The Effect of Exercise and Nutritional Interventions on Body Composition in Patients with Advanced or Metastatic Cancer: A Systematic Review
Abstract
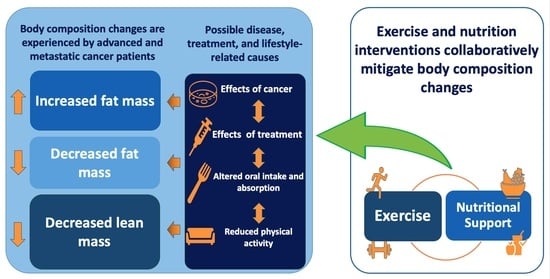
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility
2.3. Study Selection
2.4. Data Extraction
2.5. Methodological Assessment
3. Results
3.1. Study Characteristics
3.2. Intervention Characteristics
3.3. Adherence and Adverse Events
3.4. Outcome Measures
3.4.1. Lean Mass-Related Outcomes
3.4.2. Fat Mass-Related Outcomes
3.5. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koleva-Kolarova, R.G.; Oktora, M.P.; Robijn, A.L.; Greuter, M.J.W.; Reyners, A.K.L.; Buskens, E.; de Bock, G.H. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 55, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, H.T.; Suh, H.S. Combination therapy of BRAF inhibitors for advanced melanoma with BRAF V600 mutation: A systematic review and meta-analysis. J. Dermatol. Treat. 2018, 29, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Jacome, A.A.; Castro, A.C.G.; Vasconcelos, J.P.S.; Silva, M.; Lessa, M.A.O.; Moraes, E.D.; Andrade, A.C.; Lima, F.M.T.; Farias, J.P.F.; Gil, R.A.; et al. Efficacy and Safety Associated with Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma: A Meta-analysis. JAMA Netw. Open 2021, 4, e2136128. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Rimer, B.K.; Winer, E.P. Weight Gain in Women Diagnosed with Breast Cancer. J. Am. Diet. Assoc. 1997, 97, 519–529. [Google Scholar] [CrossRef]
- Galvao, D.A.; Spry, N.A.; Taaffe, D.R.; Newton, R.U.; Stanley, J.; Shannon, T.; Rowling, C.; Prince, R. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008, 102, 44–47. [Google Scholar] [CrossRef]
- Hardee, J.P.; Counts, B.R.; Carson, J.A. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am. J. Lifestyle Med. 2019, 13, 46–60. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Vigano, A.; Watanabe, S.; Bruera, E. Anorexia and cachexia in advanced cancer patients. Cancer Surv. 1994, 21, 99–115. [Google Scholar]
- Wasley, D.; Gale, N.; Roberts, S.; Backx, K.; Nelson, A.; van Deursen, R.; Byrne, A. Patients with established cancer cachexia lack the motivation and self-efficacy to undertake regular structured exercise. Psychooncology 2018, 27, 458–464. [Google Scholar] [CrossRef]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018, 23, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laviano, A.; Meguid, M.M.; Inui, A.; Muscaritoli, M.; Rossi-Fanelli, F. Therapy insight: Cancer anorexia-cachexia syndrome—When all you can eat is yourself. Nat. Clin. Pract. Oncol. 2005, 2, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Davis, G.M.; Beith, J.M.; Wilcken, N.; Currow, D.; Emery, J.; Phillips, J.; Martin, A.; Hui, R.; Harrison, M.; et al. Physical activity and fitness in women with metastatic breast cancer. J. Cancer Surviv. Res. Pract. 2014, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.T.; Petersen, M.A.; Pedersen, L.; Houmann, L.J.; Groenvold, M. Do advanced cancer patients in Denmark receive the help they need? A nationally representative survey of the need related to 12 frequent symptoms/problems. Psychooncology 2013, 22, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Prescott, E.; Andersen, L.L.; et al. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, L.P.; Silveira, M.N.; Mendes, M.C.S.; Costa, F.O.; Macedo, L.T.; de Siqueira, N.S.; Carvalheira, J.B.C. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: A retrospective evaluation. Clin. Nutr. ESPEN 2019, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Parsons, H.A.; Baracos, V.E.; Dhillon, N.; Hong, D.S.; Kurzrock, R. Body composition, symptoms, and survival in advanced cancer patients referred to a phase I service. PLoS ONE 2012, 7, e29330. [Google Scholar] [CrossRef] [Green Version]
- Barret, M.; Antoun, S.; Dalban, C.; Malka, D.; Mansourbakht, T.; Zaanan, A.; Latko, E.; Taieb, J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr. Cancer 2014, 66, 583–589. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef] [Green Version]
- Van Vugt, J.L.A.; Buettner, S.; Levolger, S.; Coebergh van den Braak, R.R.J.; Suker, M.; Gaspersz, M.P.; de Bruin, R.W.F.; Verhoef, C.; van Eijck, C.H.C.; Bossche, N.; et al. Low skeletal muscle mass is associated with increased hospital expenditure in patients undergoing cancer surgery of the alimentary tract. PLoS ONE 2017, 12, e0186547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyère, O.; Beaudart, C.; Locquet, M.; Buckinx, F.; Petermans, J.; Reginster, J.Y. Sarcopenia as a public health problem. Eur. Geriatr. Med. 2016, 7, 272–275. [Google Scholar] [CrossRef]
- Raynard, B.; Pigneur, F.; Di Palma, M.; Deluche, E.; Goldwasser, F. The prevalence of CT-defined low skeletal muscle mass in patients with metastatic cancer: A cross-sectional multicenter French study (the SCAN study). Support. Care Cancer 2022, 30, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Martinez-Tapia, C.; Heitz, D.; Geiss, R.; Albrand, G.; Falandry, C.; Gisselbrecht, M.; Couderc, A.L.; Boulahssass, R.; Liuu, E.; et al. Prevalence and prognostic impact of cachexia among older patients with cancer: A nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia Sarcopenia Muscle 2021, 12, 1477–1488. [Google Scholar] [CrossRef]
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Deng, H.Y.; Chen, Z.J.; Qiu, X.M.; Zhu, D.X.; Tang, X.J.; Zhou, Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: A comprehensive systematic review and meta-analysis. Nutrition 2021, 90, 111345. [Google Scholar] [CrossRef]
- Mallet, R.; Modzelewski, R.; Lequesne, J.; Mihailescu, S.; Decazes, P.; Auvray, H.; Benyoucef, A.; Di Fiore, F.; Vera, P.; Dubray, B.; et al. Prognostic value of sarcopenia in patients treated by Radiochemotherapy for locally advanced oesophageal cancer. Radiat. Oncol. 2020, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Smith, M.R. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology 2004, 63, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Mazurak, V.C. Evidence and mechanisms of fat depletion in cancer. Nutrients 2014, 6, 5280–5297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lu, Y.; Fang, Y. Nutritional status and related factors of patients with advanced gastrointestinal cancer. Br. J. Nutr. 2014, 111, 1239–1244. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.L.; Taaffe, D.R.; Newton, R.U.; Hart, N.H.; Lyons-Wall, P.; Galvão, D.A. Using Exercise and Nutrition to Alter Fat and Lean Mass in Men with Prostate Cancer Receiving Androgen Deprivation Therapy: A Narrative Review. Nutrients 2021, 13, 1664. [Google Scholar] [CrossRef]
- Dev, R.; Bruera, E.; Dalal, S. Insulin resistance and body composition in cancer patients. Ann. Oncol. 2018, 29, ii18–ii26. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Demark-Wahnefried, W.; Schmitz, K.H.; Alfano, C.M.; Bail, J.R.; Goodwin, P.J.; Thomson, C.A.; Bradley, D.W.; Courneya, K.S.; Befort, C.A.; Denlinger, C.S.; et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J. Clin. 2018, 68, 64–89. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Robson, T. Obesity and Cancer Metastasis: Molecular and Translational Perspectives. Cancers 2020, 12, 3798. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, J.A.; Rodrigues, C.; de Barros Rocha, L.; Rocha, R.S.B.; da Silva, M.L.; da Costa Cunha, K. Physical exercise and quality of life in patients with prostate cancer: Systematic review and meta-analysis. Support. Care Cancer 2021, 29, 4911–4919. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, E.C.W.; van den Hurk, R.M.; Blauwhoff-Buskermolen, S.; van der Vorst, M.; Becker-Commissaris, A.; de van der Schueren, M.A.E.; Buffart, L.M.; Verheul, H.M.W. Muscle mass as a target to reduce fatigue in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.; Hart, N.H.; Bolam, K.A.; Mansfield, S.; Santa Mina, D.; Winters-Stone, K.M.; Campbell, A.; Rosenberger, F.; Wiskemann, J.; Quist, M.; et al. Exercise for individuals with bone metastases: A systematic review. Crit. Rev. Oncol./Hematol. 2021, 166, 103433. [Google Scholar] [CrossRef]
- Oldervoll, L.M.; Loge, J.H.; Lydersen, S.; Paltiel, H.; Asp, M.B.; Nygaard, U.V.; Oredalen, E.; Frantzen, T.L.; Lesteberg, I.; Amundsen, L.; et al. Physical exercise for cancer patients with advanced disease: A randomized controlled trial. Oncologist 2011, 16, 1649–1657. [Google Scholar] [CrossRef] [Green Version]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef]
- Kang, D.W.; Fairey, A.S.; Boule, N.G.; Field, C.J.; Wharton, S.A.; Courneya, K.S. Effects of Exercise on Cardiorespiratory Fitness and Biochemical Progression in Men with Localized Prostate Cancer Under Active Surveillance: The ERASE Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1487–1495. [Google Scholar] [CrossRef]
- Kim, J.S.; Taaffe, D.R.; Galvao, D.A.; Hart, N.H.; Gray, E.; Ryan, C.J.; Kenfield, S.A.; Saad, F.; Newton, R.U. Exercise in advanced prostate cancer elevates myokine levels and suppresses in-vitro cell growth. Prostate Cancer Prostatic Dis. 2022, 25, 86–92. [Google Scholar] [CrossRef]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Blackwood, H.A.; Hall, C.C.; Balstad, T.R.; Solheim, T.S.; Fallon, M.; Haraldsdottir, E.; Laird, B.J. A systematic review examining nutrition support interventions in patients with incurable cancer. Support. Care Cancer 2020, 28, 1877–1889. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittus, K.L.; Gramling, R.E.; Ades, P.A. Exercise interventions for individuals with advanced cancer: A systematic review. Prev. Med. 2017, 104, 124–132. [Google Scholar] [CrossRef]
- Hall, C.C.; Cook, J.; Maddocks, M.; Skipworth, R.J.E.; Fallon, M.; Laird, B.J. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: A systematic review. Support. Care Cancer 2019, 27, 2371–2384. [Google Scholar] [CrossRef] [Green Version]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Efficacy of Exercise Interventions in Patients with Advanced Cancer: A Systematic Review. Arch. Phys. Med. Rehabil. 2018, 99, 2595–2620. [Google Scholar] [CrossRef] [Green Version]
- Payne, C.; Larkin, P.J.; McIlfatrick, S.; Dunwoody, L.; Gracey, J.H. Exercise and nutrition interventions in advanced lung cancer: A systematic review. Curr. Oncol. 2013, 20, e321–e337. [Google Scholar] [CrossRef] [Green Version]
- Sheill, G.; Guinan, E.M.; Peat, N.; Hussey, J. Considerations for Exercise Prescription in Patients With Bone Metastases: A Comprehensive Narrative Review. PM R 2018, 10, 843–864. [Google Scholar] [CrossRef]
- Salakari, M.R.; Surakka, T.; Nurminen, R.; Pylkkanen, L. Effects of rehabilitation among patients with advances cancer: A systematic review. Acta Oncol. 2015, 54, 618–628. [Google Scholar] [CrossRef]
- Courneya, K.S.; Sellar, C.M.; Stevinson, C.; McNeely, M.L.; Peddle, C.J.; Friedenreich, C.M.; Tankel, K.; Basi, S.; Chua, N.; Mazurek, A.; et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J. Clin. Oncol. 2009, 27, 4605–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormie, P.; Galvao, D.A.; Spry, N.; Joseph, D.; Taaffe, D.R.; Newton, R.U. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: Longitudinal results of a pilot study. Support. Care Cancer 2014, 22, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Van den Dungen, I.A.; Verhagen, C.A.; van der Graaf, W.T.; van den Berg, J.P.; Vissers, K.C.; Engels, Y. Feasibility and impact of a physical exercise program in patients with advanced cancer: A pilot study. J. Palliat. Med. 2014, 17, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Uth, J.; Hornstrup, T.; Schmidt, J.F.; Christensen, J.F.; Frandsen, C.; Christensen, K.B.; Helge, E.W.; Brasso, K.; Rorth, M.; Midtgaard, J.; et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand. J. Med. Sci. Sports 2014, 24 (Suppl. S1), 105–112. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]
- Cormie, P.; Newton, R.U.; Spry, N.; Joseph, D.; Taaffe, D.R.; Galvao, D.A. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013, 16, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.J.; Cheng, J.C.; Lee, J.M.; Huang, P.M.; Huang, G.H.; Chen, C.C. A Walk-and-Eat Intervention Improves Outcomes for Patients with Esophageal Cancer Undergoing Neoadjuvant Chemoradiotherapy. Oncologist 2015, 20, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Naufahu, J.; Tewfik, S.; Bhatnagar, S.; Garg, R.; Tewfik, I. A Prospective Randomized Controlled Trial to Study the Impact of a Nutrition-Sensitive Intervention on Adult Women with Cancer Cachexia Undergoing Palliative Care in India. Integr. Cancer Ther. 2017, 16, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvao, D.A.; Taaffe, D.R.; Spry, N.; Cormie, P.; Joseph, D.; Chambers, S.K.; Chee, R.; Peddle-McIntyre, C.J.; Hart, N.H.; Baumann, F.T.; et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med. Sci. Sports Exerc. 2018, 50, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villumsen, B.R.; Jorgensen, M.G.; Frystyk, J.; Hordam, B.; Borre, M. Home-based ‘exergaming’ was safe and significantly improved 6-min walking distance in patients with prostate cancer: A single-blinded randomised controlled trial. BJU Int. 2019, 124, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Stuecher, K.; Bolling, C.; Vogt, L.; Niederer, D.; Schmidt, K.; Dignass, A.; Banzer, W. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: A single-blind RCT. Support. Care Cancer 2019, 27, 2159–2169. [Google Scholar] [CrossRef]
- Van der Werf, A.; Langius, J.A.E.; Beeker, A.; Ten Tije, A.J.; Vulink, A.J.; Haringhuizen, A.; Berkhof, J.; van der Vliet, H.J.; Verheul, H.M.W.; de van der Schueren, M.A.E. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: A randomized controlled trial. Clin. Nutr. 2020, 39, 3005–3013. [Google Scholar] [CrossRef]
- Storck, L.J.; Ruehlin, M.; Gaeumann, S.; Gisi, D.; Schmocker, M.; Meffert, P.J.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effect of a leucine-rich supplement in combination with nutrition and physical exercise in advanced cancer patients: A randomized controlled intervention trial. Clin. Nutr. 2020, 39, 3637–3644. [Google Scholar] [CrossRef]
- Bjerre, E.D.; Weller, S.; Poulsen, M.H.; Madsen, S.S.; Bjerre, R.D.; Ostergren, P.B.; Borre, M.; Brasso, K.; Midtgaard, J. Safety and Effects of Football in Skeletal Metastatic Prostate Cancer: A Subgroup Analysis of the FC Prostate Community Randomised Controlled Trial. Sports Med. Open 2021, 7, 27. [Google Scholar] [CrossRef]
- Mikkelsen, M.K.; Lund, C.M.; Vinther, A.; Tolver, A.; Johansen, J.S.; Chen, I.; Ragle, A.M.; Zerahn, B.; Engell-Noerregaard, L.; Larsen, F.O.; et al. Effects of a 12-Week Multimodal Exercise Intervention Among Older Patients with Advanced Cancer: Results from a Randomized Controlled Trial. Oncologist 2022, 27, 67–78. [Google Scholar] [CrossRef]
- Sheean, P.; Matthews, L.; Visotcky, A.; Banerjee, A.; Moosreiner, A.; Kelley, K.; Chitambar, C.R.; Papanek, P.E.; Stolley, M. Every Day Counts: A randomized pilot lifestyle intervention for women with metastatic breast cancer. Breast Cancer Res. Treat. 2021, 187, 729–741. [Google Scholar] [CrossRef]
- Allen, S.K.; Brown, V.; White, D.; King, D.; Hunt, J.; Wainwright, J.; Emery, A.; Hodge, E.; Kehinde, A.; Prabhu, P.; et al. Multimodal Prehabilitation during Neoadjuvant Therapy Prior to Esophagogastric Cancer Resection: Effect on Cardiopulmonary Exercise Test Performance, Muscle Mass and Quality of Life-A Pilot Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Alexander, N.B.; Djuric, Z.; Zhou, J.; Tao, Y.; Schipper, M.; Feng, F.Y.; Eisbruch, A.; Worden, F.P.; Strath, S.J.; et al. Maintaining physical activity during head and neck cancer treatment: Results of a pilot controlled trial. Head Neck 2016, 38 (Suppl. S1), E1086–E1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schink, K.; Gassner, H.; Reljic, D.; Herrmann, H.J.; Kemmler, W.; Schwappacher, R.; Meyer, J.; Eskofier, B.M.; Winkler, J.; Neurath, M.F.; et al. Assessment of gait parameters and physical function in patients with advanced cancer participating in a 12-week exercise and nutrition programme: A controlled clinical trial. Eur. J. Cancer Care 2020, 29, e13199. [Google Scholar] [CrossRef] [PubMed]
- Balstad, T.R.; Brunelli, C.; Pettersen, C.H.; Schonberg, S.A.; Skorpen, F.; Fallon, M.; Kaasa, S.; Bye, A.; Laird, B.J.A.; Stene, G.B.; et al. Power Comparisons and Clinical Meaning of Outcome Measures in Assessing Treatment Effect in Cancer Cachexia: Secondary Analysis From a Randomized Pilot Multimodal Intervention Trial. Front. Nutr. 2020, 7, 602775. [Google Scholar] [CrossRef]
- Ballmer, P.E.; Uster, A.; Ruehlin, M.; Imoberdorf, R.; Pless, M. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2019, 38, 476. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Method 2020, 1–7. [Google Scholar] [CrossRef]
- Wilson, R.L.; Newton, R.U.; Taaffe, D.R.; Hart, N.H.; Lyons-Wall, P.; Galvao, D.A. Weight Loss for Obese Prostate Cancer Patients on Androgen Deprivation Therapy. Med. Sci. Sports Exerc. 2021, 53, 470–478. [Google Scholar] [CrossRef]
- Dawson, J.K.; Dorff, T.B.; Todd Schroeder, E.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef]
- Hanson, E.D.; Nelson, A.R.; West, D.W.; Violet, J.A.; O’Keefe, L.; Phillips, S.M.; Hayes, A. Attenuation of Resting but Not Load-Mediated Protein Synthesis in Prostate Cancer Patients on Androgen Deprivation. J. Clin. Endocrinol. Metab. 2017, 102, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Ferro, M.; Terracciano, D.; Buonerba, C.; Lucarelli, G.; Bottero, D.; Perdonà, S.; Autorino, R.; Serino, A.; Cantiello, F.; Damiano, R.; et al. The emerging role of obesity, diet and lipid metabolism in prostate cancer. Future Oncol. 2017, 13, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes, S.J.; Daly, R.M.; Fraser, S.F. The clinical importance of quantifying body fat distribution during androgen deprivation therapy for prostate cancer. Endocr. Relat. Cancer 2017, 24, R35–R48. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Rathbone, E.; Brown, J.E. Management of cancer treatment-induced bone loss. Nat. Rev. Rheumatol. 2013, 9, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Yoon, S.L.; Williams, J.J. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—A comprehensive review. Eur. J. Clin. Nutr. 2015, 69, 1290–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Study Details | Population | Experimental Groups | Intervention | Body Composition Outcomes | Pre vs. Post Training Mean ± SD or MD ± SD/(95%CI) |
|---|---|---|---|---|---|
| Exercise only | |||||
| Cormie et al. [69] Australia RCT | Population: Prostate cancer with bone metastases (n = 20) Stage: Metastatic: 100% | EX (n allocated = 10; n completed outcome = 8): Supervised resistance training, home-based aerobic training. CON (n allocated = 10; n completed outcome = 7): Self-directed exercise | 12-week intervention Exercise component (FITT): F: RT = 2 days/week; AT = NR. I: RT = 8–12 RM; AT = moderate intensity. Time: RT = 60 min, 8–12 reps, 2–4 sets; AT = 150 min/week. Type: Supervised machine weight resistance training that did not target areas of bone metastases, self-directed home-based aerobic exercise. Adherence: attended 93% of supervised exercise sessions. | Lean mass (kg) (DXA) | Within-group differences: EX: PRE: 57.2 ± 7.8 vs. POST: 57.8 ± 8.0 CON: PRE: 53.2 ± 9.7 vs. POST: 52.5 ± 8.0 Between-group differences: EX vs. CON: MD: 1.7 (0.2 to 3.2) ¥↑ |
| Appendicular lean mass (kg) (DXA) | Within-group differences: EX: PRE: 24.3 ± 3.7 vs. POST: 24.5 ± 3.7 CON: PRE: 21.4 ± 3.9 vs. POST: 20.9 ± 3.3 Between-group differences: EX vs. CON: MD: 1.0 (0.4 to 1.6) ¥↑ | ||||
| Fat mass (kg) (DXA) | Within-group differences: EX: PRE: 27.7 ± 5.6 vs. POST: 27.8 ± 6.0 CON: PRE: 27.2 ± 5.7 vs. POST: 27.5 ± 6.5 Between-group differences: EX vs. CON: MD: −0.3 (−1.4 to 0.9) | ||||
| Trunk fat mass (kg) (DXA) | Within-group differences: EX: PRE: 14.7 ± 3.4 vs. POST: 14.6 ± 3.7 CON: PRE: 15.0 ± 3.4 vs. POST: 15.0 ± 3.8 Between-group differences: EX vs. CON: MD: 0.0 (−0.6 to 0.6) | ||||
| Visceral fat mass (kg) (DXA) | Within-group differences: EX: PRE: 0.89 ± 0.20 vs. POST: 0.89 ± 0.23 CON: PRE: 0.96 ± 0.19 vs. POST: 0.96 ± 0.19 Between-group differences: EX vs. CON: MD: 0.01 (−55.3 to 58.6) | ||||
| Body fat percent (%) (DXA) | Within-group differences: EX: PRE: 31.7 ± 4.9 vs. POST: 31.5 ± 5.1 CON: PRE: 32.7 ± 2.2 vs. POST: 33.0 ± 3.3 Between-group differences: EX vs. CON: MD: −0.4 (−1.9 to 1.2) | ||||
| Uth et al. [67] Denmark RCT | Population: Advanced or locally advanced prostate cancer (n = 57) Stage: ≥T3: 70.2% | EX (n allocated = 29, n completed outcome = 26): Supervised football training CON (n allocated = 28, n completed outcome = 23): Usual care control. | 12-week intervention Exercise component (FITT): F: 2–3 days/week I: Not prescribed but a mean HR of 84.6 ± 3.9% of individual max HR was achieved. Time: 45 min Type: Football drills and game. Adherence: attended 76.5 ± 24.2% of supervised exercise sessions. | Lean mass (kg) (DXA) | Within-group differences: EX: MD: 0.5 (0.1 to 0.9) ¥↑ CON: MD: −0.2 (−0.6 to 0.2) Between-group differences: EX vs. CON: MD: 0.7 (0.1 to 1.2) ¥↑ |
| Fat mass (kg (DXA) | Within-group differences: EX: MD: −0.6 (−1.4 to 0.1) CON: MD: 0.0 (−0.5 to 0.5) Between-group differences: EX vs. CON: MD: −0.6 (−1.5 to 0.2) | ||||
| Body fat percent (%) (DXA) | Within-group differences: EX: MD: −0.7 (−1.3 to 0.0) CON: MD: 0.1 (−0.4 to 0.5) Between-group differences: EX vs. CON: MD: −0.7 (−1.5 to 0.2) | ||||
| Galvao et al. [73] Australia RCT | Population: Prostate cancer with bone metastases(n = 57) Stage: Metastatic: 100% | EX (n allocated = 28, n completed outcome = 23): Supervised aerobic and resistance exercise. CON (n allocated = 29, n completed outcome = 26): Usual care control. | 12-week intervention Exercise component (FITT): F: 3 days/week I: AT = 60–85% HRmax; RT = 10–12 RM Time: 60 min sessions; AT = 20–30 min; RT = 10–12 reps, 3 sets. Type: Exercises did not target bone metastases sites. AT = choice or walking, cycling, rowing; RT = machine based. Adherence: attended 89% of supervised exercise sessions. | Lean mass (kg) (DXA) | Within-group differences: EX: PRE: 56.6 ± 8.1 vs. POST: 56.2 ± 8.0 CON: PRE: 55.6 ± 7.8 vs. POST: 55.4 ± 7.5 Between-group differences: EX vs. CON: MD: −0.3 (−1.3 to 0.7) |
| Fat mass (kg) (DXA) | Within-group differences: EX: PRE: 28.7 ± 8.1 vs. POST: 29.0 ± 7.8 CON: PRE: 28.3 ± 6.9 vs. POST: 29.0 ± 6.4 Between-group differences: EX vs. CON: MD: −0.2 (−1.2 to 0.7) | ||||
| Villumsen et al. [74] Denmark RCT | Population: Locally advanced or advanced stage prostate cancer (n = 46) Stage: Bone metastases: 34.8% Lymph node metastases: 6.5% | EX (n allocated = 23, n completed outcome = 21): Home-based exergaming CON (n allocated = 23, n completed outcome = 20): Usual care control inclusive of physical activity advice. | 12-week intervention Exercise component (FITT): F: 3 days/week I: NR Time: 60 min Type: Exergaming using both aerobic and strength exercises, free weights. Adherence: Completed on average 153.5 min/week from a prescribed 180 min/week. | Lean mass (%) (BIA) | Within-group differences: EX: NR CON: NR Between-group differences: EX vs. CON: MD: 0.91 (−0.2 to 2.0) |
| Fat mass (% (BIA) | Within-group differences: EX: NR CON: NR Between-group differences: EX vs. CON: MD: −0.9 (−2.0 to 0.2) | ||||
| Stuecher et al. [75] Germany RCT | Population: Stage III or IV gastrointestinal tract cancers(n = 44) Stage: Metastatic: NR | EX (n allocated = 22, n completed outcome = 13): Self-directed walking. CON (n allocated = 22, n completed outcome = 15): Usual care control. | 12-week intervention Exercise component (FITT): F: 3–5 days/week I: 11–13 RPE Time: 150 min/week Type: Home-based walking. Adherence: 81.3% completed the home-based program as prescribed. | Lean mass (%) (BIA) | Within-group differences: EX: MD: 3.4 ± 4.6 CON: MD: 0.64 ± 3.4 Between-group differences: EX vs. CON: MD: NR, p = 0.02. ¥↑ |
| Phase angle (°) (BIA) | Within-group differences: EX: MD: 0.13 ± 0.91 CON: MD: −0.01 ± 0.69 Between-group differences: EX vs. CON: MD: NR, p = 0.2 | ||||
| Bjerre et al. [78] Denmark RCT | Population: Prostate cancer with bone metastases (n = 41) Stage: Metastatic: 100% | EX (n allocated = 22, n completed outcome = 21): Community-based football intervention CON (n allocated = 19, n completed outcome = 15): Usual care | 6-month intervention Exercise component (FITT): F: 2 days/week I: NR Time: 60 min Type: Supervised group-based football training involving bodyweight training, football skills and football match play. Adherence: attended 63% of supervised group sessions (at week-12); attended 54% of supervised group sessions (at 6-months). | Lean mass (kg) (DXA) | Within-group differences: EX: MD: −0.3 (−1.1 to 0.5) CON: MD: −0.4 (−1.3 to 0.6) Between-group differences: EX vs. CON: MD: −0.2 (−1.4 to 0.9) |
| Fat mass (kg) (DXA) | Within-group differences: EX: MD: −0.4 (−1.3 to 0.6) CON: MD: −0.2 (−1.4 to 1.0) Between-group differences: EX vs. CON: MD: 0.4 (−1.1 to 1.8) | ||||
| Combined exercise and nutrition | |||||
| Xu et al. [70] Taiwan RCT | Population: Locally advanced tumors of the esophagus (n = 56) Stage: Stage 1: 3.6% Stage 2: 7.1% Stage 3: 82.1% | EX + NU (n allocated = 28, n completed outcome = 28): Supervised walking and nutrition counselling. CON (n allocated = 28, n completed outcome = 28): Usual care control. | 4–5-week intervention Exercise component (FITT): F: 3 days/week I: 60% age predicted maximum HR Time: 25 min Type: Walking Nutrition component: Weekly nutrition counselling. Adherence: EX: Completed 8.4 ± 3.6 of supervised walking sessions.NU: attended 100% of nutrition sessions. | Lean mass (kg) (BIA) | Within-group differences: EX + NU: MD: −0.7 ± 1.9 CON: MD: −2.0 ± 3.0 Between-group differences: EX + NU vs. CON: MD: 1.3 (−0.05 to 2.66) |
| Kapoor et al. [72] India RCT | Population: Females with advanced cancer (n = 63) Stage: NR | EX + NU (n allocated = 30, n completed outcome = 17): Multimodal (Nutrition counselling, oral nutrition supplement, physical activity recommendation) CON (n allocated = 33, n completed outcome = 15): Nutrition counselling and physical activity recommendation | 6-month intervention Exercise component (FITT): F: NR I: Not prescribed but reported: EX + NU: PRE: 33.6 ± 3.9 METs vs. POST 31.9 ± 2.7 METs (p = 0.274); CON: PRE: 30.7 ± 2.7 METs vs. POST 28.0 ± 2.5 METs (p = 0.004). Time: NR. Type: Low levels of PA, e.g., walking and participation in household activities. Nutrition component: Bi-weekly nutrition counselling visits. 100 g/day of IAtta oral nutrition supplement (mixture of roasted bengal gram flour, roasted barley flour, roasted soybean flour, flaxseed powered, dried amaranthus spinosus powder). Adherence: EX: NR. NU: NR. EX + NU: 51% completed the intervention as prescribed. | Body fat percent (%) (skinfolds) | Within-group differences: EX + NU: PRE: 20.5 ± 5.2 vs. POST: 23.7 ± NR ¥↑ CON: PRE: 25.4 ± 6.5 vs. POST: 24.5 ± NR ¥↓ Between-group differences: EX + NU vs. CON: MD: NR; p = 0.001 ¥↑ |
| Uster et al. [68] Switzerland RCT | Population: Metastatic or locally advanced tumors of gastrointestinal and lung tracts (n = 58) Stage: Stage III: 2% Stage IV: 98% | EX + NU (n allocated = 29, n completed outcome = 24): Multimodal (Supervised group-based resistance and balance training, nutrition counseling) CON (n allocated = 29, n completed outcome = 20): Usual care control. | 3-month intervention Exercise component (FITT): F: 2 days/week I: RT= 60–80% of 1-RM; Balance = NR. Time: 60 min, RT = 10 reps, 2 sets, Balance= 1–2 min per move. Type: RT = resistance machines; balance mat. Nutrition component: Minimum of 3 nutritional counselling during intervention encouraging patients to consume 1.2 g protein/kg body weight/day, with emphasis on consuming protein after exercise sessions. Adherence: EX: attended 67% of supervised exercise sessions. NU: 89.7% completed the minimum nutritional counseling sessions. EX + NU: 100% consumed at least 9–10 g of protein after each exercise session. | Phase angle (°) (BIA) | Within-group differences: EX + NU: NR. CON: NR. Between-group differences: EX + NU vs. CON: MD: NR. |
| Zhao et al. [82] United States of America Non-RCT | Population: Stage III-IV Head and neck squamous cell carcinoma (n = 20) Stage: Stage III: 22% Stage IV: 78% | EX + NU (n = 11): Multimodal (Supervised and unsupervised aerobic and resistance training, nutrition counselling) CON (n = 7): Standard of care inclusive of nutritional counselling. | 14-week intervention (7 weeks supervised, 7 weeks unsupervised) Exercise component (FITT): F: Supervised period = 3 days/week; unsupervised period = 5 days/week I: 11–13 RPE Time: 60 min sessions; AT = 30 min; RT = 8–12 reps, 3 sets. Type: AT = walking; RT = free weights Nutrition component: Baseline nutrition counselling. Adherence: EX: attended 72% of supervised exercise sessions. NU: NR. | Lean mass (%) (DXA) | Within-group differences: EX + NU: MD: 7 weeks: 0.2 ± 0.5 vs. 14 weeks: 4.7 ± 1.5 CON: MD: 7 weeks: 1.0 ± 0.7 vs. 14 weeks: 4.0 ± 0.9 Between-group differences: EX + NU vs. CON: NR; p > 0.05. |
| Schink et al. [83] Germany Non-RCT | Population: Advanced solid tumours (n = 131) Stage: Stage III: 26% Stage IV: 74% | EX + NU (n allocated = 96; n completed outcome = 58): Multimodal (supervised whole-body electromyostimulation, nutrition counselling) CON (n allocated = 35; n completed outcome = 27): Usual care control with nutrition counselling. | 12-week intervention Exercise component (FITT): F: 2 days/week I: 85 Hz, 350 μs inducing a 6 s stimulation and 4 s rest. Time: 12–20 min, 6 reps per min. Type: whole-body electromyostimulation with additional light exercises. Nutrition component: Nutrition counselling encouraging >1 g/kg day of protein and minimum energy intake of 25 kcal/kg/day. Adherence: EX: attended 86.6 ± 10.8% of supervised sessions. NU: EX + NU = 67.4% and CON = 69% consumed the protein intake recommendation or more. EX + NU =74.2% and 75.8 consumed the kcal intake recommendations. | Skeletal muscle mass (kg) (BIA) | Within-group differences: EX + NU: NRCON: NRBetween-group differences: EX + NU vs.CON: MD: 0.53 (0.05 to 0.98) ¥ ↑ |
| Fat mass (%) (BIA) | Within-group differences: EX + NU: NR CON: NRBetween-group differences: EX + NU vs. CON: MD: 0.51 (−0.46 to 1.47) | ||||
| Phase angle (°) (BIA) | Within-group differences: EX + NU: NR CON: NR Between-group differences: EX + NU vs. CON: MD: 0.07 (−0.06 to 0.19) | ||||
| Schink et al. [84] Germany Non-RCT | Population: Advanced solid tumours (n = 80) Stage: Stage III: 24.4% Stage IV: 75.6% | EX + NU (n allocated = 58; n completed outcome = 26): Multimodal (supervised whole-body electromyostimulation, nutrition counselling) CON (n allocated = 22; n completed outcome = 15): Usual care control with nutrition counselling. | 12-week intervention Exercise component (FITT): F: 2 days/week I: 85 Hz, 350 μs inducing a 6 s stimulation and 4 s rest. Time: 12–20 min, 6 reps per min. Type: whole-body electromyostimulation with additional light exercises. Nutrition component: Nutrition counselling encouraging >1 g/kg day and minimum energy intake of 25 kcal/kg/day. Adherence: EX: attended 88.9 ± 8.7% of supervised sessions. NU: NR | Skeletal muscle mass (kg) (BIA) | Within-group differences: EX + NU: NR CON: NR Between-group differences: EX + NU vs. CON: MD: 0.99 (0.09 to 1.90) ¥↑ |
| van der Werf et al. [76] Netherlands RCT | Population: Metastatic colon cancer (n = 107) Stage: Metastatic: 100% | NU + PA: (n allocated = 52; n completed outcome T1 = 50; N completed outcome T2 = 39): Nutrition counselling and PA CON (n allocated = 55; n completed outcome T1 = 52; n completed outcome T2 = 33): Usual care inclusive of regular care dietician referral. | T0-T1 = mean 9 ± 3 weeks; T0-T2 = mean 19 ± 3 weeks Exercise component (FITT): F: 5 days/week I: moderate intensity Time: ≥30 min Type: self-directed PA. Nutrition component: Nutrition counselling with the goal of 1.2 g protein/kg body weight/day and at least ≥25 g protein per meal. Adherence: PA: T1 = 24%; T2 = 16% achieved PA recommendations. NU: T1 = 61%; T2 = 40% achieved protein intake recommendations. T1 = 61%; T2 = 49% achieved energy intake recommendations. | Skeletal muscle area (cm2) (CT) | Within-group differences: NU + PA: NR CON: NR Between-group differences: NU + PA vs. CON: MD: T0-T1: 0.3 (−3.5 to 4.0) vs. T1-T2: 0.3 (−3.4 to 4.0) |
| Muscle density (Hounsfield units) (CT) | Within-group differences: NU + PA: NR CON: NR Between-group differences: NU + PA vs. CON: MD: T0-T1: 0.2 (−1.8 to 2.2) vs. T1-T2: −0.1 (−2.2 to 2.0) | ||||
| Storck et al. [77] Switzerland RCT | Population: Metastatic or locally advanced cancers of the lungs, gastrointestinal tract, breast, ovarian, prostate, renal cell, bladder (n = 52) Stage: Metastatic: NR | EX + NU (n allocated = 27; n completed outcome = 23):Multimodal (supervised and self-directed aerobic and resistance exercise, nutrition counselling). CON (n allocated = 25; n completed outcome =18): Usual care inclusive of regular care nutrition counselling and physiotherapy. | 12-week intervention Exercise component (FITT): F: 2 days/week supervised, 1 day/week home-based. I: AT = 3–5 RPE (10 borg); RT = NR. Time: 60–90 min; AT = NR; RT = 10–15 reps, 3 sets. Type: AT= bike or treadmill; RT = circuit, resistance bands. Nutrition component: Nutrition counselling at baseline, 6 weeks, 12 weeks, and as required between times. 15–30 g/day of whey protein. Adherence: EX: attended 70.7% of supervised sessions and completed 95% of home sessions. NU: attended 106.7% nutrition counselling sessions. 71.2% consumed the protein supplements. | Phase angle (°) (BIA) | Within-group differences: EX + NU: MD: 0.08 ± NR CON: MD: −0.04 ± NR Between-group differences: EX + NU vs. CON: MD: NR (−0.39 to 0.16) |
| Lean mass (kg) (BIA) | Within-group differences: EX + NU: MD: 0.89 ± NRCON: MD: 0.46 ± NR Between-group differences: EX vs. CON: MD:NR (−2.04 to 1.18) | ||||
| Body cell mass (kg) (BIA) | Within-group differences: EX + NU: MD: 0.62 ± NR CON: MD: 0.33 ± NR Between-group differences: EX + NU vs. CON: MD:NR (−1.45 to 0.87) | ||||
| Fat mass (kg) (BIA) | Within-group differences: EX + NU: MD: 0.17 ± NR CON: MD: −0.38 ± NR Between-group differences: EX + NU vs. CON: MD:NR (−2.08 to 0.97) | ||||
| Exercise with or without nutrition, plus an additional component | |||||
| Solheim et al. [71] United Kingdom and Norway RCT | Population: Stage III/IV NSCLC or inoperable pancreatic cancer (n = 46) Stage: Pancreas stage III = 20% Pancreas stage IV = 25% NSCLC stage III = 10% NSCLC stage IV = 47.5% | EX + NU + O (n allocated = 25; n completed outcome = 23): Multimodal (self-directed exercise, nutrition counselling, oral nutrition supplement, anti-inflammatory drug). CON (n allocated = 21; n completed outcome = 18): Standard of care | 6-week intervention Exercise component (FITT): F: AT = 2 days/week; RT = 3 days/week. I: NR Time: AT = 30 min; RT = 20 min Type: AT = patient choice; AT = body weight and free weights. Nutrition component: Baseline nutrition counselling session. 220 mL of an oral nutrition supplement equating to 2 g/day of eicosapentaenoic acid. Other component: 300 mg/day of Celecoxib, an anti-inflammatory. Adherence: EX: attended 60% of exercise sessions. NU: 48% consumed the supplement O: 76% took the prescribed celecoxib. | Lean mass (cm2) (CT) | Within-group differences: EX + NU + O: MD: −2.82 ± 9.41 CON: MD: −4.97 ± 7.80 Between-group differences: EX + NU + O vs. CON: MD: NR |
| Balstad et al. [85] United Kingdom and Norway Secondary analysis of Solheim et al., 2017. | See Solheim et al. [71] | EX + NU + O (n allocated = 23; n completed outcome = 22):Multimodal (self-directed exercise, nutrition counselling, oral nutrition supplement, anti-inflammatory drug). CON (n allocated = 23; n completed outcome = 18): Standard of care | See Solheim et al. [71] | Visceral adipose tissue (cm2) (CT) | Within-group differences: EX + NU + O: PRE: 108.4 ± 67.6 vs. POST: 108.8 ± 66.1 CON: PRE: 99.9 ± 65.2 vs. POST: 94.9 ± 55.9 Between-group differences: EX + NU + O vs. CON: ES: 0.22 |
| Subcutaneous adipose tissue (cm2) (CT) | Within-group differences: EX + NU + O: PRE: 182.3 ± 114.5 vs. POST: 176.4 ± 108.5 CON: PRE: 160.6 ± 70.7 vs. POST: 149.4 ± 64.5 Between-group differences: EX + NU + O vs. CON: ES: 0.15 | ||||
| Ratio VAT:SAT | Within-group differences: EX + NU + O: PRE: 0.7 ± 0.6 vs. POST: 0.7 ± 0.5 CON: PRE: 0.7 ± 0.5 vs. POST: 0.7 ± 0.4 Between-group differences: EX + NU + O vs. CON: ES: 0.25 | ||||
| Total adipose area (cm2) (CT) | Within-group differences: EX + NU + O: PRE: 290.7 ± 154.0 vs. POST: 285.2 ± 149.5 CON: PRE: 260.5 ± 99.9 vs. POST: 244.3 ± 93.7 Between-group differences: EX + NU + O vs. CON: ES: 0.21 | ||||
| Total adipose index (cm2/m2) (CT) | Within-group differences: EX + NU + O: PRE: 99.5 ± 52.7 vs. POST: 97.4 ± 51.2 CON: PRE: 93.3 ± 36.5 vs. POST: 87.4 ± 34.2 Between-group differences: EX + NU + O vs. CON: ES: 0.21 | ||||
| Skeletal muscle mass index (cm2/m2) (CT) | Within-group differences: EX + NU + O: PRE: 45.9 ± 8.9 vs. POST: 45.0 ± 9.2 CON: PRE: 45.7 ± 8.6 vs. POST: 43.9 ± 9.4 ¥↓ Between-group differences: EX + NU + O vs. CON: ES: 0.26 | ||||
| Sheean et al. [80] United States of America RCT | Population: Metastatic breast cancer (n = 35) Stage: Metastatic: 100% | EX + NU + O (n allocated = 17; n complete outcome = 17): Multimodal (Supervised aerobic and resistance exercise, nutrition counseling) CON (n allocated = 18; n complete outcome = 18): Usual care waitlist control given monthly reminder of upcoming intervention. | 12-week intervention Exercise component (FITT): F: 4 days/week I: moderate intensity Time: 150 min/week Type: AT = patient choice; RT = resistance bands. Nutrition component: Weekly phone calls, encouraging consumption of whole grains and 5+ fruits and vegetables daily. Other component: Rooted in social cognitive theory. Adherence: EX: attended 93% for supervised sessions NU + O: 84% for telephone sessions. | Appendicular skeletal muscle index (kg/m2) (DXA) | Within-group differences: EX + NU + O: MD: − 0.1 ± 0.4 CON: MD: 0.0 ± 0.2 Between-group differences: EX + un + O vs. CON: MD: − 0.0 ± 0.3 |
| Lean mass (kg) (DXA) | Within-group differences: EX + NU + O: MD: − 0.5 ± 1.6 CON: MD: − 0.3 ± 1.4 Between-group differences: EX + NU + O vs. CON: MD: − 0.4 ± 1.5 | ||||
| Fat mass (kg) (DXA) | Within-group differences: EX + NU + O: MD: 0.3 ± 1.7 CON: MD: 0.3 ± 2.0 Between-group differences: EX + NU + O vs. CON: MD: 0.3 ± 1.8 | ||||
| Body fat percent (%) (DXA) | Within-group differences: EX + NU + O: MD: 0.5 ± 1.3 CON: MD: 0.3 ± 1.2 Between-group differences: EX + NU + O vs. CON: MD: 0.4 ± 1.2 | ||||
| Visceral fat mass (g) (DXA) | Within-group differences: EX + NU + O: MD: − 99 ± 181 ¥↓ CON: MD: − 81 ± 162 ¥↓ Between-group differences: EX + NU + O vs. CON: MD: − 89 ± 168 ¥↓ | ||||
| Mikkelsen et al. [79] Denmark RCT | Population: Pancreatic cancer, biliary tract cancer, small cell lung cancer (n = 84) Stage: Locally advanced: 14.3% Metastatic: 85.7% | EX + NU + O (n allocated = 43; n complete outcome = 29): Multimodal intervention (exercise + protein + PA+ counselling) CON (n allocated = 41; n completed outcome = 34): Usual care. | 12-week intervention Exercise components (FITT): F: 2 days/week I: 10–15 RM Time: 60 min (Volume: 10–15 reps, 2–3 sets) Type: Supervised group-based resistance training. Individualized home-based walking program controlled with a pedometer. Nutrition component: Post-exercise protein supplementation intake (12–18 g) 2 days/week. Other components: Nurse-led support and counselling (holistic assessment of function) Adherence: EX: attended 69% of supervised exercise sessions and 75% adherence to the walking program. NU: NR | Lean mass (kg) (DXA) | Within-group differences: EX + NU + O: PRE: 47.3 ± 8.1 vs. POST: 48.7 ± 9.1¥↑ CON: PRE: 47 ± 9.2 vs. POST: 46.4 ± 9.1 Between-group differences: EX + NU + O vs. CON: MD: 0.9 ± 0.4 ¥↑ |
| Fat mass (kg) (DXA) | Within-group differences: EX + NU + O: PRE: 20.8 ± 8.1 vs. POST: 21.6 ± 7.6 CON: PRE: 22.4 ± 9.4 vs. POST: 22.7 ± 10 Between-group differences: EX + NU + O vs. CON: MD: 0.2 ± 0.6 | ||||
| Lean mass (kg) (BIA) | Within-group differences: EX + NU + O: PRE:44.1 ± 8.5 vs. POST: 44.4 ± 9.6 CON: PRE: 42.9 ± 10.5 vs. POST: 41.9 ± 8.8 Between-group differences: EX + NU + O vs. CON: MD: −0.9 ± 1.3 | ||||
| Fat mass (kg) (BIA) | Within-group differences: EX + NU + O: PRE:17.2 ± 8.8 vs. POST: 17.4 ± 8.5 CON: PRE: 18.5 ± 10.2 vs. POST: 18.9 ± 11.1 Between-group differences: EX + NU + O vs. CON: MD: 1.0 kg ± 1.0 | ||||
| Kim et al. [48] Australia RCT | Population: Prostate cancer (n = 40) Stage: Metastatic: 100% | EX + O (n allocated = 20; n complete outcome =13): Supervised aerobic and resistance training with psychological support CON (n allocated = 20; n complete outcome = 12): self-directed exercise | 6-month intervention Exercise component (FITT): F: 3 days/week I: RT: 6–12 RM, HITT: RPE 8 AT: RPE 6 Time: RT: 2–5 sets, 6 exercises. HITT 3–6 bouts of 30–60 s. AT: 10–40 min. Progressive increase in time and volume. Type: Supervised RT and HITT 2 days per week and continuous cycling AT 1 day per week. Other component Psychological support Adherence: EX + O: attended 82.5 ± 13.0% of supervised exercise sessions. | Lean mass (kg) (DXA) | Within-group differences: EX + O: PRE: 53.1 ± 10.4 vs. POST: 50.6 (95%CI: 49.4 to 51.9) CON: PRE: 49.1 ± 8.2 vs. POST 50.7 (95%CI 49.4 to 51.9) Between-group differences: EX + O vs. CON: MD: NR |
| Lean mass (%) (DXA) | Within-group differences: EX + O: PRE: 57.0 ± 3.9 vs. POST: 58.4 (57.1 to 59.6) CON: PRE: 59.8 ± 4.0 vs. POST: 57.7 (56.4 to 59) Between-group differences: EX + O vs. CON: MD: NR | ||||
| Lean mass index (kg/m2) (DXA) | Within-group differences: EX + O: PRE: 17.6 ± 1.9 vs. POST: 17.2 (16.8 to 17.4) CON: PRE: 16.7 ± 2.1 vs. POST: 17.0 (16.6 to 17.4) Between-group differences: EX + O vs. CON: MD: NR | ||||
| Fat mass (kg) (DXA) | Within-group differences: EX + O: PRE: 33.4 ± 10.5 vs. POST: 29.8 (27.9 to 31.8) CON: PRE: 26.9 ± 6.7 vs. POST: 32.1 (30.0 to 34.1) Between-group differences: EX + O vs. CON: MD: NR | ||||
| Body fat percent (%) (DXA) | Within-group differences: EX + O: PRE: 37.1 ± 4.4 vs. POST: 35.9 (34.4 to 37.5) CON: PRE: 34.4 ± 4.7 vs. POST: 36.7 (35.1 to 38.2) Between-group differences: EX + O vs. CON: MD: NR | ||||
| Allen et al. [81] United Kingdom RCT | Population: Locally advanced esophagogastric cancer patients (n = 54) Stage: T1 = 1(2) T2 = 12 (22) T3 = 38 (70) T4 = 3 (6) N0 = 18 (33) N1 = 17 (31) N2 = 16 (30) N3 = 3 (6) | EX + NU + On allocated = 26; n complete outcome = 24: Prehabilitation Multimodal intervention (exercise + nutrition + psychological support) CON (n allocated = 28; n complete outcome = 28): Usual care with encouragement to get fitter during treatment. | 15-week intervention Exercise component (FITT): F: Supervised in-clinic 2 days/week + Home-based 3 days/week I: AT: 40–60 HRR or 11–14 RPE and RT: 12–14 RPE Time: 60 min (Volume: 12 reps, 2 sets) Type: Prehabilitation supervised in clinic and unsupervised home-based AT and RT and flexibility. Nutrition component: Needs-based nutritional intervention with frequent, tailored, dietetic input from dieticians. Other component Psychological support: 6 face-to-face sessions with discussion of health status, strengths, recognition, resilience, or goal setting. Adherence: EX: attended 76 ± 14% of supervised exercise sessions and 65 ± 27% of home-based.NU + O: NR | Skeletal muscle index (cm2/m2) (CT) | Within-group differences: EX + NU + O: MD: −11.6 (95%CI –14.2 to –9.0) CON: MD: −15.6 (95%CI –18.7 to –15.4) Between-group differences: EX + NU + O vs. CON: MD: NR¥↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, O.; Wilson, R.L.; Gonzalo-Encabo, P.; Kang, D.-W.; Christopher, C.N.; Bentley, T.; Dieli-Conwright, C.M. The Effect of Exercise and Nutritional Interventions on Body Composition in Patients with Advanced or Metastatic Cancer: A Systematic Review. Nutrients 2022, 14, 2110. https://doi.org/10.3390/nu14102110
Barnes O, Wilson RL, Gonzalo-Encabo P, Kang D-W, Christopher CN, Bentley T, Dieli-Conwright CM. The Effect of Exercise and Nutritional Interventions on Body Composition in Patients with Advanced or Metastatic Cancer: A Systematic Review. Nutrients. 2022; 14(10):2110. https://doi.org/10.3390/nu14102110
Chicago/Turabian StyleBarnes, Oscar, Rebekah L. Wilson, Paola Gonzalo-Encabo, Dong-Woo Kang, Cami N. Christopher, Thomas Bentley, and Christina M. Dieli-Conwright. 2022. "The Effect of Exercise and Nutritional Interventions on Body Composition in Patients with Advanced or Metastatic Cancer: A Systematic Review" Nutrients 14, no. 10: 2110. https://doi.org/10.3390/nu14102110
APA StyleBarnes, O., Wilson, R. L., Gonzalo-Encabo, P., Kang, D.-W., Christopher, C. N., Bentley, T., & Dieli-Conwright, C. M. (2022). The Effect of Exercise and Nutritional Interventions on Body Composition in Patients with Advanced or Metastatic Cancer: A Systematic Review. Nutrients, 14(10), 2110. https://doi.org/10.3390/nu14102110