Mega-Dose Vitamin C Ameliorates Nonalcoholic Fatty Liver Disease in a Mouse Fast-Food Diet Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experimental Design
2.2. Histopathology
2.3. Primary Antibodies and Chemicals
2.4. Immunohistochemistry
2.5. Western Blot Analysis
2.6. Quantitative RT-PCR
2.7. Serum Biochemistry for Measuring Triglyceride, Free Fatty Acid, ALT, and Vitamin C Levels
2.8. Extraction and Measurements of Hepatic Triglycerides and Free Fatty Acids
2.9. Statistical Analysis
3. Results
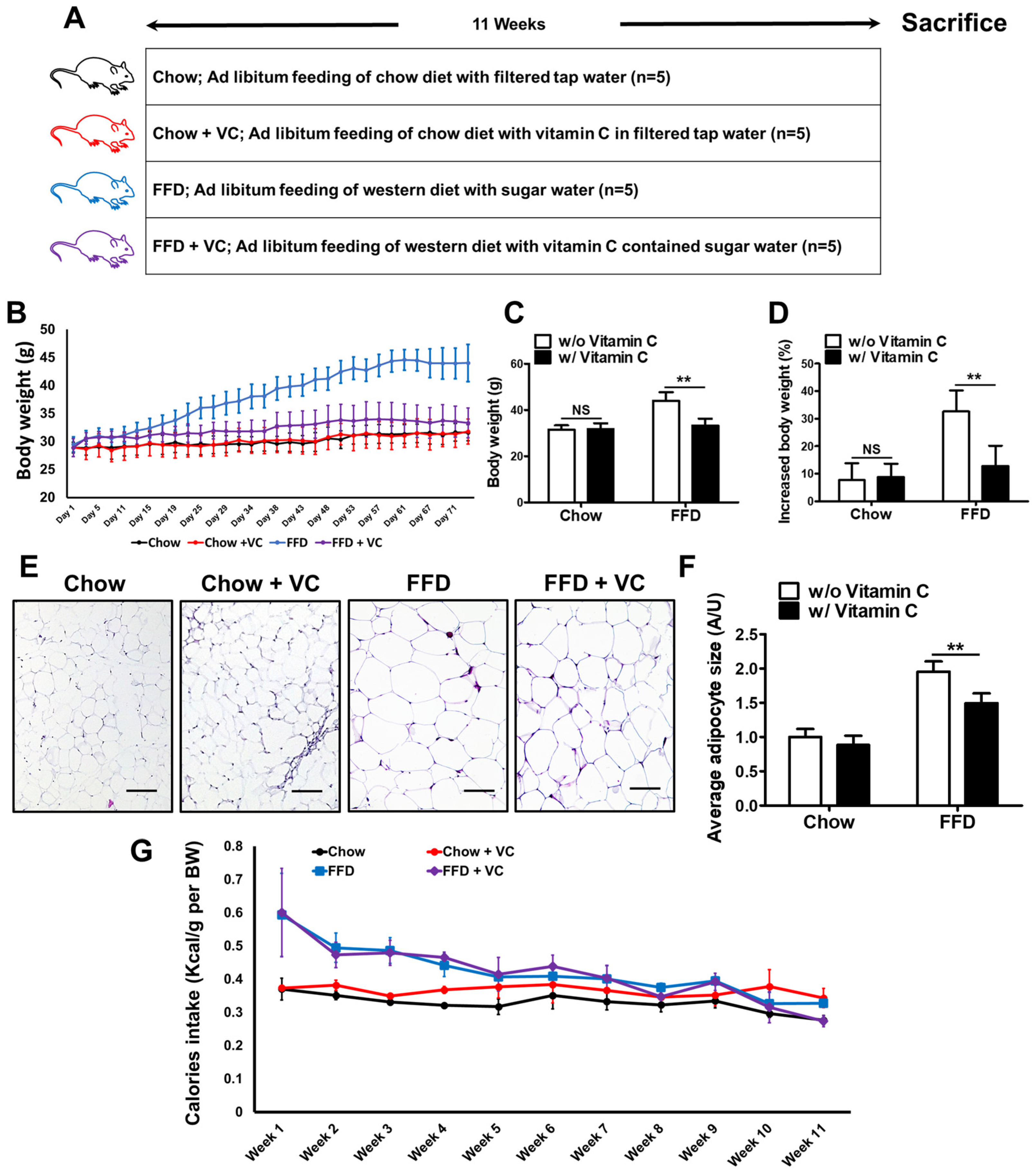
3.1. Mega-Dose Vitamin C Treatment Significantly Decreased Fast-Food-Diet Mediated Body Weight Gain
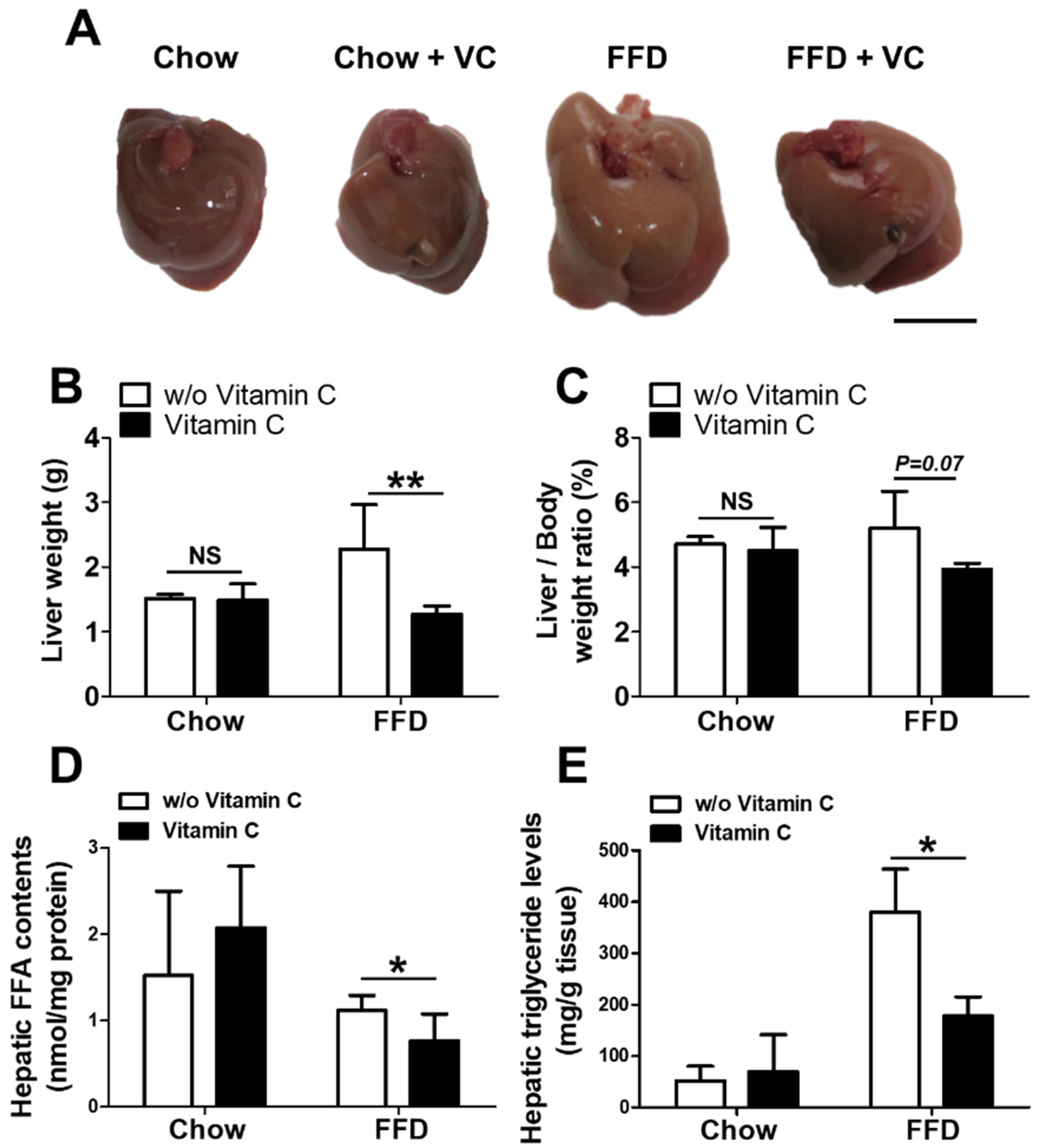
3.2. Mega-Dose Vitamin C Treatments Reduced Hepatic Triglyceride Accumulation in the Fast-Food-Diet Fed Mice
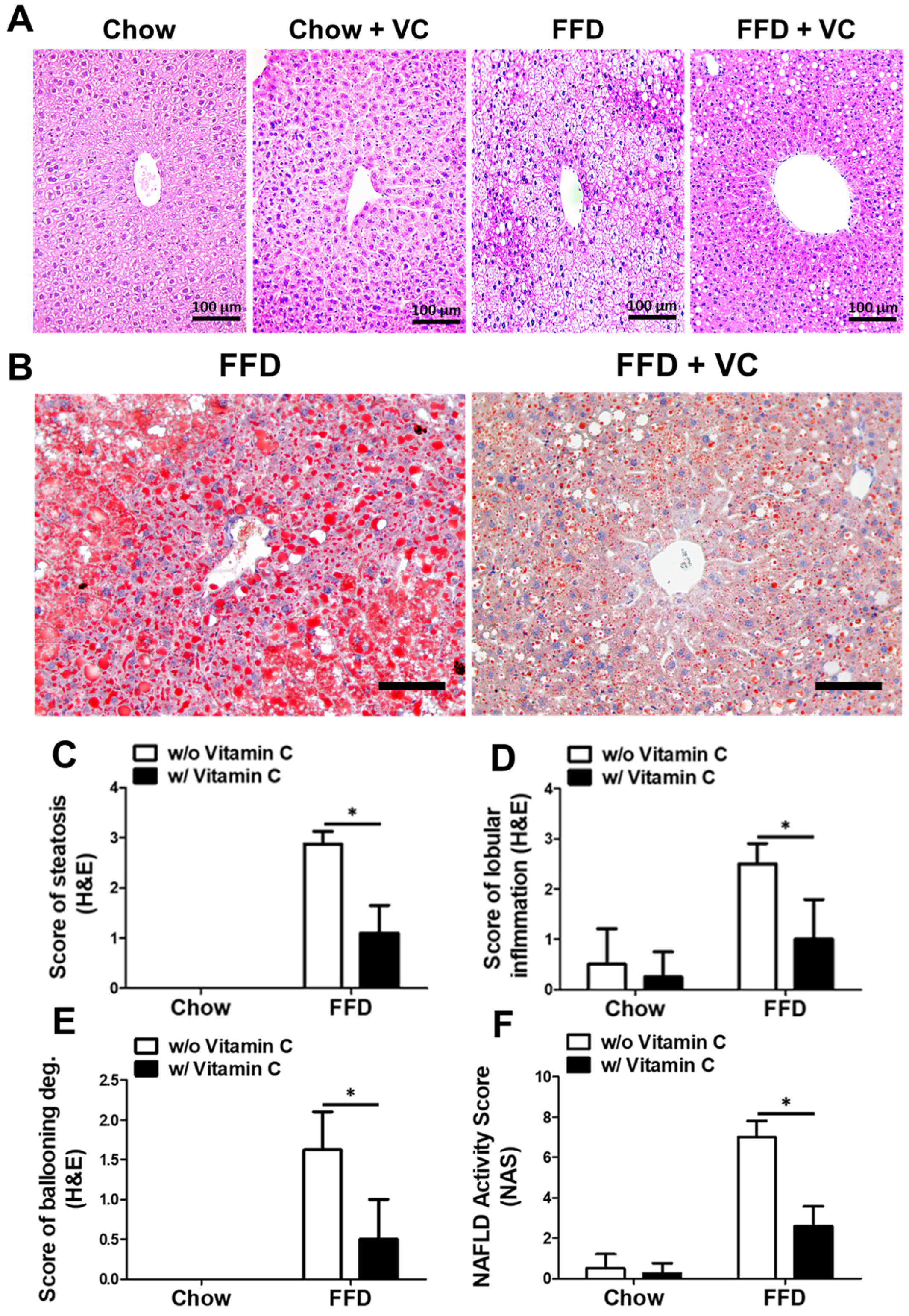
3.3. Mega-Dose Vitamin C Treatment Attenuated NAFLD Progression Microscopically
3.4. Mega-Dose Vitamin C Treatment Attenuated Liver Injuries in the FFD-Fed Mice
3.5. Mega-Dose Vitamin C Treatment Protects the Liver from NASH-Mediated Inflammatory Responses
3.6. Mega-Dose Vitamin C Treatment Attenuated NASH Progression Independent of Catabolic Pathways
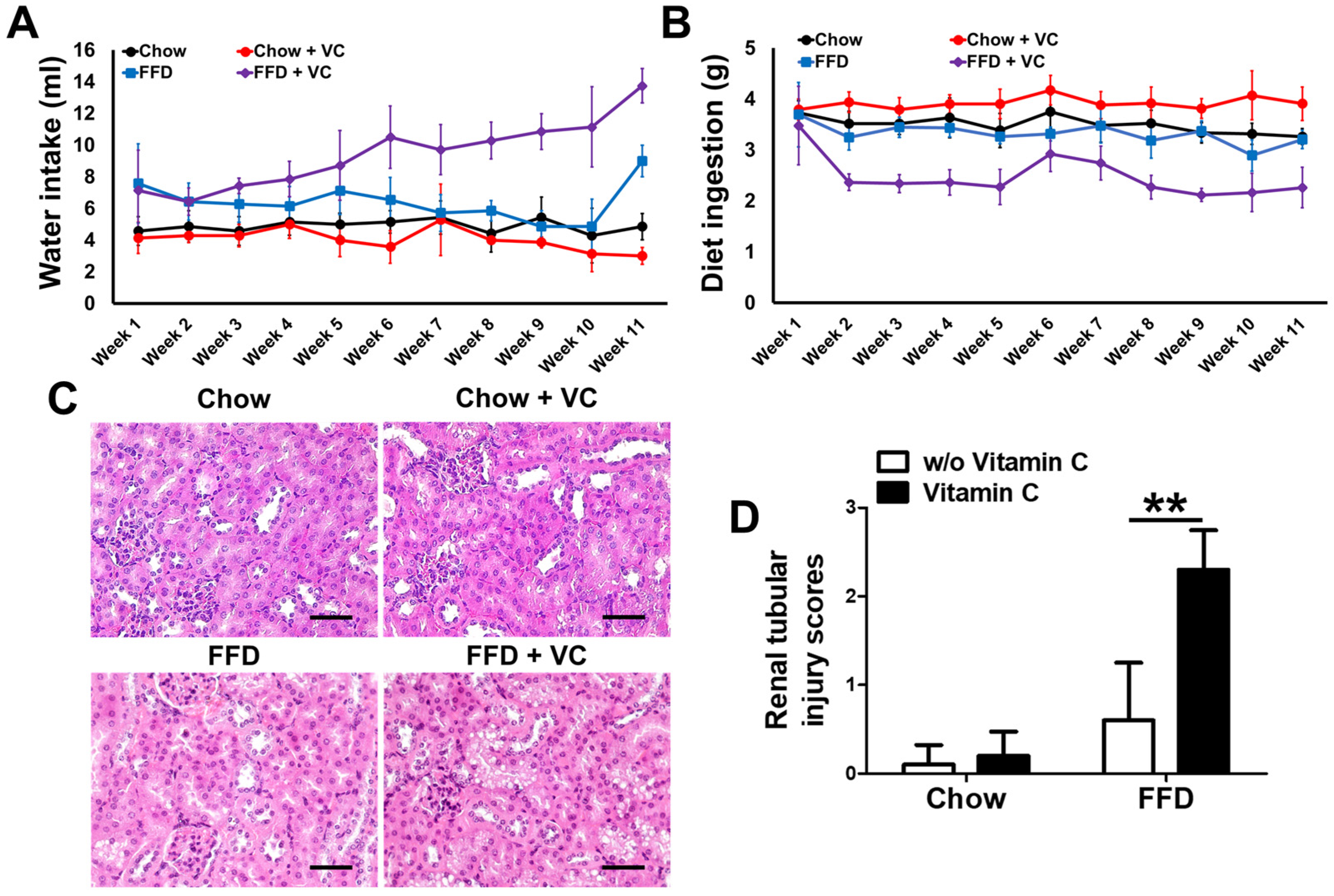
3.7. Mega-Dose Vitamin C Treatment Inhibits Diet Ingestion via Increased Water Intake with Compensatory Increases in Urine Volume
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, A.; Keeffe, E.B. Nonalcoholic fatty liver disease. Rev. Gastroenterol. Disord. 2002, 2, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Schlueter, A.K.; Johnston, C.S. Vitamin C: Overview and update. J. Evid.-Based Complement. Altern. Med. 2011, 16, 49–57. [Google Scholar] [CrossRef]
- May, C.N.; Bellomo, R.; Lankadeva, Y.R. Therapeutic potential of mega-dose vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br. J. Pharmacol. 2021, 178, 3864–3868. [Google Scholar] [CrossRef]
- Frei, B.; Lawson, S. Vitamin C and cancer revisited. Proc. Natl. Acad. Sci. USA 2008, 105, 11037–11038. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Curhan, G.; Forman, J.P.; Ascherio, A.; Choi, H.K. Vitamin C intake and serum uric acid concentration in men. J. Rheumatol. 2008, 35, 1853–1858. [Google Scholar]
- Brzezińska, O.; Styrzyński, F.; Makowska, J.; Walczak, K. Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review. Nutrients 2021, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wang, C.; Xu, Z.; He, Y.; Hou, Y.; Li, B. A Novel Standardized Method of Renal Biopsy in Mice. Kidney Dis. 2021, 7, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Cakir, B.; Liu, C.-H.; Fu, Z.; Huang, S.; Cho, S.S.; Britton, W.R.; Sun, Y.; Puder, M.; Hellström, A. Free fatty acid receptor 4 activation protects against choroidal neovascularization in mice. Angiogenesis 2020, 23, 385–394. [Google Scholar] [CrossRef]
- Mandrekar, P.; Ambade, A.; Lim, A.; Szabo, G.; Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011, 54, 2185–2197. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lopez, N.; Tarabra, E.; Toledo, M.; Garcia-Macia, M.; Sahu, S.; Coletto, L.; Batista-Gonzalez, A.; Barzilai, N.; Pessin, J.E.; Schwartz, G.J. System-wide benefits of intermeal fasting by autophagy. Cell Metab. 2017, 26, 856–871.e855. [Google Scholar] [CrossRef] [Green Version]
- Park, J.K.; Shao, M.; Kim, M.Y.; Baik, S.K.; Cho, M.Y.; Utsumi, T.; Satoh, A.; Ouyang, X.; Chung, C.; Iwakiri, Y. An endoplasmic reticulum protein, Nogo-B, facilitates alcoholic liver disease through regulation of kupffer cell polarization. Hepatology 2017, 65, 1720–1734. [Google Scholar] [CrossRef]
- Lassailly, G.; Saleh, M.B.; Leleu-Chavain, N.; Ningarhari, M.; Gantier, É.; Carpentier, R.; Artru, F.; Gnemmi, V.; Bertin, B.; Maboudou, P. Nucleotide-binding oligomerization domain 1 (NOD1) modulates liver ischemia reperfusion through the expression adhesion molecules. J. Hepatol. 2019, 70, 1159–1169. [Google Scholar] [CrossRef]
- Farrell, G.C.; Van Rooyen, D.; Gan, L.; Chitturi, S. NASH is an inflammatory disorder: Pathogenic, prognostic and therapeutic implications. Gut Liver 2012, 6, 149. [Google Scholar] [CrossRef] [Green Version]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef]
- Russo, E.; Leoncini, G.; Esposito, P.; Garibotto, G.; Pontremoli, R.; Viazzi, F. Fructose and uric acid: Major mediators of cardiovascular disease risk starting at pediatric age. Int. J. Mol. Sci. 2020, 21, 4479. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.; Schwarz, C.; Funk, G.-C. Osmotic diuresis due to urea as the cause of hypernatraemia in critically ill patients. Nephrol. Dial. Transplant. 2012, 27, 962–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economidou, D.; Stavrinou, E.; Giamalis, P.; Dimitriadis, C.; Economou, S.; Memmos, D. Acute kidney injury due to osmotic nephrosis following intraoperative placement of an intraperitoneal antiadhesive barrier. Am. J. Kidney Dis. 2011, 57, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Varela-Echavarría, A.; de Oca-Luna, R.M.; Barrera-Saldaña, H.A. Uricase protein sequences: Conserved during vertebrate evolution but absent in humans. FASEB J. 1988, 2, 3092–3096. [Google Scholar] [CrossRef]
- Garay, R.P.; El-Gewely, M.R.; Labaune, J.-P.; Richette, P. Therapeutic perspectives on uricases for gout. Jt. Bone Spine 2012, 79, 237–242. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, Y.; Zhan, C.G.; Liao, F. Uricases as therapeutic agents to treat refractory gout: Current states and future directions. Drug Dev. Res. 2012, 73, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [Green Version]
- Asgharpour, A.; Cazanave, S.C.; Pacana, T.; Seneshaw, M.; Vincent, R.; Banini, B.A.; Kumar, D.P.; Daita, K.; Min, H.-K.; Mirshahi, F. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 2016, 65, 579–588. [Google Scholar] [CrossRef] [Green Version]



| Features | Grades/Score | Proportion of Damaged Tubules |
|---|---|---|
| Renal tubular injury | 0 | <5% |
| 1 | 5–33% | |
| 2 | 33–67% | |
| 3 | 67%> |
| Target Gene | Primer Sequences (5′-3′) | |
|---|---|---|
| 18s | Forward | ACGGAAGGGCACCACCAGGA |
| Reverse | CACCACCACCCACGGAATCG | |
| IL-1β | Forward | CTTTGAAGTTGACGGACCC |
| Reverse | TGAGTGATACTGCCTGCCTG | |
| IL-6 | Forward | GCTAAGGACCAAGACCATCCAAT |
| Reverse | GCTTAGGCATAACGCACTAGGTTT | |
| CD-11b | Forward | GTGTCCGCAAGAACACCAAG |
| Reverse | GGACAGGGTCTAAAGCCAGG | |
| ICAM-1 | Forward | ATTCGTTTCCGGAGAGTGTG |
| Reverse | CAGCACCGTGAATGTGATCT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Lee, Y.-J.; Baek, S.-M.; Kang, K.-K.; Kim, T.-U.; Yim, J.-H.; Kim, H.-Y.; Han, S.-H.; Choi, S.-K.; Park, S.-J.; et al. Mega-Dose Vitamin C Ameliorates Nonalcoholic Fatty Liver Disease in a Mouse Fast-Food Diet Model. Nutrients 2022, 14, 2195. https://doi.org/10.3390/nu14112195
Lee S-W, Lee Y-J, Baek S-M, Kang K-K, Kim T-U, Yim J-H, Kim H-Y, Han S-H, Choi S-K, Park S-J, et al. Mega-Dose Vitamin C Ameliorates Nonalcoholic Fatty Liver Disease in a Mouse Fast-Food Diet Model. Nutrients. 2022; 14(11):2195. https://doi.org/10.3390/nu14112195
Chicago/Turabian StyleLee, Seoung-Woo, Young-Jin Lee, Su-Min Baek, Kyung-Ku Kang, Tae-Un Kim, Jae-Hyuk Yim, Hee-Yeon Kim, Se-Hyeon Han, Seong-Kyoon Choi, Sang-Joon Park, and et al. 2022. "Mega-Dose Vitamin C Ameliorates Nonalcoholic Fatty Liver Disease in a Mouse Fast-Food Diet Model" Nutrients 14, no. 11: 2195. https://doi.org/10.3390/nu14112195
APA StyleLee, S.-W., Lee, Y.-J., Baek, S.-M., Kang, K.-K., Kim, T.-U., Yim, J.-H., Kim, H.-Y., Han, S.-H., Choi, S.-K., Park, S.-J., Kim, T.-H., & Park, J.-K. (2022). Mega-Dose Vitamin C Ameliorates Nonalcoholic Fatty Liver Disease in a Mouse Fast-Food Diet Model. Nutrients, 14(11), 2195. https://doi.org/10.3390/nu14112195







