Fermented Carica papaya and Morinda citrifolia as Perspective Food Supplements for the Treatment of Post-COVID Symptoms: Randomized Placebo-Controlled Clinical Laboratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Clinical Conditions and Treatment in the Post-COVID Period
2.3. Food Supplements under Investigation and Protocols of the Application
2.4. Biological Material Collection and Processing
2.5. Haematological and Biochemical Analyses
2.6. Reagents and Assay Kits for Laboratory Study
2.7. Redox and Oxidation Marker Assays
2.8. ATP Assay
2.9. Immunological Assays
2.10. Oral-Nasal-Pharyngeal Microbiota Determination
2.11. Questionnaire and Physicality Tests
2.12. Statistical Analysis
3. Results
3.1. Effects of Fermented Fruits Supplementation on Self-Assessment, Cardiac Functions, and Physical Endurance of Post-COVID Patients
3.1.1. Comparable Effects of Fermented Fruit Supplementation and Placebo on Self-Assessment of Clinical Symptoms of Post-COVID Patients
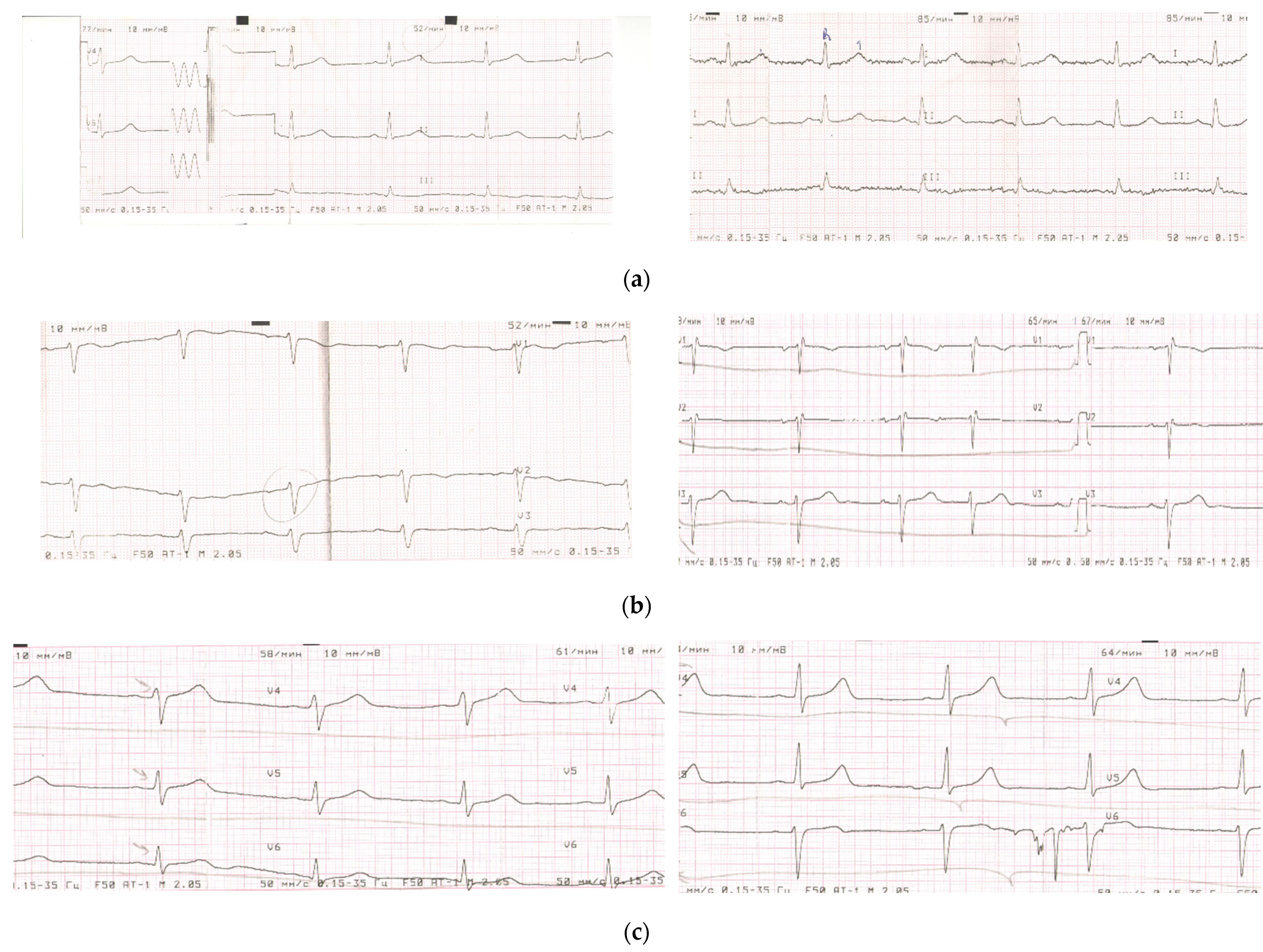
3.1.2. Comparable Effects of Fermented Fruit Supplementation (FFS) and Placebo on Electrocardiography of Patients in Post-COVID Period
3.1.3. Comparable Effects of Fermented Fruit Supplementation and Placebo on Subjective Dyspnea and Physical Load of Patients in Post-COVID Period
3.2. Effects of FFS versus Placebo on Cellular Immunity (Functions of Circulating Phagocytes) and Oral-Nasal-Pharyngeal Microbiota
3.3. Effects of FFS and Placebo on Plasma Levels of Cytokines/Chemokines Regulating Immune Response
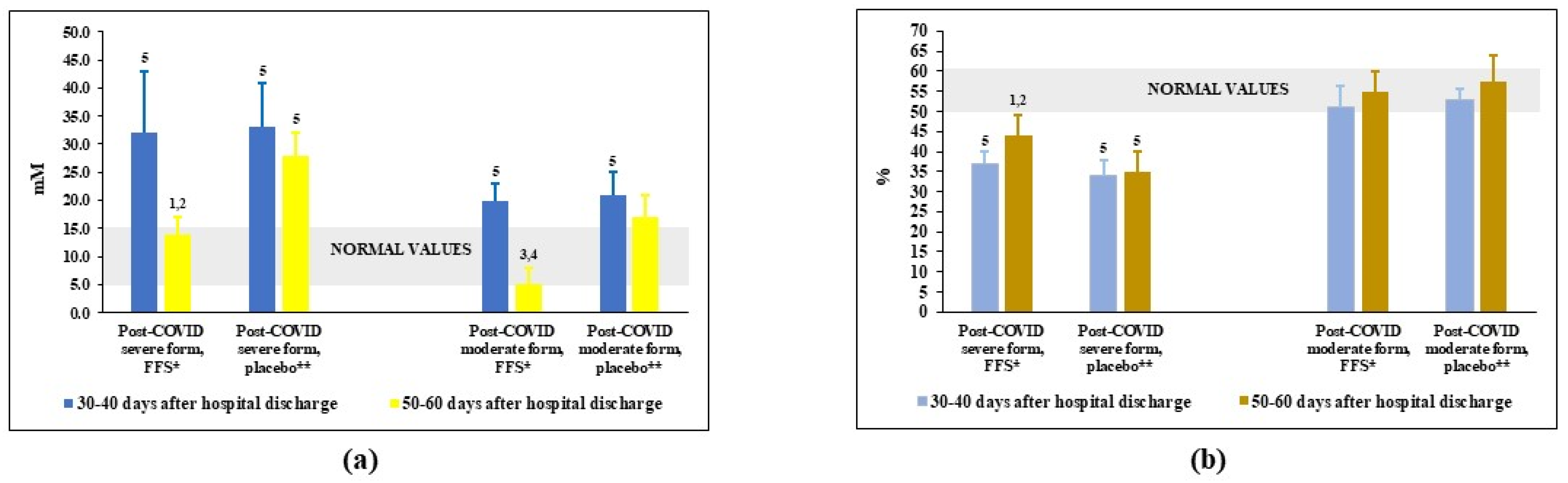
3.4. Effects of FFS and Placebo on the Redox Balance in Plasma of Post-COVID Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. COVID Disease Diagnosis and Assessment of Clinical Conditions
- (a)
- Hospital admission: from polyclinics at the 3d day after infection/symptoms-10 patients (10.9%), at the 5th day-38 patients (41.8%), at the 5th–7th day-7 patients (7.8%), at the 8th–12th day-20 (21.9%), and after 12th day-16 patients (17.6%) were transported to the hospital by the emergency cars.
- (b)
- Admission to Emergency Unit of the Hospital: all patients of these groups were moved to emergency unit due to severe life threatening clinical status and the need of invasive or non-invasive lung ventilation. The criteria for emergency procedures were SpO2 ≤ 93%, t° ≥ 39 °C, respiratory rate ≥ 30 min−1, unstable hemodynamics (systolic blood pressure < 90 mm Hg and diastolic blood pressure < 60 mm Hg), reduced consciousness, and agitation.
- (c)
- Frequent co-morbidities: obesity of different degrees-37 patients (40.6%), chronic cardio-vascular diseases-34 patients (37.5%), diabetes mellitus-26 patients (28.6%), chronic bronchitis-19 patients (20.9%), anaemia-4 patients (4.4%), and bronchial asthma-3 patients (3.3%).
- (d)
- Computer tomography: 25% of lung tissue damage was revealed in 3 patients (3.3%), 26–50% of lung tissue damage-74 patients (81.3%), and > 50% of lung tissue damage-14 patients (15.4%).
- (e)
- Major complaints: general malaise, dry cough, shortness of breath, chest pain, weakness, fever ≥ 39 °C, chills, anosmia, and pain in the lumbar region.
- (f)
- Discharge from the hospital: practically all patients recruited for the trial were released from the hospital after general stabilisation of their clinical conditions at the 30th–40th day after admission. Thirty patients of Group 1 and 9 patients of Group 2 still needed respiratory support.
- (a)
- Hospital admission: from polyclinics at the 3d day after infection/symptoms-27 patients (27.8%), at the 5th day-45 patients (46.4%), at the 5th–7th day-29 patients (29.9%), at the 8th–12th day-16 patients (16.5%). The criteria for hospitalization were as follows: SpO2 ≤ 93–95%; t° ≥ 38 °C; respiratory rate (breaths/min) ≥ 22.
- (b)
- Frequent co-morbidities: diabetes mellitus-15 patients (15.5%), chronic bronchial diseases-14 patients (14.4%), arterial hypertonia-35 patients (36.1%), and obesity-19 patients (19.6%).
- (c)
- Computer tomography: 18–25% of lung tissue damage was revealed in 73 patients (54.3%), 26–30% of lung tissue damage was seen in 23 patients (24.8%).
- (d)
- Major complaints: dry cough, dyspnea, chest pain, weakness, general malaise, fever ≥ 38 °C, chills, and anosmia.
- (e)
- Discharge from the hospital: practically all patients recruited for the trial were released from the hospital after general stabilisation of their clinical conditions at the 20th–30th day after admission. None of these patients were on oxygen supplementation at the discharge.
Appendix B
Appendix C
| Group | Number of Patients | Total Amount of Microbes on Epithelia (lg CFU */mL) | Number of Isolated and Identified Microbial Strains | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Klebsiella pneumoniae | Candida albicans | |||||||||||
| Pharyngeal Epithelia | Oral Epithelia | Nasal Epithelia | |||||||||||
| 30–40 cyтки ** | 50–60 cyтки | 30–40 cyтки | 50–60 cyтки | 30–40 cyтки | 50–60 cyтки | 30–40 cyтки | 50–60 cyтки | 30–40 cyтки | 50–60 cyтки | 30–40 cyтки | 50–60 cyтки | ||
| Post-COVID severe, FFS ** | 64 | 5.5 ± 1.0 1 | 4.5 ± 1.0 1 | 4.5 ± 1.0 1 | 4.0 ± 1.0 1 | 4.5 ± 1.0 1 | 4.0 ± 1.0 1 | 46 | 41 (89%) | 18 | 8 (44%) | 21 | 11 (52%) |
| Post-COVID severe, placebo | 27 | 5.0 ± 1.0 1 | 4.5 ± 1.0 1 | 4.5 ± 1.0 1 | 4.5 ± 1.0 1 | 4.0 ± 1.0 1 | 4.0 ± 1.0 1 | 19 | 15 (79%) | 9 | 4 (44%) | 9 | 5 (56%) |
| Post-COVID moderate, FFS | 68 | 3.5 ± 1.02 | 3.0 ± 1.0 2 | 3.0 ± 1.0 2 | 3.0 ± 1.0 2 | 3.5 ± 1.0 2 | 3.0 ± 1.0 2 | 16 | 8 (50%) | 3 | 1 (33%) | 9 | 3 (33%) |
| Post-COVID moderate, placebo | 29 | 3.5 ± 1.0 2 | 3.0 ± 1.0 2 | 3.0 ± 1.0 2 | 3.0 ± 1.0 2 | 3.5 ± 1.0 2 | 3.5 ± 1.0 2 | 6 | 3 (50%) | 1 | 0 | 4 | 2 (50%) |
| Donors | 25 | 2.5 ± 1.0 | 2.5 ± 1.0 | 2.5 ± 1.0 | - | - | - | ||||||
References
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; Hanghicel, P.; Sitar-Taut, A.; Suharoschi, R.; Vulturar, R.; et al. Endothelial dysfunction, inflammation, and oxidative stress in COVID-19-mechanisms and therapeutic targets. Oxid. Med. Cell. Longev. 2021, 2021, 8671713. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Ulstrand Fuglebjerg, N.J.; Oestergaard Jensen, T.; Hoyer, N.; Koch Ryrso, C.; Lindergaard, B.; Barella Harboe, Z. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Inter. J. Infect. Dis. 2020, 99, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.-M.; Rojas, M.; Salinas, M.L.; Rodriguez, Y.; Roa, G.; Lozano, M.; Rodriguez-Jimenez, M.; Montoya, N.; Zapata, E.; Post-COVID Study Group; et al. Post-COVID syndrome. A case series and comprehensive review. Autoimm. Rev. 2021, 20, 102947. [Google Scholar] [CrossRef]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, 13746. [Google Scholar] [CrossRef]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The role of micronutrients in support of the immune response against viral infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, e000085. [Google Scholar] [CrossRef]
- Slominski, R.M.; Stefan, J.; Athar, M.; Holick, M.F.; Jetten, A.M.; Raman, C.; Slominski, A.T. COVID-19 and vitamin D: A lesson from the skin. Exp. Dermatol. 2020, 29, 885–890. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llang, T. Handbook of COVID-19 Prevention and Treatment; ALNAP: London, UK, 2020; Available online: https://covid19.alnap.org/help-library/handbook-of-%20covid-19-prevention-and-treatment (accessed on 19 February 2022).
- Manna, P.R.; Gray, Z.C.; Reddy, P.H. Healthy immunity on preventive medicine for combating COVID-19. Nutrients 2022, 14, 1004. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozen, M.; Kocabas Sandal, G.; Dinleyici, E.C. Probiotics for the prevention of pediatric upper respiratory tract infections: A systematic review. Expert Opin. Biol. Ther. 2015, 15, 9–20. [Google Scholar] [CrossRef]
- Benatrehina, P.A.; Pan, L.; Naman, B.; Li, J.; Kinghorn, A.D. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement. Med. 2018, 8, 267–277. [Google Scholar] [CrossRef]
- Caliskan, U.K.; Karakus, M.M. Evaluation of botanicals as potential COVID-19 symptoms terminator. World J. Gastroenterol. 2021, 27, 6551–6571. [Google Scholar] [CrossRef]
- Aucoin, M.; Cooley, K.; Saunders, P.R.; Carè, J.; Anheyer, D.; Medina, D.N.; Cardozo, V.; Remy, D.; Hannan, N.; Garber, A. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Integr. Med. 2020, 7, 203–217. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Tariq, A.; Jiang, X.; Ahmed, Z.; Zhihao, Z.; Idrees, M.; Azizullah, A.; Adnan, M.; Bussmann, R.W. Food as medicine: A possible preventive measure against coronavirus disease (COVID-19). Phytother. Res. 2020, 34, 3124–3136. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Kaur, R.; Bhardwaj, A.; Bhardwaj, V.; Ohri, P.; Sharma, A.; Ahmad, A.; Bhardwaj, R.; Ahmad, P. Herbal immune-boosters: Substantial warriors of pandemic COVID-19 battle. Phytomedicine 2021, 85, 153361. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M.; et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Ng, C.C.; Su, H.; Tzeng, W.S.; Shyu, Y.T. Probiotic potential of noni juice fermented with lactic acid bacteria and bifidobacteria. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S6), 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Y.; Lin, Y.L.; Yang, D.J.; Liu, C.W.; Hsu, C.L.; Tzang, B.S.; Chen, Y.C. Hepatoprotection of noni juice against chronic alcohol consumption: Lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation. J. Agric. Food Chem. 2013, 61, 11016–11024. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Peng, L.; Jensen, C.J.; Deng, S.; West, B.J. Noni juice reduces lipid peroxidation-derived DNA adducts in heavy smokers. Food Sci. Nutr. 2013, 1, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-N.; Pawlus, A.D.; Jung, H.-A.; Keller, W.J.; McLaughlin, J.L.; Kinghorn, A.D. Chemical constituents of the fruits of Morinda citrifolia (noni) and their antioxidant activity. J. Nat. Prod. 2005, 68, 592–595. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, J.; Li, X.; Tang, X.; Ma, S.; Lv, Y.; Yang, S. Noni (Morinda citrifolia L.) wine prevents the oxidative stress and obesity in mice induced by high-fat diet. J. Food Biochem. 2020, 44, e13460. [Google Scholar] [CrossRef]
- Palu, A.K.; Kim, A.H.; West, B.J.; Deng, S.; Jensen, J.; White, L. The effects of Morinda citrifolia L. (noni) on the immune system: Its molecular mechanisms of action. J. Ethnopharmacol. 2008, 115, 502–506. [Google Scholar] [CrossRef]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (noni) alleviates DNCB-induced atopic dermatitis and NC/Nga mice through modulating immune balance and skin barrier function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, X.M.; Zhao, T.; Wang, J.-L.; Li, M.-M.; Lyu, S.; Zhou, Q.; Chen, G.-Y. Chemical constituents from fermented Noni juice. Zhongguo Zhong Yao Za Zhi 2019, 44, 4015–4020. [Google Scholar]
- Almeida-Souza, F.; da Silva Freitas de Souza, C.; Taniwaki, N.N.; Silva, J.J.; de Oliveira, R.M.; Abreu-Silva, A.L.; da Silva Calabrese, K. Morinda citrifolia Linn. fruit (noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c. Nitric Oxide 2016, 58, 51–58. [Google Scholar] [CrossRef]
- Almeida-Souza, F.; de Oliveira, A.E.R.; Abreu-Silva, A.L.; da Silva Calabrese, K. In vitro activity of Morinda citrifolia Linn. fruit juice against the axenic amastigote form of Leishmania amazonensis and its hydrogen peroxide induction capacity in BALB/c peritoneal macrophages. BMC Res. Notes 2018, 11, 492. [Google Scholar] [CrossRef] [Green Version]
- Nerurkar, P.V.; Nishioka, A.; Eck, P.O.; Johns, L.M.; Volper, E.; Nerurkar, V.R. Regulation of glucose metabolism via hepatic forkhead transcription factor 1 (FoxO1) by Morinda citrifolia (noni) in high-fat diet-induced obese mice. Br. J. Nutr. 2012, 108, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerurkar, P.V.; Hwang, P.W.; Saksa, E. Anti-diabetic potential of noni: The Yin and the Yang. Molecules 2015, 20, 17684–17719. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.L.; Hwang, J.T.; Yi, S.H.; Nam, Y.D.; Lim, S.I. Antidiabetic effect of Morinda citrifolia (Noni) fermented by Cheonggukjang in KK-A(y) diabetic mice. Evid. Based Complement. Altern. Med. 2012, 2012, 163280. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, P.; Manavalan, A.; Wu, X.; Kim, J.-C.; Pang, C.; Cao, S.; Kang, K.S.; et al. Identification of anti-inflammatory compounds from Hawaiian noni (Morinda citrifolia L.) fruit juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef]
- Youn, U.J.; Park, E.J.; Kondratyuk, T.P.; Sang-Ngern, M.; Wall, M.M.; Wei, Y.; Pezzuto, J.M.; Chang, L.C. Anti-inflammatory and quinone reductase inducing compounds from fermented noni (Morinda citrifolia) juice exudates. J. Nat. Prod. 2016, 79, 1508–1513. [Google Scholar] [CrossRef]
- Dussossoy, E.; Bichon, F.; Bony, E. Pulmonary anti-inflammatory effects and spasmolytic properties of Costa Rican noni juice (Morinda citrifolia L.). J. Ethnopharmacol. 2016, 192, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Isitor, G.N.; Maxwell, A.; Bhogadi, V.; Ramdath, D.D. Wound-healing activity of Morinda citrifolia fruit juice on diabetes-induced rats. J. Wound Care 2007, 16, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Li, D.; Jaminet, S.-C.; Cao, S. Activation of the Nrf2 cell defense pathway by ancient foods: Disease prevention by important molecules and microbes lost from the modern western diet. PLoS ONE 2016, 11, e0148042. [Google Scholar] [CrossRef]
- Senger, D.R.; Cao, S. Diabetic wound healing and activation of Nrf2 by herbal medicine. J. Nat. Sci. 2016, 2, e247. [Google Scholar]
- Pachauri, S.D.; Verma, P.R.; Dwivedi, A.K.; Tota, S.; Khandelwal, K.; Saxena, J.K.; Nath, C. Ameliorative effect of Noni fruit extract on streptozotocin-induced memory impairment in mice. Behav. Pharmacol. 2013, 24, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Hamabe, W.; Kamiya, K.; Satake, T.; Yamamoto, J.; Tokuyama, S. Preventive effect of Morinda citrifolia fruit juice on neuronal damage induced by focal ischemia. Biol. Pharm. Bull. 2009, 32, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, J.; Hosung, L.; Uwaya, A.; Isami, F.; Ohno, M.; Mikami, T. Morinda citrifolia fruit reduces stress-induced impairment of cognitive function accompanied by vasculature improvement in mice. Physiol. Behav. 2010, 101, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.O.; de Fátima Braga Magalhães, I.; Mondêgo-Oliveira, R.; de Sá, J.C.; Rocha, A.L.; Abreu-Silva, A.L. One plant, many uses: A review of the pharmacological applications of Morinda citrifolia. Phytother. Res. 2017, 31, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) fruit—Phytochemistry, pharmacology, safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C. Changes in phenols, polysaccharides and volatile profile of noni (Morinda citrifolia L.) juice during fermentation. Molecules 2021, 26, 2604. [Google Scholar] [CrossRef]
- Wall, M.M.; Miller, S.; Siderhurst, M.S. Volatile changes in Hawaiian noni fruit, Morinda citrifolia L. during ripening and fermentation. J. Sci. Food Agric. 2018, 98, 3391–3399. [Google Scholar] [CrossRef]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef]
- Sy, J.B.A.; Hsu, T.-C.; Limaye, A.; Liu, J.-R. Oral administration with a traditional fermented multi-fruit beverage modulates non-specific and antigen-specific immune responses in BALB/c mice. PLoS ONE 2020, 15, e0233047. [Google Scholar] [CrossRef]
- Rimbach, G.; Park, Y.C.; Guo, Q.; Moini, H.; Qureshi, N.; Saliou, C.; Takayama, K.; Virgili, F.; Packer, L. Nitric oxide synthesis and TNF-alpha secretion in RAW 264.7 macrophages: Mode of action of a fermented papaya preparation. Life Sci. 2000, 67, 679–694. [Google Scholar] [CrossRef]
- Collard, E.; Roy, S. Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid. Redox Signal. 2010, 13, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Kharaeva, Z.F.; Zhanimova, R.L.; Mustafaev, M.S.; De Luca, C.; Mayer, W.; Thai, J.C.S.; Tuan, R.T.S.; Korkina, L. Effects of standardised fermented papaya gel on clinical symptoms, inflammatory cytokines, and nitric oxide metabolites in patients with chronic periodontitis: An open randomised clinical study. Med. Inflamm. 2016, 2016, 9379840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Dickerson, R.; Das Ghatak, P.; Gordillo, G.M.; Chaffe, S.; Saha, A.; Khanna, S.; Roy, S. May dietary supplementation augment respiratory burst in wound-site inflammatory cells? Antioxid. Redox Signal. 2018, 28, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.; Deshapande, B.; Gnyawali, U.; Lynch, D.; Gordillo, G.M.; Schuster, D.; Osei, K.; Roy, S. Correction of aberrant NADPH oxidase activity in blood derived mononuclear cells from type II diabetes mellitus patients by a naturally fermented papaya preparation. Antioxid. Redox Signal. 2012, 17, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, R.; Banejee, J.; Rauckhorst, A.; Pfeiffer, D.R.; Gordillo, G.M.; Khanna, S.; Osei, K.; Roy, S. Does oral supplementation of a fermented papaya preparation correct respiratory burst function of innate immune cells in type 2 diabetes mellitus patients? Antioxid. Redox Signal. 2015, 22, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osato, J.A.; Korkina, L.G.; Santiago, L.A.; Afanas’ev, I.B. Effects of bio-normalizer (a food supplementation) on free radical production by human blood neutrophils, erythrocytes, and rat peritoneal macrophages. Nutrition 1995, 11, 568–572. [Google Scholar]
- Fibach, E.; Ginsburg, I. The antioxidant effect of fermented papaya preparation in the oral cavity. Phytother. Res. 2015, 29, 1317–1322. [Google Scholar] [CrossRef]
- Lubrano, C.; Valacchi, G.; Specchia, P.; Gnessi, L.; Rubanenko, E.P.; Shuginina, E.A.; Trukhanov, A.I.; Korkina, L.G.; De Luca, C. Integrated haematological profiles of redox status, lipid, and inflammatory protein biomarkers in benign obesity and unhealthy obesity with metabolic syndrome. Oxid. Med. Cell. Longev. 2015, 2015, 490613. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, G.; Land, J.M.; Keir, G.; Thompson, E.J.; Heals, S.J.R. Adaptation of the nitrate reductase and Griess reaction methods for the measurement of serum nitrate plus nitrite levels. Ann. Clin. Biochem. 1997, 34, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kharaeva, Z.F.; Mustafaev, M.S.; Khazhmetov, A.V.; Gazaev, I.H.; Blieva, L.Z.; Mayer, W.; De Luca, C.; Korkina, L.G. Anti-bacterial and anti-inflammatory effects of toothpaste with Swiss medicinal herbs towards patients suffering from gingivitis and initial stage of periodontitis: From clinical efficacy to mechanisms. Dent. J. 2020, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Kharaeva, Z.; Hokonova, T.; Elmurzaeva, J.; Dzamihova, I.; Mayer, W.; De Luca, C.; Trakhtman, I.; Korkina, L. Effects of heavy isotopes (2H1 and 18O16) depleted water consumption on physical recovery and metabolic and immunological parameters of healthy volunteers under regular fitness load. Sports 2021, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, G.E.; Gould, J.C. Simplified technique for counting the number of bacteria in urine and other fluids. J. Clin. Pathol. 1965, 18, 480–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Borg, E.; Kaijser, A. A comparison between three rating scales for perceived exertion and two different work tests. Scand. J. Med. Sci. Sport 2006, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodrigez, Y.; Zapata, E.; Ramirez-Santana, C.; Anaya, J.-M. Persistent autoimmune activation and proinflammatory state in post-COVID syndrome. J. Infect. Dis. 2022, jiac017. [Google Scholar] [CrossRef]
- Hasichaolu; Zhang, X.; Li, X.; Li, X.; Li, D. Circulating cytokines and lymphocyte subsets in patients who have recovered from COVID-19. BioMed. Res. Int. 2020, 2020, 7570981. [Google Scholar]
- Laija-Martinez, J.; Huang, F.; Del-Rio-Navarro, B.E.; Sancez-Munoz, F.; Munoz-Hernandez, O.; Giacoman-Martinez, A.; Hall-Mondragon, M.S.; Espinosa-Velazquez, D. IL-17A and TNF-α as potential biomarkers for acute respiratory distress syndrome and mortality in patients with obesity and COVID-19. Med. Hypotheses 2020, 144, 109935. [Google Scholar] [CrossRef]
- Li, Q.; Xu, W.; Li, W.-X.; Huang, C.-L.; Chen, L. Dynamics of cytokines and lymphocyte subsets associated with the poor prognosis of severe COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12536–12544. [Google Scholar]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]



| Group | Number of Patients | Age Range, Years | Sex | |
|---|---|---|---|---|
| F | M | |||
| Group 1. Post-COVID after severe COVID, fermented fruits supplementation (FFS) | 64 | 38–69 | 35 | 29 |
| Group 2. Post-COVID after severe COVID, placebo | 27 | 39–67 | 13 | 14 |
| Group 3. Post-COVID after moderate COVID, fermented fruits supplementation (FFS) | 68 | 38–65 | 32 | 36 |
| Group 4. Post-COVID after moderate COVID, placebo | 29 | 36–64 | 15 | 14 |
| Group 5. Healthy donors, control | 25 | 35–59 | 12 | 13 |
| Question | Answer, Score | Average Score before the Trial (m ± S.D.), FFS * | Average Score after the Trial (m ± S.D.), FFS | Average Score before the Trial (m ± S.D.), Placebo ** | Average Score after the Trial (m ± S.D.), Placebo | Fisher’s Exact Test, Significance (p) |
|---|---|---|---|---|---|---|
| Weakness | 2—great | 2.0±0.0 | 1.1 ± 0.1 | 2.0 ± 0.0 | 1.6 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Impairment of physical working capacity | 2—great | 2.0 ± 0.0 | 1.1 ± 0.2 | 2.0 ± 0.0 | 1.6 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Impairment of mental working capacity | 2—great | 2.0 ± 0.0 | 1.1 ± 0.1 | 2.0 ± 0.0 | 1.6 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Memory impairment | 2—great | 2.0 ± 0.0 | 1.4 ± 0.3 | 2.0 ± 0.0 | 1.6 ± 0.1 | 0.05 < p < 0.1 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Attention concentration impairment | 2—great | 2.0 ± 0.0 | 1.3 ± 0.3 | 2.0 ± 0.0 | 1.6 ± 0.1 | 0.05 < p < 0.1 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Headache | 2—frequent | 1.4 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 0.05 < p < 0.1 |
| 1—rare | ||||||
| 0— none | ||||||
| Dizziness | 2—frequente | 1.4 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 0.05 < p < 0.1 |
| 1—rare | ||||||
| 0—non | ||||||
| Night sleeping impairment | 2—great | 1.1 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Sleepiness during day time | 2—great | 1.1 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Emotional instability (tearfulness, irritation, aggression) | 2—great | 1.1 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Depression or apathy | 2—frequent | 1.1 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | p < 0.05 |
| 1—rare | ||||||
| 0—none | ||||||
| Anxiety, suspiciousness | 1—Yes | 1.2 ± 0.2 | 0.2 ± 0.1 | 1.3 ± 0.3 | 0.3 ± 0.1 | p > 0.05 |
| 0—No | ||||||
| Heart pain | 2—great | 1.1 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.2 | 0.9 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Heart arythmia | 2—great | 1.1 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Tachycardia | 2—great | 1.1 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.3 | 1.0 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Night sweating | 1—Yes | 0.6 ± 0.1 | 0.04 ± 0.01 | 0.6 ± 0.1 | 0.3 ± 0.1 | p < 0.05 |
| 0—No | ||||||
| Joint pain | 2—great | 1.3 ± 0.3 | 0.4 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Muscle pain | 2—great | 1.3 ± 0.3 | 0.5 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Dryness of skin and epithelia | 2—great | 2.0 ± 0.0 | 1.2 ± 0.3 | 2.0 ± 0.0 | 1.2 ± 0.2 | p > 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Change of bodyweight | 1—increased | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | p > 0.05 |
| 1—decreased | ||||||
| 0—no change | ||||||
| Hair loss | 1—Yes | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | p > 0.05 |
| 0—No | ||||||
| Total average score | - | 1.2 ± 0.2 | 0.5 ± 0.1 | 1.2 ± 0.2 | 0.9 ± 0.1 | p < 0.05 |
| Question | Answer, Score | Average Score before the Trial (m ± S.D.), FFS * Group | Average Score after the Trial (m ± S.D.), FFS Group | Average Score before the Trial (m ± S.D.), Placebo ** | Average Score after the Trial (m ± S.D.), Placebo | Fisher’s Exact Test, Significance (p) |
|---|---|---|---|---|---|---|
| Weakness | 2—great | 1.3 ± 0.3 | 0.3 ± 0.3 | 1.3 ± 0.3 | 0.8 ± 0.5 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Impairment of physical working capacity | 2—great | 0.8 ± 0.5 | 0.3 ± 0.3 | 1.0 ± 0.4 | 0.7 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Impairment of mental working capacity | 2—great | 1.2 ± 0.4 | 0.3 ± 0.3 | 1.1 ± 0.4 | 0.7 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Memory impairment | 2—great | 1.1 ± 0.4 | 0.5 ± 0.3 | 1.1 ± 0.4 | 0.9 ± 0.6 | 0.05 < p < 0.1 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Attention concentration impairment | 2—great | 1.1 ± 0.4 | 0.5 ± 0.5 | 1.1 ± 0.9 | 0.9 ± 0.4 | 0.05 < p < 0.1 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Headache | 2—frequent | 0.1 ± 0.1 | 0.02 ± 0.02 | 0.2 ± 0.02 | 0.06 ± 0.06 | 0.05 < p < 0.1 |
| 1—rare | ||||||
| 0—none | ||||||
| Dizziness | 2—frequent | 0.6 ± 0.6 | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 | p > 0.05 |
| 1—rare | ||||||
| 0—none | ||||||
| Night sleeping impairment | 2—great | 0.5 ± 0.5 | 0.2 ± 0.2 | 0.5 ± 0.5 | 0.3 ± 0.3 | p > 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Sleepiness during day time | 2—great | 1.1 ± 0.4 | 0.6 ± 0.4 | 0.9 ± 0.9 | 0.5 ± 0.5 | p > 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Emotional instability (tearfulness, irritation, aggression) | 2—great | 1.6 ± 0.4 | 0.4 ± 0.4 | 1.8 ± 0.2 | 0.7 ± 0.6 | p > 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Depression or apathy | 2—frequent | 1.6 ± 0.4 | 0.4 ± 0.4 | 1.8 ± 0.2 | 0.7 ± 0.6 | p > 0.05 |
| 1—rare | ||||||
| 0—none | ||||||
| Anxiety, suspiciousness | 1—Yes | 0.4 ± 0.4 | 0.1 ± 0.1 | 0.5 ± 0.5 | 0.3 ± 0.2 | p > 0.05 |
| 0—No | ||||||
| Heart pain | 2—great | 0.2 ± 0.2 | 0.02 ± 0.02 | 0.2 ± 0.2 | 0.1 ± 0.1 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Heart arrhythmia | 2—great | 1.0 ± 0.2 | 0.4 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Tachycardia | 2—great | 1.0 ± 0.2 | 0.4 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Night sweating | 1—Yes | 0.3 ± 0.2 | 0 | 0.3 ± 0.2 | 0.03 ± 0.03 | p > 0.05 |
| 0—No | ||||||
| Joint pain | 2—great | 0.6 ± 0.2 | 0.2 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.2 | p > 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Muscle pain | 2—great | 1.0 ± 0.2 | 0.4 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Dryness of skin and epithelia | 2—great | 1.9 ± 0.1 | 1.0 ± 0.2 | 1.8 ± 0.2 | 1.7 ± 0.2 | p < 0.05 |
| 1—moderate | ||||||
| 0—absent | ||||||
| Change of bodyweight | 1—increased | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | p > 0.05 |
| 1—decreased | ||||||
| no change—0 | ||||||
| Hair loss | 1—Yes | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | p > 0.05 |
| 0—No | ||||||
| Total average score | - | 1.3 ± 0.3 | 0.3 ± 0.1 | 1.2 ± 0.2 | 0.6 ± 0.1 | p < 0.05 |
| Group | Number of Patients | Dysmetabolic Changes in the Myocardium Number of Patients | Cardiac Arrhythmias | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Partial Blockade of the Left/Right Bundle of Hiss Number of Patients | Bradycardia Number of Patients | Supraventricular Extrasystole Number of Patients | |||||||
| 30–40 Days | 50–60 Days | 30–40 Days | 50–60 Days | 30–40 Days | 50–60 Days | 30–40 Days | 50–60 Days | ||
| Post-COVID severe, FFS | 64 | 64 | 41 (64. 1%) * | 11 | 8 (72.7%) * | 8 | 4 (50.0%) * | 21 | 8 (38.0%) |
| Post-COVID severe, placebo | 27 | 27 | 19 (70.4%) * | 5 | 4 (80.0%) * | 9 | 5 (55.5%) * | 9 | 4 (44.4%) |
| Post-COVID moderate, FFS | 68 | 40 | 17 (42.5%) * | 8 | 3 (37.5%) * | 3 | 1 (33.3%) * | 9 | 4 (44.4%) |
| Post-COVID moderate, placebo | 29 | 19 | 16 (84.2%) * | 4 | 3 (75.0%) * | 3 | 2 (66.6%) * | 4 | 3 (75%) |
| Group | Number of Patients | Number of Patients on Respiratory Support before the Trial * | Number of Patients on Respiratory Support after the Trial * | Subjective Dyspnea, Borg’s Score before the Trial | Subjective Dyspnea, Borg’s Score after the Trial | 6MWT, Number of Patients with Oxygen Desaturation > 4%, (%) before the Trial | 6MWT, Number of Patients with Oxygen Desaturation > 4%, (%) after the Trial |
|---|---|---|---|---|---|---|---|
| Group 1, severe COVID, post-COVID, FFS * | 64 | 21 | 6 (28.5%) ** | 7.1 ± 1.1 | 9.4 ± 1.9 1 | 15 | 8 (53.3%) ** |
| Group 2, severe COVID, post-COVID, placebo | 27 | 9 | 4 (44.4%) ** | 7.3 ± 0.8 | 9.1 ± 1.4 1 | 7 | 5 (71.4%) ** |
| Group 3, moderate COVID, post-COVID, FFS | 68 | 0 | 0 | 10.8 ± 1.3 | 11.8 ± 1.3 1,2 | ND | ND |
| Group 4, moderate COVID, post-COVID, placebo | 29 | 0 | 0 | 10.5 ± 1.0 | 10.8 ± 1.3 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharaeva, Z.; Shokarova, A.; Shomakhova, Z.; Ibragimova, G.; Trakhtman, P.; Trakhtman, I.; Chung, J.; Mayer, W.; De Luca, C.; Korkina, L. Fermented Carica papaya and Morinda citrifolia as Perspective Food Supplements for the Treatment of Post-COVID Symptoms: Randomized Placebo-Controlled Clinical Laboratory Study. Nutrients 2022, 14, 2203. https://doi.org/10.3390/nu14112203
Kharaeva Z, Shokarova A, Shomakhova Z, Ibragimova G, Trakhtman P, Trakhtman I, Chung J, Mayer W, De Luca C, Korkina L. Fermented Carica papaya and Morinda citrifolia as Perspective Food Supplements for the Treatment of Post-COVID Symptoms: Randomized Placebo-Controlled Clinical Laboratory Study. Nutrients. 2022; 14(11):2203. https://doi.org/10.3390/nu14112203
Chicago/Turabian StyleKharaeva, Zaira, Albina Shokarova, Zalina Shomakhova, Galina Ibragimova, Pavel Trakhtman, Ilya Trakhtman, Jessie Chung, Wolfgang Mayer, Chiara De Luca, and Liudmila Korkina. 2022. "Fermented Carica papaya and Morinda citrifolia as Perspective Food Supplements for the Treatment of Post-COVID Symptoms: Randomized Placebo-Controlled Clinical Laboratory Study" Nutrients 14, no. 11: 2203. https://doi.org/10.3390/nu14112203






