Supplementation with High or Low Iron Reduces Colitis Severity in an AOM/DSS Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Protocol
2.2. Morphological Analysis
2.3. Histological Analysis
2.4. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
2.5. cDNA Extraction and Reverse Transcription (RT)-PCR
2.6. Western Blot
2.7. Statistical Analysis
3. Results
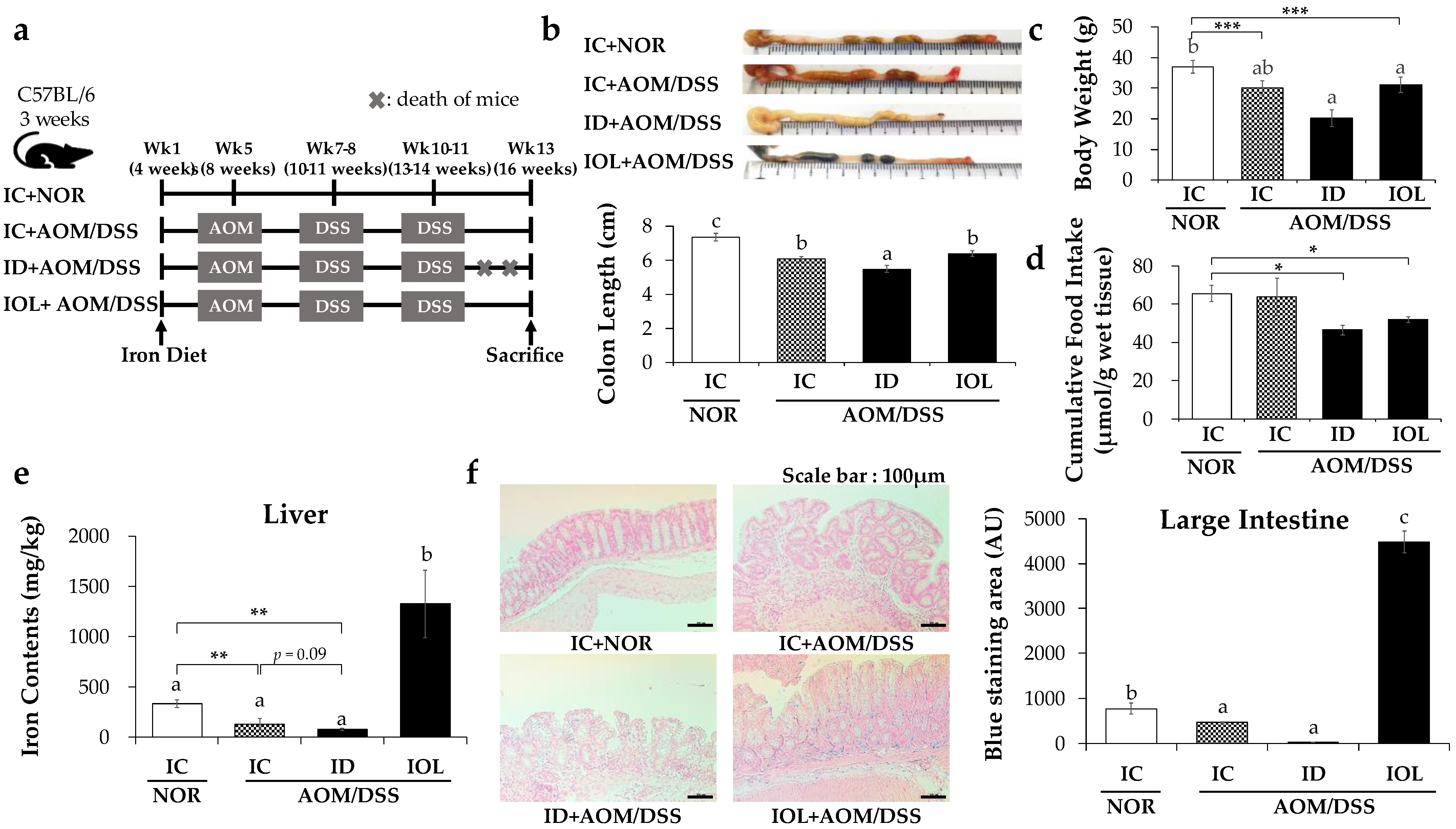
3.1. Effects of ID and IOL Diets on Colon Length, Body Weight, and Body Iron Levels in the AOM/DSS Model
3.2. Iron Metabolic Gene Expression Was Altered in the AOM/DSS-Induced Cancer Model and Further by the ID and IOL Diets
3.3. IOL and ID Diets Alleviated the Development of Colonic Tumors
3.4. IOL Diet Downregulated AOM/DSS-Induced Inflammatory Gene Expression
3.5. Iron Overload Diet Enhanced Antioxidant System through Upregulating the Antioxidant Signaling Pathway
3.6. Iron-Deficient and -Overload Status with AOM/DSS Affected Tumor Suppression through PI3K/AKT Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Stidham, R.W.; Higgins, P.D. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2018, 31, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Bopanna, S.; Ananthakrishnan, A.N.; Kedia, S.; Yajnik, V.; Ahuja, V. Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 269–276. [Google Scholar] [CrossRef]
- Park, J.H.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun. Rev. 2017, 16, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol. Clin. 2006, 35, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Azer, S.A. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur. J. Gastroenterol. Hepatol. 2013, 25, 271–281. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [PubMed]
- Von Drygalski, A.; Adamson, J.W. Iron metabolism in man. J. Parenter. Enter. Nutr. 2013, 37, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Buratti, P.; Cairo, G.; Recalcati, S. The transferrin receptor: The cellular iron gate. Metallomics 2017, 9, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef]
- Forciniti, S.; Greco, L.; Grizzi, F.; Malesci, A.; Laghi, L. Iron metabolism in cancer progression. Int. J. Mol. Sci. 2020, 21, 2257. [Google Scholar] [CrossRef]
- Wurzelmann, J.I.; Silver, A.; Schreinemachers, D.M.; Sandler, R.S.; Everson, R.B. Iron intake and the risk of colorectal cancer. Cancer Epidemiol. Prev. Biomark. 1996, 5, 503–507. [Google Scholar]
- Tseng, M.; Sandler, R.S.; Greenberg, E.R.; Mandel, J.S.; Haile, R.W.; Baron, J.A. Dietary iron and recurrence of colorectal adenomas. Cancer Epidemiol. Biomark. Prev. 1997, 6, 1029–1032. [Google Scholar]
- Kobayashi, Y.; Ohfuji, S.; Kondo, K.; Fukushima, W.; Sasaki, S.; Kamata, N.; Yamagami, H.; Fujiwara, Y.; Suzuki, Y.; Hirota, Y.; et al. Association between dietary iron and zinc intake and development of ulcerative colitis: A case–control study in Japan. J. Gastroenterol. Hepatol. 2019, 34, 1703–1710. [Google Scholar] [CrossRef]
- Senesse, P.; Meance, S.; Cottet, V.; Faivre, J.; Boutron-Ruault, M.-C. High Dietary Iron and Copper and Risk of Colorectal Cancer: A Case-Control Study in Burgundy, France. Nutr. Cancer 2004, 49, 66–71. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk—A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef]
- Khalili, H.; de Silva, P.S.; Ananthakrishnan, A.N.; Lochhead, P.; Joshi, A.; Garber, J.J.; Richter, J.R.; Sauk, J.; Chan, A.T. Dietary Iron and Heme Iron Consumption, Genetic Susceptibility, and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Cornish, A.J.; Law, P.J.; Timofeeva, M.; Palin, K.; Farrington, S.M.; Palles, C.; Jenkins, M.A.; Casey, G.; Brenner, H.; Chang-Claude, J.; et al. Modifiable pathways for colorectal cancer: A mendelian randomisation analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 55–62. [Google Scholar] [CrossRef]
- Kabat, G.; Miller, A.; Jain, M.; Rohan, T.E. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. Br. J. Cancer 2007, 97, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Mahalhal, A.; Burkitt, M.D.; Duckworth, C.A.; Hold, G.L.; Campbell, B.J.; Pritchard, D.M.; Probert, C.S. Long-Term Iron Deficiency and Dietary Iron Excess Exacerbate Acute Dextran Sodium Sulphate-Induced Colitis and Are Associated with Significant Dysbiosis. Int. J. Mol. Sci. 2021, 22, 73646. [Google Scholar] [CrossRef]
- Seril, D.N.; Liao, J.; Ho, K.-L.K.; Warsi, A.; Yang, C.S.; Yang, G.-Y. Dietary Iron Supplementation Enhances DSS-Induced Colitis and Associated Colorectal Carcinoma Development in Mice. Dig. Dis. Sci. 2002, 47, 1266–1278. [Google Scholar] [CrossRef]
- Seril, D.N.; Liao, J.; Yang, G.-Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef]
- Yilmaz, B.; Li, H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef]
- Ashmore, J.H.; Rogers, C.J.; Kelleher, S.L.; Lesko, S.M.; Hartman, T.J. Dietary Iron and Colorectal Cancer Risk: A Review of Human Population Studies. Crit. Rev. Food Sci. Nutr. 2016, 56, 1012–1020. [Google Scholar] [CrossRef]
- Rada, B.; Gardina, P.; Myers, T.G.; Leto, T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011, 4, 158–171. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.; Rampton, D.; Blake, D. Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Aliment. Pharmacol. Ther. 2000, 14, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Carrier, J.; Aghdassi, E.; Platt, I.; Cullen, J.; Allard, J.P. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment. Pharmacol. Ther. 2001, 15, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Soendergaard, C.; Vikner, M.E.; Weiss, G. Rational Management of Iron-Deficiency Anaemia in Inflammatory Bowel Disease. Nutrients 2018, 10, 82. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron metabolism in cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef]
- Xue, X.; Shah, Y.M. Intestinal iron homeostasis and colon tumorigenesis. Nutrients 2013, 5, 2333–2351. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Barollo, M.; D’Incà, R.; Scarpa, M.; Medici, V.; Cardin, R.; Fries, W.; Angriman, I.; Sturniolo, G.C. Effects of iron deprivation or chelation on DNA damage in experimental colitis. Int. J. Colorectal Dis. 2004, 19, 461–466. [Google Scholar] [CrossRef]
- Chua, A.C.G.; Klopcic, B.R.S.; Ho, D.S.; Fu, S.K.; Forrest, C.H.; Croft, K.D.; Olynyk, J.K.; Lawrance, I.C.; Trinder, D. Dietary Iron Enhances Colonic Inflammation and IL-6/IL-11-Stat3 Signaling Promoting Colonic Tumor Development in Mice. PLoS ONE 2013, 8, e78850. [Google Scholar] [CrossRef]
- Wang, S.-W.; Sun, Y.-M. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer. Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, F.; Li, G.; Li, G.; Yang, X.; Liu, L.; Zhang, R.; Zhang, B.; Feng, Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Keshavarzian, A.; Gounaris, E.; Melson, J.E.; Cheon, E.C.; Blatner, N.R.; Chen, Z.E.; Tsai, F.-N.; Lee, G.; Ryu, H.; et al. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages, and epithelial cells in colitis-induced cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2342–2354. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Yang, Y.; Gu, C. Iron metabolism and its contribution to cancer. Int. J. Oncol. 2019, 54, 1143–1154. [Google Scholar] [CrossRef]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar] [CrossRef]
- Fujita, N.; Sugimoto, R.; Takeo, M.; Urawa, N.; Mifuji, R.; Tanaka, H.; Kobayashi, Y.; Iwasa, M.; Watanabe, S.; Adachi, Y.; et al. Hepcidin expression in the liver: Relatively low level in patients with chronic hepatitis C. Mol. Med. 2007, 13, 97–104. [Google Scholar] [CrossRef]
- Heath, J.L.; Weiss, J.M.; Lavau, C.P.; Wechsler, D.S. Iron Deprivation in Cancer—Potential Therapeutic Implications. Nutrients 2013, 5, 2836–2859. [Google Scholar] [CrossRef]
- Viennois, E.; Ingersoll, S.A.; Ayyadurai, S.; Zhao, Y.; Wang, L.; Zhang, M.; Han, M.K.; Garg, P.; Xiao, B.; Merlin, D. Critical role of PepT1 in promoting colitis-associated cancer and therapeutic benefits of the anti-inflammatory PepT1-mediated tripeptide KPV in a murine model. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 340–357. [Google Scholar] [CrossRef]
- Katkoori, V.R.; Suarez-Cuervo, C.; Shanmugam, C.; Jhala, N.C.; Callens, T.; Messiaen, L.; Posey, J., 3rd; Bumpers, H.L.; Meleth, S.; Grizzle, W.E.; et al. Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J. Gastrointest. Oncol. 2010, 1, 76–89. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Cortesini, C.; Fantappiè, O.; Messerini, L.; Sardi, I.; Lasagna, N.; Perna, F.; Fabbroni, V.; Di Felice, A.; Perigli, G.; et al. Cyclooxygenase-2 Activation Mediates the Proangiogenic Effect of Nitric Oxide in Colorectal Cancer. Clin. Cancer Res. 2004, 10, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Atreya, R.; Neurath, M. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Vitali, R.; Palone, F.; Pierdomenico, M.; Negroni, A.; Cucchiara, S.; Aloi, M.; Oliva, S.; Stronati, L. Dipotassium glycyrrhizate via HMGB1 or AMPK signaling suppresses oxidative stress during intestinal inflammation. Biochem. Pharmacol. 2015, 97, 292–299. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.-T.; Prawan, A.; Liu, Y.; Hao, X.; Yu, S.; Cheung, W.K.L.; Chan, J.Y.; Reddy, B.S.; Yang, C.S.; et al. Increased Susceptibility of Nrf2 Knockout Mice to Colitis-Associated Colorectal Cancer. Cancer Prev. Res. 2008, 1, 187–191. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Li, Z.-H.; Li, Q.; Huang, W.-S.; Kang, L.; Wang, J.-P. Resveratrol affects protein kinase C activity and promotes apoptosis in human colon carcinoma cells. Asian Pac. J. Cancer Prev. 2012, 13, 6017–6022. [Google Scholar] [CrossRef]
- Lui, G.Y.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget 2015, 6, 18748. [Google Scholar] [CrossRef]
- Pusatcioglu, C.K.; Nemeth, E.; Fantuzzi, G.; Llor, X.; Freels, S.; Tussing-Humphreys, L.; Cabay, R.J.; Linzmeier, R.; Ng, D.; Clark, J.; et al. Systemic and tumor level iron regulation in men with colorectal cancer: A case control study. Nutr. Metab. 2014, 11, 21. [Google Scholar] [CrossRef]
- Lavilla, I.; Costas, M.; San Miguel, P.; Millos, J.; Bendicho, C. Elemental fingerprinting of tumorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis. BioMetals 2009, 22, 863–875. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hue, J.-J.; Kang, B.S.; Park, H.; Nam, S.Y.; Yun, Y.W.; Kim, J.-S.; Lee, B.J. Effects of selenium on colon carcinogenesis induced by azoxymethane and dextran sodium sulfate in mouse model with high-iron diet. Lab. Anim. Res. 2011, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Picard, V.; Epsztejn, S.; Santambrogio, P.; Cabantchik, Z.I.; Beaumont, C. Role of ferritin in the control of the labile iron pool in murine erythroleukemia cells. J. Biol. Chem. 1998, 273, 15382–15386. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Lombardi, D.; Russo, P.; Palescandolo, E.; De Luca, A.; Santini, D.; Baldi, F.; Rossiello, L.; Dell’Anna, M.L.; Mastrofrancesco, A.; et al. Ferritin Contributes to Melanoma Progression by Modulating Cell Growth and Sensitivity to Oxidative Stress. Clin. Cancer Res. 2005, 11, 3175–3183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shpyleva, S.I.; Tryndyak, V.P.; Kovalchuk, O.; Starlard-Davenport, A.; Chekhun, V.F.; Beland, F.A.; Pogribny, I.P. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res. Treat. 2011, 126, 63–71. [Google Scholar] [CrossRef]
- Horniblow, R.D.; Bedford, M.; Hollingworth, R.; Evans, S.; Sutton, E.; Lal, N.; Beggs, A.; Iqbal, T.H.; Tselepis, C. BRAF mutations are associated with increased iron regulatory protein-2 expression in colorectal tumorigenesis. Cancer Sci. 2017, 108, 1135–1143. [Google Scholar] [CrossRef]
- Brookes, M.J.; Hughes, S.; Turner, F.E.; Reynolds, G.; Sharma, N.; Ismail, T.; Berx, G.; McKie, A.T.; Hotchin, N.; Anderson, G.J. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut 2006, 55, 1449–1460. [Google Scholar] [CrossRef]
- Prutki, M.; Poljak-Blazi, M.; Jakopovic, M.; Tomas, D.; Stipancic, I.; Zarkovic, N. Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett. 2006, 238, 188–196. [Google Scholar] [CrossRef]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422. [Google Scholar] [CrossRef]
- Simão, M.; Camacho, A.; Ostertag, A.; Cohen-Solal, M.; Pinto, I.J.; Porto, G.; Hang Korng, E.; Cancela, M.L. Iron-enriched diet contributes to early onset of osteoporotic phenotype in a mouse model of hereditary hemochromatosis. PLoS ONE 2018, 13, e0207441. [Google Scholar] [CrossRef]
- Cao, L.-L.; Liu, H.; Yue, Z.; Liu, L.; Pei, L.; Gu, J.; Wang, H.; Jia, M. Iron chelation inhibits cancer cell growth and modulates global histone methylation status in colorectal cancer. BioMetals 2018, 31, 797–805. [Google Scholar] [CrossRef]
- Ramos, E.; Kautz, L.; Rodriguez, R.; Hansen, M.; Gabayan, V.; Ginzburg, Y.; Roth, M.-P.; Nemeth, E.; Ganz, T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology 2011, 53, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D.; Vafa, M.R.; Haile, D.J.; Wessling-Resnick, M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood 2003, 102, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, F.; Jenkitkasemwong, S.; Gulec, S.; Knutson, M.D. Iron Loading Increases Ferroportin Heterogeneous Nuclear RNA and mRNA Levels in Murine J774 Macrophages. J. Nutr. 2009, 139, 434–438. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.J.; Ostini, L.; Wallace, D.F.; John, A.N.; Watters, D.J.; Subramaniam, V.N. Iron loading and oxidative stress in the Atm−/− mouse liver. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G554–G560. [Google Scholar] [CrossRef]
- Wareing, M.; Ferguson, C.J.; Delannoy, M.; Cox, A.G.; McMahon, R.F.T.; Green, R.; Riccardi, D.; Smith, C.P. Altered dietary iron intake is a strong modulator of renal DMT1 expression. Am. J. Physiol.-Ren. Physiol. 2003, 285, F1050–F1059. [Google Scholar] [CrossRef]
- Hansen, J.B.; Tonnesen, M.F.; Madsen, A.N.; Hagedorn, P.H.; Friberg, J.; Grunnet, L.G.; Heller, R.S.; Nielsen, A.Ø.; Størling, J.; Baeyens, L.; et al. Divalent Metal Transporter 1 Regulates Iron-Mediated ROS and Pancreatic β Cell Fate in Response to Cytokines. Cell Metab. 2012, 16, 449–461. [Google Scholar] [CrossRef]
- Hansen, J.B.; Dos Santos, L.R.B.; Liu, Y.; Prentice, K.J.; Teudt, F.; Tonnesen, M.; Jonas, J.-C.; Wheeler, M.B.; Mandrup-Poulsen, T. Glucolipotoxic conditions induce β-cell iron import, cytosolic ROS formation and apoptosis. J. Mol. Endocrinol. 2018, 61, 69–77. [Google Scholar] [CrossRef]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar] [CrossRef]
- Gustafson, W.; Weiss, W. Myc proteins as therapeutic targets. Oncogene 2010, 29, 1249–1259. [Google Scholar] [CrossRef]
- Tashiro, E.; Tsuchiya, A.; Imoto, M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007, 98, 629–635. [Google Scholar] [CrossRef]
- Liu, F.; Ji, F.; Ji, Y.; Jiang, Y.; Sun, X.; Lu, Y.; Zhang, L.; Han, Y.; Liu, X. In-depth analysis of the critical genes and pathways in colorectal cancer. Int. J. Mol. Med. 2015, 36, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, P.; Srivastava, A. Iron: Key player in cancer and cell cycle? J. Trace Elem. Med. Biol. 2020, 62, 126582. [Google Scholar] [CrossRef] [PubMed]
- Simonart, T.; Heenen, M.; Degraef, C.; Andrei, G.; Mosselmans, R.; Hermans, P.; Van Vooren, J.-P.; Noel, J.-C.; Boelaert, J.R.; Snoeck, R. Iron chelators inhibit the growth and induce the apoptosis of Kaposi’s sarcoma cells and of their putative endothelial precursors. J. Investig. Dermatol. 2000, 115, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Defrère, S.; Van Langendonckt, A.; Vaesen, S.; Jouret, M.; González Ramos, R.; Gonzalez, D.; Donnez, J. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum. Reprod. 2006, 21, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Mathahs, M.M.; Broadhurst, K.A.; Weydert, J. Chronic iron overload stimulates hepatocyte proliferation and cyclin D1 expression in rodent liver. Transl. Res. 2006, 148, 55–62. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Rishi, G.; Huang, G.; Subramaniam, V.N. Cancer: The role of iron and ferroptosis. Int. J. Biochem. Cell Biol. 2021, 141, 106094. [Google Scholar] [CrossRef]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The Application of Ferroptosis in Diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef]
- Viennois, E.; Chen, F.; Merlin, D. NF-κB pathway in colitis-associated cancers. Transl. Gastrointest. Cancer 2013, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.-W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKβ Links Inflammation and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.-J.; Kim, J.M.; Kim, I.-K.; Ko, S.H.; Kim, J.S. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J. Gastroenterol. Hepatol. 2014, 29, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liang, W.; Wang, T.; Sui, J.; Wang, J.; Deng, Z.; Chen, D. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol. Res. 2019, 144, 66–72. [Google Scholar] [CrossRef]
- Koh, S.-J.; Kim, J.M.; Kim, I.-K.; Kim, N.; Jung, H.C.; Song, I.S.; Kim, J.S. Fluoxetine inhibits NF-κB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G9–G19. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar] [CrossRef]
- Gochman, E.; Mahajna, J.; Shenzer, P.; Dahan, A.; Blatt, A.; Elyakim, R.; Reznick, A.Z. The expression of iNOS and nitrotyrosine in colitis and colon cancer in humans. Acta Histochem. 2012, 114, 827–835. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Alaa, R.; Catherine, D.; Gabriela, L.; Alexander, T.; Nicholas, B.; Catherine, C.; Margaret, S.; David, M. Nonsteroidal Anti-inflammatory Drugs and Cyclooxygenase-2 Inhibitors for Primary Prevention of Colorectal Cancer: A Systematic Review Prepared for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2007, 146, 376–389. [Google Scholar] [CrossRef]
- Kaler, P.; Godasi, B.N.; Augenlicht, L.; Klampfer, L. The NF-κB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1β. Cancer Microenviron. 2009, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calvé, A.; Santos, M.M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.-Y.; Kim, J.H.; Kang, J.-E.; Park, M.H.; Kim, E.-J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Schenk, H.; Klein, M.; Erdbrügger, W.; Dröge, W.; Schulze-Osthoff, K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc. Natl. Acad. Sci. USA 1994, 91, 1672–1676. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Aranda, R.; Martín, M.G.; Doroshow, J.H.; Binder, S.W.; Chu, F.-F. Mice with combined disruption of Gpx1 andGpx2 genes have colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281, G848–G855. [Google Scholar] [CrossRef]
- Moon, M.S.; McDevitt, E.I.; Zhu, J.; Stanley, B.; Krzeminski, J.; Amin, S.; Aliaga, C.; Miller, T.G.; Isom, H.C. Elevated Hepatic Iron Activates NF-E2–Related Factor 2–Regulated Pathway in a Dietary Iron Overload Mouse Model. Toxicol. Sci. 2012, 129, 74–85. [Google Scholar] [CrossRef]
- Li, C.; Deng, X.; Xie, X.; Liu, Y.; Friedmann Angeli, J.P.; Lai, L. Activation of Glutathione Peroxidase 4 as a Novel Anti-inflammatory Strategy. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Rushworth, S.A.; MacEwan, D.J.; O’Connell, M.A. Lipopolysaccharide-Induced Expression of NAD(P)H:Quinone Oxidoreductase 1 and Heme Oxygenase-1 Protects against Excessive Inflammatory Responses in Human Monocytes. J. Immunol. 2008, 181, 6730–6737. [Google Scholar] [CrossRef]
- Dasari, A.; Messersmith, W.A. New Strategies in Colorectal Cancer: Biomarkers of Response to Epidermal Growth Factor Receptor Monoclonal Antibodies and Potential Therapeutic Targets in Phosphoinositide 3-Kinase and Mitogen-Activated Protein Kinase Pathways. Clin. Cancer Res. 2010, 16, 3811–3818. [Google Scholar] [CrossRef]
- Zhang, J.; Roberts, T.M.; Shivdasani, R.A. Targeting PI3K Signaling as a Therapeutic Approach for Colorectal Cancer. Gastroenterology 2011, 141, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, P.; Uittenboogaard, A.; Thijs, A.M.J.; Westgeest, H.M.; Boere, I.A.; Lambrechts, S.; van de Stolpe, A.; Bekkers, R.L.M.; Piek, J.M.J. The effectiveness of monotherapy with PI3K/AKT/mTOR pathway inhibitors in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2021, 163, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shcherba, M.; Pendurti, G.; Liang, Y.; Piperdi, B.; Perez-Soler, R. Targeting the PI3K/AKT/mTOR pathway: Potential for lung cancer treatment. Lung Cancer Manag. 2014, 3, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, E.; Chaudhuri, A.; Leiphrakpam, P.D.; Haferbier, K.L.; Brattain, M.G.; Chowdhury, S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer 2014, 14, 145. [Google Scholar] [CrossRef]
- Pan, Q.; Lou, X.; Zhang, J.; Zhu, Y.; Li, F.; Shan, Q.; Chen, X.; Xie, Y.; Su, S.; Wei, H.; et al. Genomic variants in mouse model induced by azoxymethane and dextran sodium sulfate improperly mimic human colorectal cancer. Sci. Rep. 2017, 7, 25. [Google Scholar] [CrossRef]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Kang, H.W.; Kim, J.M.; Cha, M.Y.; Jung, H.C.; Song, I.S.; Kim, J.S. Deguelin, an Akt inhibitor, down-regulates NF-κB signaling and induces apoptosis in colon cancer cells and inhibits tumor growth in mice. Dig. Dis. Sci. 2012, 57, 2873–2882. [Google Scholar] [CrossRef]
- Agarwal, E.; Brattain, M.G.; Chowdhury, S. Cell survival and metastasis regulation by Akt signaling in colorectal cancer. Cell. Signal. 2013, 25, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.-Y.; Cen, W.-J.; Zhou, X.-Z.; Li, Y.-R.; Kong, W.-D.; Jiang, J.-W. Iron overload induces apoptosis of murine preosteoblast cells via ROS and inhibition of AKT pathway. Oral Dis. 2017, 23, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jing, X.; Guo, J.; Sun, K.; Deng, Y.; Zhang, Y.; Guo, F.; Ye, Y. Icariin Protects Bone Marrow Mesenchymal Stem Cells Against Iron Overload Induced Dysfunction Through Mitochondrial Fusion and Fission, PI3K/AKT/mTOR and MAPK Pathways. Front. Pharmacol. 2019, 10, 163. [Google Scholar] [CrossRef]
- Salama, S.A.; Omar, H.A. Modulating NF-κB, MAPK, and PI3K/AKT signaling by ergothioneine attenuates iron overload-induced hepatocellular injury in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22729. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Kabel, A.M. Taxifolin ameliorates iron overload-induced hepatocellular injury: Modulating PI3K/AKT and p38 MAPK signaling, inflammatory response, and hepatocellular regeneration. Chem.-Biol. Interact. 2020, 330, 109230. [Google Scholar] [CrossRef]
- Dixon, K.M.; Lui, G.Y.L.; Kovacevic, Z.; Zhang, D.; Yao, M.; Chen, Z.; Dong, Q.; Assinder, S.J.; Richardson, D.R. Dp44mT targets the AKT, TGF-β and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epithelial cells and prostate cancer cells. Br. J. Cancer 2013, 108, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, Z.; Chikhani, S.; Lui, G.Y.; Sivagurunathan, S.; Richardson, D.R. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid. Redox Signal. 2013, 18, 874–887. [Google Scholar] [CrossRef]





| Ingredient | AIN-76A Diet Base | ||
|---|---|---|---|
| (g/1000 g Diet) | IC | ID | IOL |
| Casein | 200 | 200 | 200 |
| Corn starch | 150 | 150 | 150 |
| Sucrose | 499.99 | 499.99 | 490.09 |
| Corn oil | 50 | 50 | 50 |
| Cellulose | 50 | 50 | 50 |
| Vitamin mixture | 10 | 10 | 10 |
| AIN 76a mineral mix | 35 | - | 35 |
| AIN 76a mineral mix | - | 35 | - |
| (Fe-deficient) | |||
| Choline bitartrate | 2 | 2 | 2 |
| DL-Methionine | 3 | 3 | 3 |
| Butylated hydroxytoluene | 0.01 | 0.01 | 0.01 |
| FeSO4, 7H2O | - | - | 9.9 |
| Total(g) | 1000 | 1000 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Kim, M.; Kim, Y.; Lee, S. Supplementation with High or Low Iron Reduces Colitis Severity in an AOM/DSS Mouse Model. Nutrients 2022, 14, 2033. https://doi.org/10.3390/nu14102033
Moon S, Kim M, Kim Y, Lee S. Supplementation with High or Low Iron Reduces Colitis Severity in an AOM/DSS Mouse Model. Nutrients. 2022; 14(10):2033. https://doi.org/10.3390/nu14102033
Chicago/Turabian StyleMoon, Seonghwan, Minju Kim, Yeonhee Kim, and Seungmin Lee. 2022. "Supplementation with High or Low Iron Reduces Colitis Severity in an AOM/DSS Mouse Model" Nutrients 14, no. 10: 2033. https://doi.org/10.3390/nu14102033
APA StyleMoon, S., Kim, M., Kim, Y., & Lee, S. (2022). Supplementation with High or Low Iron Reduces Colitis Severity in an AOM/DSS Mouse Model. Nutrients, 14(10), 2033. https://doi.org/10.3390/nu14102033






