Dietary Nucleotides Retard Oxidative Stress-Induced Senescence of Human Umbilical Vein Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Cuture and Teatments
2.3. Morphology Observation
2.4. Cell Viability Assay
2.5. Senescence-Associated Beta-Galactosidase (SA-β-gal) Activity
2.6. Flow Cytometry
2.7. Biochemical Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
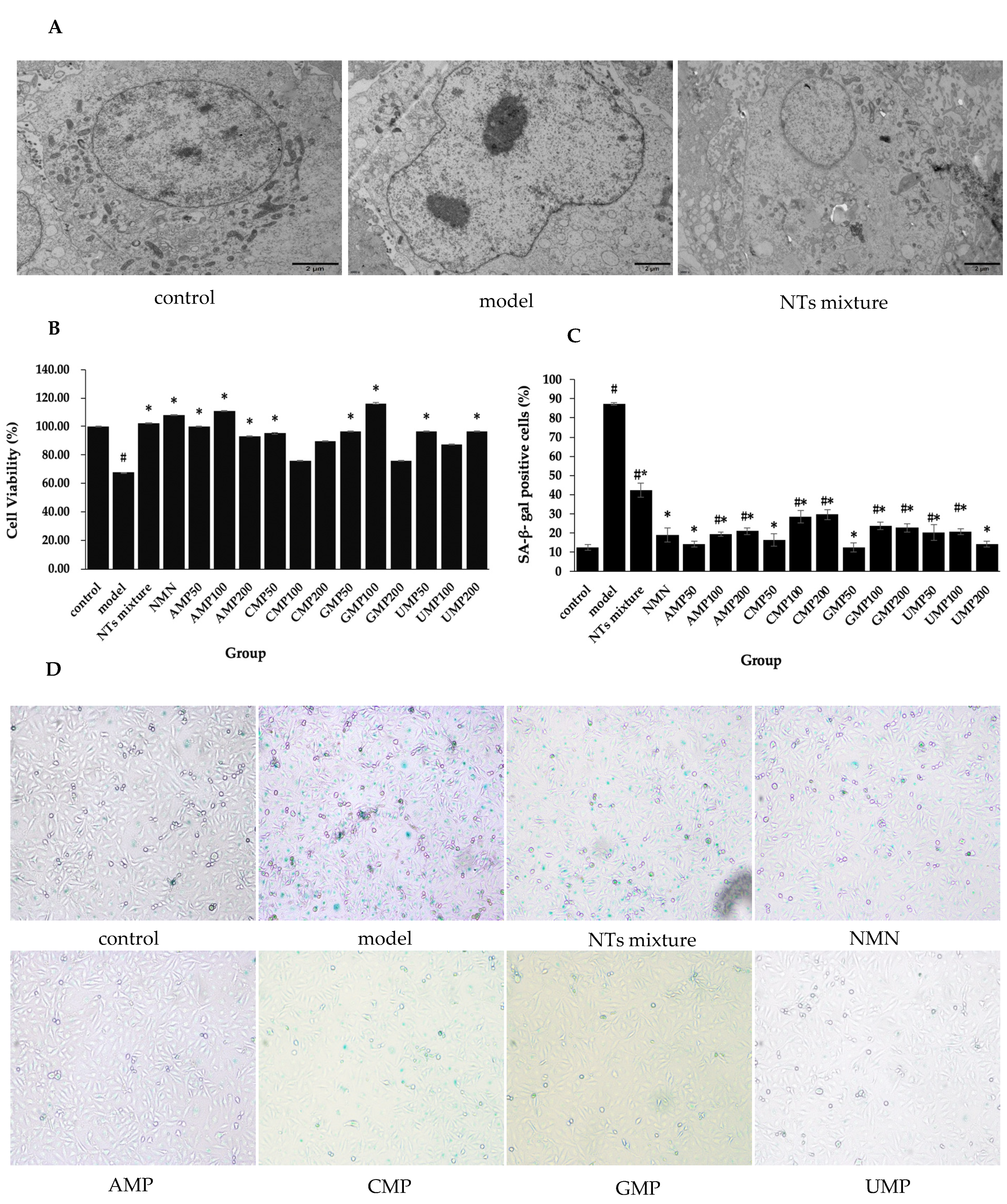
3.1. Effect of NTs on Senescent HUVECs Morphological Changes
3.2. Effect of NTs on Senescent HUVECs Viability
3.3. Effect of NTs on SA-β-gal Activity
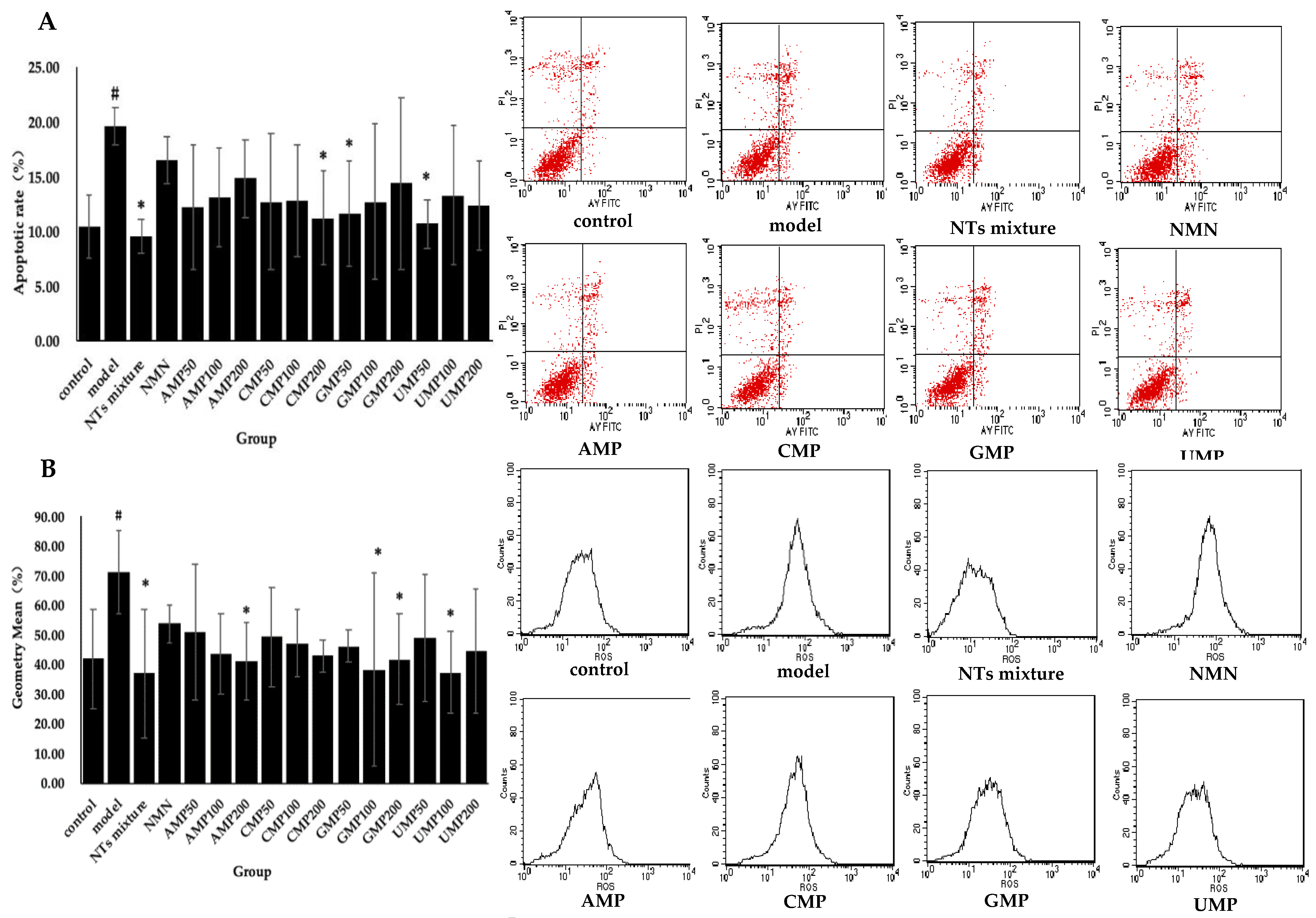
3.4. Effect of NTs on Cell Apoptosis in H2O2-Induced Senescent HUVECs
3.5. Effect of NTs on Intracellular ROS Production
3.6. Effect of NTs on Senescent HUVECs Mitochondrial Membrane Potential (JC-1)
3.7. Effect of NTs on Senescent HUVECs SASP
3.8. Effect of NTs on H2O2-Induced Decreased Antioxidant Activity in HUVECs
3.9. Effect of NTs on NAD+ Levels and NAD+/NADH
3.10. Effect of NTs on Senescent HUVECs ATP Production
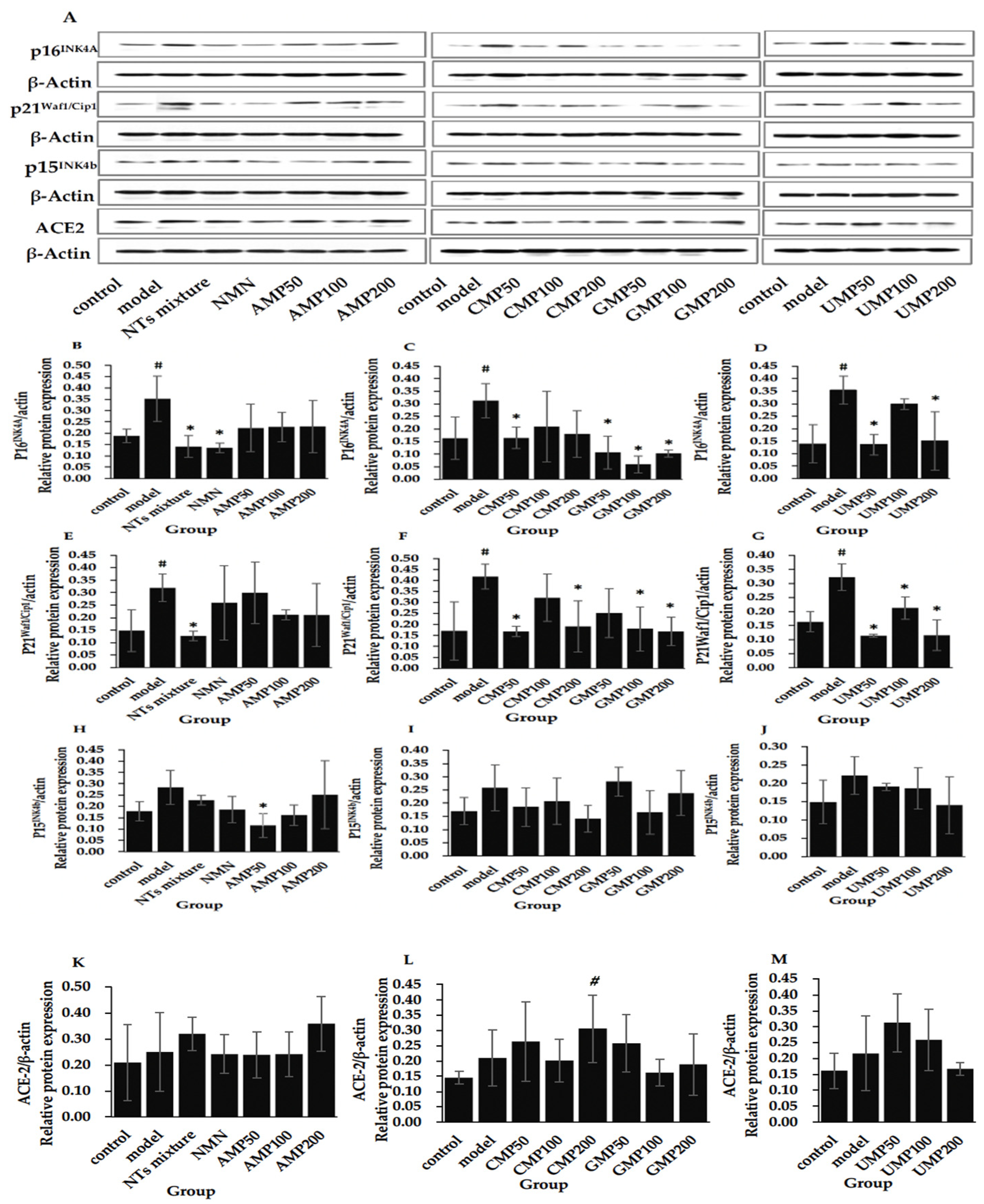
3.11. Effect of NTs on the Protein Expression of p16INK4A, p21Waf1/Cip1, p15INK4b, and ACE-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef]
- Seals, D.R.; Alexander, L.M. Vascular aging. J. Appl. Physiol. 2018, 125, 1841–1842. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Saez-Atienzar, S.; Masliah, E. Cellular senescence and Alzheimer disease: The egg and the chicken scenario. Nat. Rev. Neurosci. 2020, 21, 433–444. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Eppinga, R.N.; Hagemeijer, Y.; Verweij, N.; van der Harst, P. Telomere Length and Risk of Cardiovascular Disease and Cancer. J. Am. Coll. Cardiol. 2017, 70, 506–507. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef]
- Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The roles of senescence and telomere shortening in cardiovascular disease. Nat. Rev. Cardiol. 2013, 10, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Camici, G.G.; Savarese, G.; Akhmedov, A.; Lüscher, T.F. Molecular mechanism of endothelial and vascular aging: Implications for cardiovascular disease. Eur. Heart J. 2015, 36, 3392–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Song, P.; Zhao, Q.; Zou, M.H. Targeting senescent cells to attenuate cardiovascular disease progression. Ageing Res. Rev. 2020, 60, 101072. [Google Scholar] [CrossRef]
- Che, L.; Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Wang, R.; Cheng, Y.; Chen, H.; Fang, Z.; et al. Dietary Nucleotides Supplementation Improves the Intestinal Development and Immune Function of Neonates with Intra-Uterine Growth Restriction in a Pig Model. PLoS ONE 2016, 11, e0157314. [Google Scholar] [CrossRef]
- Td, A.; Ge, S.; Xl, A.; Mx, A.; Yong, L.A. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Carver, J.D. Dietary nucleotides: Cellular immune, intestinal and hepatic system effects. J. Nutr 1994, 124, 144s–148s. [Google Scholar] [CrossRef]
- Pérez, M.J.; Sánchez-Medina, F.; Torres, M.; Gil, A.; Suárez, A. Dietary nucleotides enhance the liver redox state and protein synthesis in cirrhotic rats. J. Nutr. 2004, 134, 2504–2508. [Google Scholar] [CrossRef] [Green Version]
- Sáez-Lara, M.J.; Manzano, M.; Angulo, A.J.; Suárez, A.; Torres, M.I.; Gómez-Llorente, C.; Gil, A.; Fontana, L. Exogenous nucleosides stimulate proliferation of fetal rat hepatocytes. J. Nutr. 2004, 134, 1309–1313. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Buentello, A.; Gatlin, D.M., 3rd. Dietary nucleotides influence immune responses and intestinal morphology of red drum Sciaenops ocellatus. Fish. Shellfish Immunol. 2011, 30, 143–147. [Google Scholar] [CrossRef]
- Holen, E.; Bjørge, O.A.; Jonsson, R. Dietary nucleotides and human immune cells. II. Modulation of PBMC growth and cytokine secretion. Nutrition 2006, 22, 90–96. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Li, Y.; Wang, J. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr. Res. 2017, 61, 1334485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Liang, R.; Guo, Q.; Wang, S.; Zhao, M.; Zhang, Z.; Wang, J.; Li, Y. Dietary nucleotides extend the life span in Sprague-Dawley rats. J. Nutr. Health Aging 2013, 17, 223–229. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, M.; Yang, R.; Zhang, Z.; Li, Y.; Wang, J. Effect of dietary nucleotides on immune function in Balb/C mice. Int. Immunopharmacol. 2013, 17, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Ran, C.; Wang, A.; Xie, M.; Xie, Y.; Ding, Q.; Zhang, Z.; Yang, Y.; Duan, M.; et al. Dietary nucleotides can directly stimulate the immunity of zebrafish independent of the intestinal microbiota. Fish. Shellfish Immunol. 2019, 86, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, A.; López-Pedrosa, J.M.; Torres, M.I.; Gil, A. Dietary nucleotides modulate mitochondrial function of intestinal mucosa in weanling rats with chronic diarrhea. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajemiroye, J.O.; da Cunha, L.C.; Saavedra-Rodríguez, R.; Rodrigues, K.L.; Naves, L.M.; Mourão, A.A.; da Silva, E.F.; Williams, N.E.E.; Martins, J.L.R.; Sousa, R.B.; et al. Aging-Induced Biological Changes and Cardiovascular Diseases. Biomed. Res. Int. 2018, 2018, 7156435. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Lee, S.G.; Park, S.H.; Kim, H.R. Sargahydroquinoic acid (SHQA) suppresses cellular senescence through Akt/mTOR signaling pathway. Exp. Gerontol. 2021, 151, 111406. [Google Scholar] [CrossRef]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Sun, S.; Li, W.; Song, M.; Ji, Q.; Wu, Z.; Liu, Z.; Fan, Y.; Liu, F.; et al. A genome-wide CRISPR-based screen identifies KAT7 as a driver of cellular senescence. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Ma, S.; Sun, S.; Li, J.; Fan, Y.; Qu, J.; Sun, L.; Wang, S.; Zhang, Y.; Yang, S.; Liu, Z.; et al. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. 2021, 31, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Yan, S.; Zhou, Y.; Yang, Q.; Pan, Y.; Zeng, X.; An, X.; Liu, Z.; Wang, L.; et al. Intracellular adenosine regulates epigenetic programming in endothelial cells to promote angiogenesis. EMBO Mol. Med. 2017, 9, 1263–1278. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, C.D.; Liu, S.; Limbad, C.; Zawadzka, A.M.; Beck, J.; Demaria, M.; Artwood, R.; Alimirah, F.; Lopez-Dominguez, J.A.; Kuehnemann, C.; et al. SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep. 2019, 28, 3329–3337.e5. [Google Scholar] [CrossRef] [Green Version]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884s–890. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.; Hosseinpourfaizi, M.A.; Houshmand, M.; Ravasi, A.A. Effect of aerobic exercise training on mtDNA deletion in soleus muscle of trained and untrained Wistar rats. Br. J. Sports Med. 2005, 39, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [Green Version]
- de Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.; Seals, D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Hill, R.B. Mitochondrial regulation of diabetic vascular disease: An emerging opportunity. Transl. Res. 2018, 202, 83–98. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Liu, X.; Xu, M.; Li, Y. Dietary Nucleotides Retard Oxidative Stress-Induced Senescence of Human Umbilical Vein Endothelial Cells. Nutrients 2021, 13, 3279. https://doi.org/10.3390/nu13093279
Zhu N, Liu X, Xu M, Li Y. Dietary Nucleotides Retard Oxidative Stress-Induced Senescence of Human Umbilical Vein Endothelial Cells. Nutrients. 2021; 13(9):3279. https://doi.org/10.3390/nu13093279
Chicago/Turabian StyleZhu, Na, Xinran Liu, Meihong Xu, and Yong Li. 2021. "Dietary Nucleotides Retard Oxidative Stress-Induced Senescence of Human Umbilical Vein Endothelial Cells" Nutrients 13, no. 9: 3279. https://doi.org/10.3390/nu13093279






