Intake in South Africa: Findings from the Adult Prospective Urban and Rural Epidemiology Cohort Study. Am. J. Clin. Nutr. 2014, 99, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Pimienta, T.G.; Batis, C.; Lutter, C.K.; Rivera, J.A. Sugar-Sweetened Beverages Are the Main Sources of Added Sugar Intake in the Mexican Population. J. Nutr. 2016, 146, 1888S–1896S. [Google Scholar] [CrossRef] [Green Version]
- Stanhope, K.L. Sugar Consumption, Metabolic Disease and Obesity: The State of the Controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Pereira, M.A. Sugar-Sweetened and Artificially-Sweetened Beverages in Relation to Obesity Risk123. Adv. Nutr. 2014, 5, 797–808. [Google Scholar] [CrossRef] [Green Version]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The Role of Artificial and Natural Sweeteners in Reducing the Consumption of Table Sugar: A Narrative Review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P. New Insights into the Health Effects of Dietary Saturated and Omega-6 and Omega-3 Polyunsaturated Fatty Acids. BMC Med. 2012, 10, 50. [Google Scholar] [CrossRef]
- Bes-Rastrollo, M.; Sayon-Orea, C.; Ruiz-Canela, M.; Martinez-Gonzalez, M.A. Impact of Sugars and Sugar Taxation on Body Weight Control: A Comprehensive Literature Review. Obesity 2016, 24, 1410–1426. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added Sugar Intake and Cardiovascular Diseases Mortality among US Adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Sluik, D.; van Lee, L.; Engelen, A.I.; Feskens, E.J.M. Total, Free, and Added Sugar Consumption and Adherence to Guidelines: The Dutch National Food Consumption Survey 2007–2010. Nutrients 2016, 8, 70. [Google Scholar] [CrossRef]
- Pfinder, M.; Heise, T.L.; Hilton Boon, M.; Pega, F.; Fenton, C.; Griebler, U.; Gartlehner, G.; Sommer, I.; Katikireddi, S.V.; Lhachimi, S.K. Taxation of Unprocessed Sugar or Sugar-added Foods for Reducing Their Consumption and Preventing Obesity or Other Adverse Health Outcomes. Cochrane Database Syst. Rev. 2020, 4, CD012333. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015; Volume 26, pp. 34–36. ISBN 978.92.4.154902 8. [Google Scholar]
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Alarcão, V.; Guiomar, S.; Mota, J. Inquérito Alimentar Nacional e de Atividade Física, IAN-AF 2015–2016: Relatório metodológico. 2017. Available online: https://ian-af.up.pt/sites/default/files/IAN-AF%20Relatorio%20Metodol%C3%B3gico.pdf (accessed on 9 February 2021).
- Goldfein, K.R.; Slavin, J.L. Why Sugar Is Added to Food: Food Science 101. Compr. Rev. Food Sci. Food Saf. 2015, 14, 644–656. [Google Scholar] [CrossRef] [Green Version]
- McLellan, A.C.; Thornalley, P.J. Glyoxalase Activity in Human Red Blood Cells Fractioned by Age. Mech. Ageing Dev. 1989, 48, 63–71. [Google Scholar] [CrossRef]
- McLellan, A.C.; Thornalley, P.J.; Benn, J.; Sonksen, P.H. Glyoxalase System in Clinical Diabetes Mellitus and Correlation with Diabetic Complications. Clin. Sci. 1994, 87, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Riboulet-Chavey, A.; Pierron, A.; Durand, I.; Murdaca, J.; Giudicelli, J.; van Obberghen, E. Methylglyoxal Impairs the Insulin Signaling Pathways Independently of the Formation of Intracellular Reactive Oxygen Species. Diabetes 2006, 55, 1289–1299. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J. The Glyoxalase System: New Developments towards Functional Characterization of a Metabolic Pathway Fundamental to Biological Life. Biochem. J. 1990, 269, 1–11. [Google Scholar] [CrossRef]
- Thornalley, P.J. Modification of the Glyoxalase System in Human Red Blood Cells by Glucose in Vitro. Biochem. J. 1988, 254, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Andrade, C.; Fogliano, V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–279. [Google Scholar] [CrossRef]
- Cuccurullo, C.; Iezzi, A.; Fazia, M.L.; de Cesare, D.; di Francesco, A.; Muraro, R.; Bei, R.; Ucchino, S.; Spigonardo, F.; Chiarelli, F.; et al. Suppression of Rage as a Basis of Simvastatin-Dependent Plaque Stabilization in Type 2 Diabetes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2716–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal and Glyoxalase I in Atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449. [Google Scholar] [CrossRef]
- Lee, H.J.; Howell, S.K.; Sanford, R.J.; Beisswenger, P.J. Methylglyoxal Can Modify GAPDH Activity and Structure. Ann. N. Y. Acad. Sci. 2005, 1043, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Loske, C.; Neumann, A.; Cunningham, A.M.; Nichol, K.; Schinzel, R.; Riederer, P.; Münch, G. Cytotoxicity of Advanced Glycation Endproducts Is Mediated by Oxidative Stress. J. Neural. Transm. 1998, 105, 1005–1015. [Google Scholar] [CrossRef]
- Rosca, M.G.; Mustata, T.G.; Kinter, M.T.; Ozdemir, A.M.; Kern, T.S.; Szweda, L.I.; Brownlee, M.; Monnier, V.M.; Weiss, M.F. Glycation of Mitochondrial Proteins from Diabetic Rat Kidney Is Associated with Excess Superoxide Formation. Am. J. Physiol. Ren. Physiol. 2005, 289, F420–F430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Juurlink, B.H.J. Increased Methylglyoxal and Oxidative Stress in Hypertensive Rat Vascular Smooth Muscle Cells. Hypertension 2002, 39, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Funk, S.D.; Yurdagul, A.; Orr, A.W. Hyperglycemia and Endothelial Dysfunction in Atherosclerosis: Lessons from Type 1 Diabetes. Int. J. Vasc. Med. 2012, 2012, 569654. [Google Scholar] [CrossRef] [Green Version]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal Promotes Oxidative Stress and Endothelial Dysfunction. Pharm. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef]
- Su, Y.; Lei, X.; Wu, L.; Liu, L. The Role of Endothelial Cell Adhesion Molecules P-Selectin, E-Selectin and Intercellular Adhesion Molecule-1 in Leucocyte Recruitment Induced by Exogenous Methylglyoxal. Immunology 2012, 137, 65–79. [Google Scholar] [CrossRef]
- Rodrigues, T.; Matafome, P.; Santos-Silva, D.; Sena, C.; Seiça, R. Reduction of Methylglyoxal-Induced Glycation by Pyridoxamine Improves Adipose Tissue Microvascular Lesions. Available online: https://www.hindawi.com/journals/jdr/2013/690650/ (accessed on 9 February 2021).
- Sacramento, J.F.; Martins, F.O.; Rodrigues, T.; Matafome, P.; Ribeiro, M.J.; Olea, E.; Conde, S.V. A2 Adenosine Receptors Mediate Whole-Body Insulin Sensitivity in a Prediabetes Animal Model: Primary Effects on Skeletal Muscle. Front. Endocrinol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Crisóstomo, J.; Rodrigues, T.; Rodrigues, L.; Sena, C.M.; Pereira, P.; Seiça, R. Methylglyoxal Causes Structural and Functional Alterations in Adipose Tissue Independently of Obesity. Arch. Physiol. Biochem. 2012, 118, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.S.; Rauber, F.; Leffa, P.S.; Sangalli, C.N.; Campagnolo, P.D.B.; Vitolo, M.R. Ultra-Processed Food Consumption and Its Effects on Anthropometric and Glucose Profile: A Longitudinal Study during Childhood. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 177–184. [Google Scholar] [CrossRef]
- Collison, K.S.; Zaidi, M.Z.; Subhani, S.N.; Al-Rubeaan, K.; Shoukri, M.; Al-Mohanna, F.A. Sugar-Sweetened Carbonated Beverage Consumption Correlates with BMI, Waist Circumference, and Poor Dietary Choices in School Children. BMC Public Health 2010, 10, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.K.; Gearan, E.C.; Schwartz, C. Added Sugars in School Meals and the Diets of School-Age Children. Nutrients 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.; Chiriboga, D.; Hebert, J.R.; Li, Y.; Li, W.; Campbell, M.; Gendreau, K.; Ockene, I.S. Association between Dietary Carbohydrates and Body Weight. Am. J. Epidemiol. 2005, 161, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, H.; Fïto, M.; Covas, M.I. REGICOR investigators Association of Fast Food Consumption with Energy Intake, Diet Quality, Body Mass Index and the Risk of Obesity in a Representative Mediterranean Population. Br. J. Nutr. 2007, 98, 1274–1280. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.S.; del-Ponte, B.; Assunção, M.C.F.; Santos, I.S. Consumption of Ultra-Processed Foods and Body Fat during Childhood and Adolescence: A Systematic Review. Public Health Nutr. 2018, 21, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Ley, S.H.; Sun, Q.; Hu, F.B.; Malik, V.S. Cross-Sectional Association between Sugar-Sweetened Beverage Intake and Cardiometabolic Biomarkers in US Women. Br. J. Nutr. 2018, 119, 570–580. [Google Scholar] [CrossRef] [Green Version]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. Intake of High-Fructose Corn Syrup Sweetened Soft Drinks, Fruit Drinks and Apple Juice Is Associated with Prevalent Arthritis in US Adults, Aged 20–30 Years. Nutr. Diabetes 2016, 6, e199. [Google Scholar] [CrossRef] [Green Version]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. Intakes of Apple Juice, Fruit Drinks and Soda Are Associated with Prevalent Asthma in US Children Aged 2–9 Years. Public Health Nutr. 2016, 19, 123–130. [Google Scholar] [CrossRef] [Green Version]
- DeChristopher, L.R.; Tucker, K.L. Excess Free Fructose, High-Fructose Corn Syrup and Adult Asthma: The Framingham Offspring Cohort. Br. J. Nutr. 2018, 119, 1157–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeChristopher, L.R.; Tucker, K.L. Excess Free Fructose, Apple Juice, High Fructose Corn Syrup and Childhood Asthma Risk—the National Children’s Study. Nutr. J. 2020, 19. [Google Scholar] [CrossRef]
- Choo, V.L.; Viguiliouk, E.; Mejia, S.B.; Cozma, A.I.; Khan, T.A.; Ha, V.; Wolever, T.M.S.; Leiter, L.A.; Vuksan, V.; Kendall, C.W.C.; et al. Food Sources of Fructose-Containing Sugars and Glycaemic Control: Systematic Review and Meta-Analysis of Controlled Intervention Studies. BMJ 2018, 363, k4644. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, P.G.; Greenspan, P. Inhibition of Nonenzymatic Protein Glycation by Pomegranate and Other Fruit Juices. J. Med. Food 2014, 17, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampersaud, G.C.; Valim, M.F. 100% Citrus Juice: Nutritional Contribution, Dietary Benefits, and Association with Anthropometric Measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.; Furuno, S.; Shirai, H.; Kawakami, M.; Muramatsu, M.; Kobayashi, Y.; Yamagishi, S. Assessment of the Concentrations of Various Advanced Glycation End-Products in Beverages and Foods That Are Commonly Consumed in Japan. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Anhê, F.F.; Varin, T.; Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From Plant Pigments to Health Benefits at Mitochondrial Level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, S.; Lomer, M.C.E.; Marciani, L.; Hoad, C.L.; Pritchard, S.E.; Paul, J.; Gowland, P.A.; Spiller, R.C. Processing Apples to Puree or Juice Speeds Gastric Emptying and Reduces Postprandial Intestinal Volumes and Satiety in Healthy Adults. J. Nutr. 2020, 150, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Sierra, N.; Marqués-Cardete, R.; Gurrea-Martínez, A.; Grau-Del Valle, C.; Morillas, C.; Hernández-Mijares, A.; Bañuls, C. Effect of Fibre-Enriched Orange Juice on Postprandial Glycaemic Response and Satiety in Healthy Individuals: An Acute, Randomised, Placebo-Controlled, Double-Blind, Crossover Study. Nutrients 2019, 11, 3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spetter, M.S.; de Graaf, C.; Viergever, M.A.; Smeets, P.A.M. Anterior Cingulate Taste Activation Predicts Ad Libitum Intake of Sweet and Savory Drinks in Healthy, Normal-Weight Men. J. Nutr. 2012, 142, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Pineli, L.D.L.; de Aguiar, L.A.; Fiusa, A.; de Assunção Botelho, R.B.; Zandonadi, R.P.; Melo, L. Sensory Impact of Lowering Sugar Content in Orange Nectars to Design Healthier, Low-Sugar Industrialized Beverages. Appetite 2016, 96, 239–244. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro-Alfredo, T.; Caramelo, B.; Arbeláez, D.; Amaro, A.; Barra, C.; Silva, D.; Oliveira, S.; Seiça, R.; Matafome, P. Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients 2021, 13, 2956. https://doi.org/10.3390/nu13092956
Monteiro-Alfredo T, Caramelo B, Arbeláez D, Amaro A, Barra C, Silva D, Oliveira S, Seiça R, Matafome P. Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients. 2021; 13(9):2956. https://doi.org/10.3390/nu13092956
Chicago/Turabian StyleMonteiro-Alfredo, Tamaeh, Beatriz Caramelo, Daniela Arbeláez, Andreia Amaro, Cátia Barra, Daniela Silva, Sara Oliveira, Raquel Seiça, and Paulo Matafome. 2021. "Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats" Nutrients 13, no. 9: 2956. https://doi.org/10.3390/nu13092956
APA StyleMonteiro-Alfredo, T., Caramelo, B., Arbeláez, D., Amaro, A., Barra, C., Silva, D., Oliveira, S., Seiça, R., & Matafome, P. (2021). Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients, 13(9), 2956. https://doi.org/10.3390/nu13092956







