Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability Assay
2.3. Annexin V Assay
2.4. Protein Extraction and Western Blot Analysis
2.5. Wound-Healing Scratch Assay
2.6. Transmigration Assay
2.7. Statistical Analysis
3. Results
3.1. OLE Reduces Seminoma Cells Viability, by Promoting Apoptosis
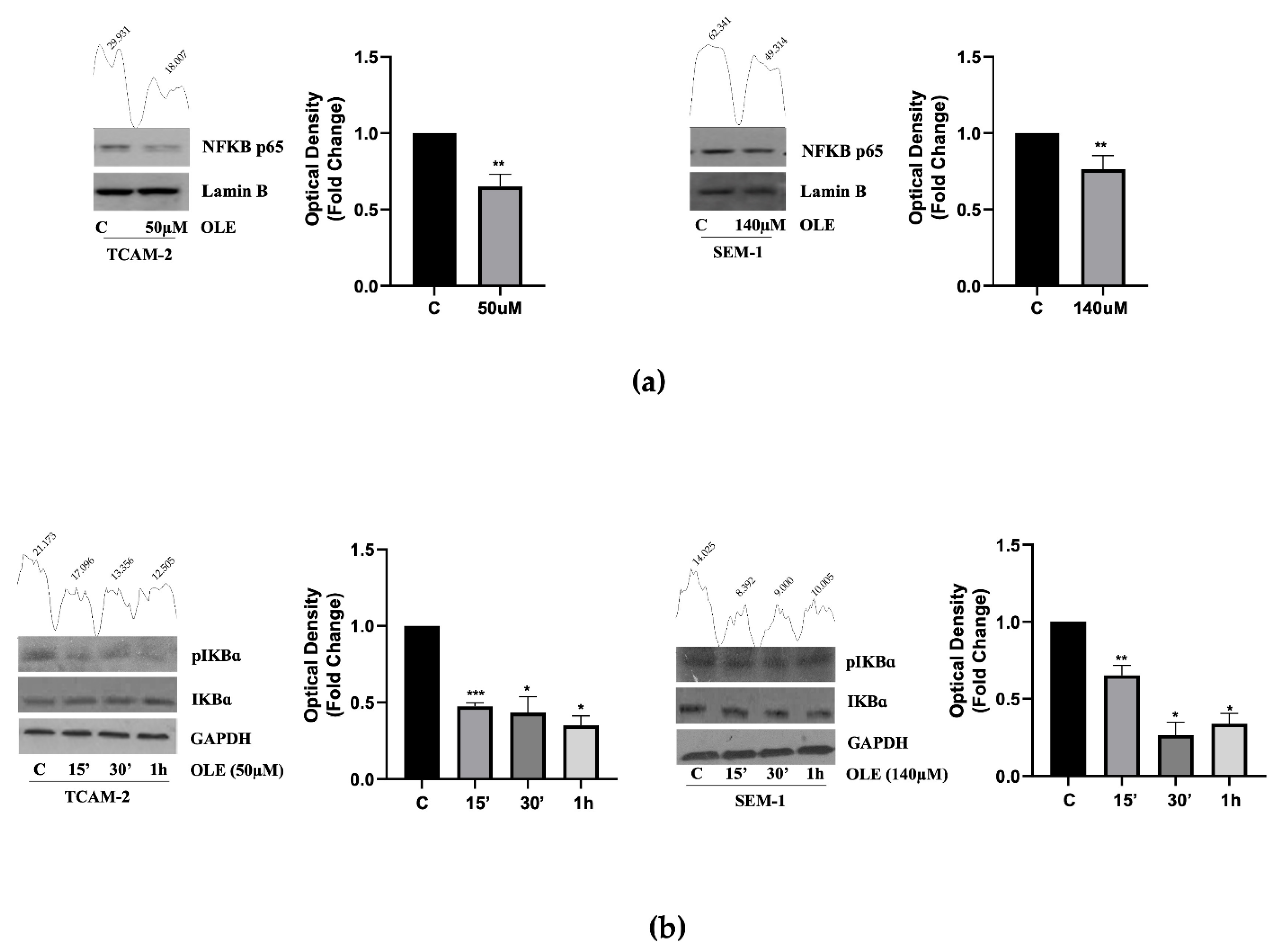
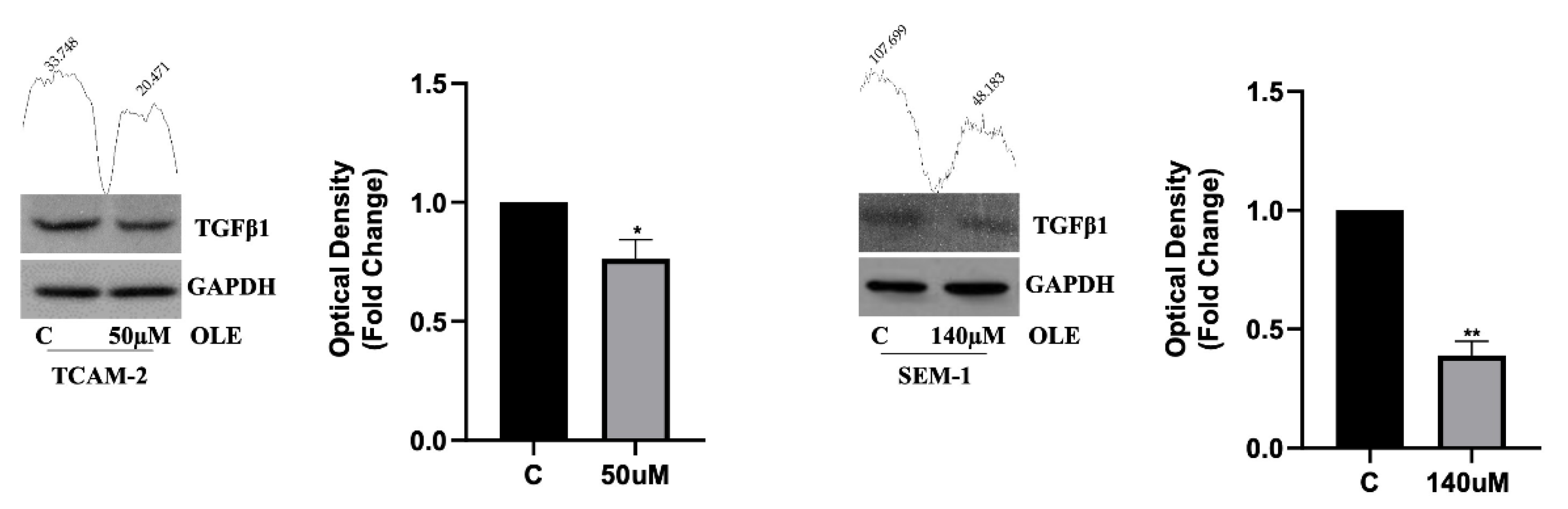
3.2. In Seminoma Cells, OLE Reduces NF-κB Nuclear Translocation by Affecting Its Upstream Pathway
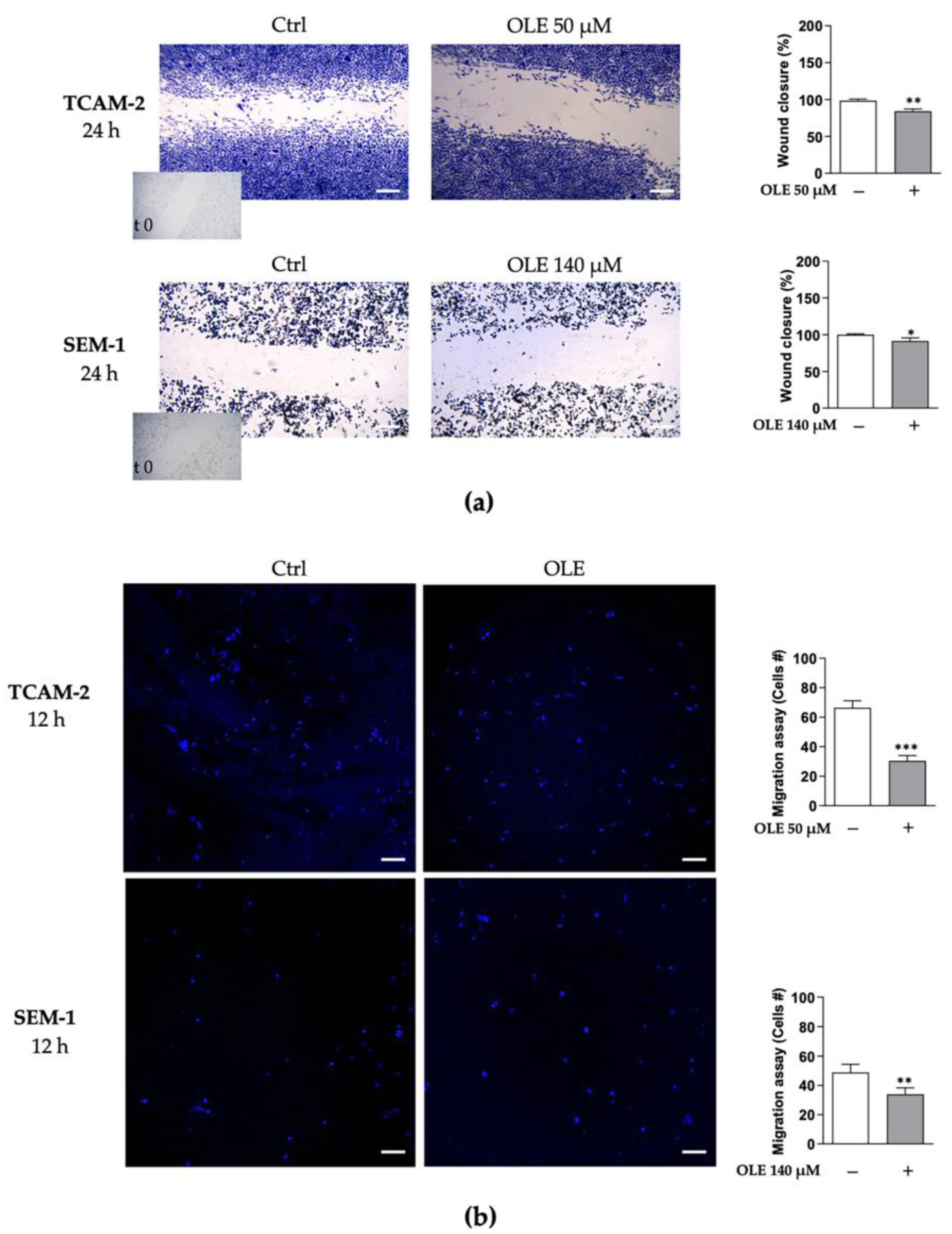
3.3. OLE Counteracts Seminoma Cells’ Migratory Capability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elhrlich, Y.; Margel, D.; Lubin, M.A.; Baniel, J. Advances in the treatment of testicular cancer. Transl. Androl. Urol. 2015, 4, 381–390. [Google Scholar] [CrossRef]
- Shen, A.H.; Howell, D.; Edwards, E.; Warde, P.; Matthew, A.; Jones, J.M. The experience of patients with early-stage testicular cancer during the transition from active treatment to follow-up surveillance. Urol. Oncol. 2016, 34, 168.e11–168.e20. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, A.A.; Rusner, C.; Britton, T.; Braulin, M.; McGlynn, K.A.; Stang, A. Testicular cancer among US men aged 50 years and older. Cancer Epidemiol. 2018, 55, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.; Svingen, T.; Miles, H.; Chetty, T.; Stukenborg, J.B.; Mitchell, R.T. Environmental Impacts on Male Reproductive Development: Lessons from Experimental Models. Horm. Res. Paediatr. 2021, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.; Eggener, S.E.; Bass, E.B.; Chelnick, D.M.; Daneshmand, S.; Feldman, D.; Gilligan, T.; Karam, J.A.; Leibovich, B.; Liauw, S.L.; et al. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. J. Urol. 2019, 202, 272–281. [Google Scholar] [CrossRef] [Green Version]
- El-Zaatari, Z.M.; Ro, J.Y. Mediastinal Germ Cell Tumors: A Review and Update on Pathologic, Clinical, and Molecular Features. Adv. Anat. Pathol. 2021, 28, 335–350. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Duca, Y.; Barbagallo, F.; Burgio, G.; Compagnone, M.; Di Cataldo, A.; Calogero, A.E.; Condorelli, R.A. Hypogonadism and sexual dysfunction in testicular tumor survivors. Front. Endocrinol. 2019, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Paoli, D.; Pallotti, F.; Lenzi, A.; Lombardo, F. Fatherhood and sperm DNA damage in testicular cancer patients. Front. Endocrinol. 2018, 9, 506. [Google Scholar] [CrossRef]
- Ion, D.; Niculescu, A.G.; Pădurau, D.N.; Andronic, O.; Mușat, F.; Grumezescu, A.M.; Bolocan, A. An Up-to-Date Review of Natural Nanoparticles for Cancer Management. Pharmaceutics 2022, 14, 18. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure Properties, Acquisition Protocols, and Biological Activities of Oleuropein Aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, C.; Hull, J. The Anti-cancer Effect of Olea europaea L. Products: A Review. Curr. Nutr. Rep. 2021, 10, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Aktas, H.G.; Ayan, H. Oleuropein: A Potential Inhibitor for Prostate Cancer Cell Motility by Blocking Voltage-Gated Sodium Channels. Nutr. Cancer 2021, 73, 1758–1767. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [Green Version]
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative effect of oleuropein in prostate cell lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Sheikhshabani, H.S.; Amini-Farsani, Z.; Rahmati, S.; Jazaeri, A.; Mohammadi-Samani, M.; Asghaezade, S. Oleuropein reduces cisplatin resistance in ovarian cancer by targeting apoptotic pathway regulators. Life Sci. 2021, 278, 119525. [Google Scholar] [CrossRef]
- Benot-Dominguez, R.; Tupone, M.G.; Castelli, V.; D’Angelo, M.; Benedetti, E.; Quintiliani, M.; Cinque, B.; Forte, I.M.; Cifone, M.G.; Ippoliti, R.; et al. Olive leaf extract impairs mitochondria by pro-oxidant activity in MDA-MB-231 and OVCAR-3 cancer cells. Biomed. Pharmacother. 2021, 134, 111139. [Google Scholar] [CrossRef]
- Xing, Y.; Cui, D.; Wang, S.; Wang, P.; Xing, X.; Li, H. Oleuropein represses the radiation resistance of ovarian cancer by inhibiting hypoxia and microRNA-299-targetted heparanase expression. Food Funct. 2017, 8, 2857–2864. [Google Scholar] [CrossRef]
- Russell, S.M.; Lechner, M.G.; Mokashi, A.; Megiel, C.; Jang, J.K.; Taylor, C.R.; Looijenga, L.H.J.; French, C.A.; Epstein, A.L. Establishment and Characterization of a new Human Extragonadal Germ Cell Line, SEM-1, and its Comparison with TCam-2 and JKT-1. Urology 2013, 81, 464.e1–464.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcidiacono, B.; Iiritano, S.; Chiefari, E.; Brunetti, F.S.; Gu, G.; Foti, D.P.; Brunetti, A. Cooperation between HMGA1, PDX-1, and MafA is Essential for Glucose-Induced Insulin Transcription in Pancreatic Beta Cells. Front. Endocrinol. 2015, 5, 237. [Google Scholar] [CrossRef] [Green Version]
- Vizza, D.; Perri, A.; Toteda, G.; Lupinacci, S.; Leone, F.; Gigliotti, P.; Lofaro, D.; La Russa, A.; Bonofiglio, R. Nerve growth factor exposure promotes tubular epithelial-mesenchymal transition via TGF-β1 signaling activation. Growth Factors 2015, 33, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lupinacci, S.; Perri, A.; Toteda, G.; Vizza, D.; Puoci, F.; Parisi, O.I.; Giordano, F.; Lofaro, D.; La Russa, A.; Bonifiglio, M.; et al. Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling. Cell Biol. Toxicol. 2019, 35, 95–109. [Google Scholar] [CrossRef]
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, Distribution, Metabolism, and Excretion of the Main Olive Tree Phenols and Polyphenols: A Literature Review. J. Agric. Food Chem. 2021, 69, 5281–5296. [Google Scholar] [CrossRef] [PubMed]
- Grobholz, R.; Zentgraf, H.; Köhrmann, K.U.; Bleyl, U. Bax, Bcl-2, Fas and Fas-L antigen expression in human seminoma: Correlation with the apoptotic index. APMIS 2002, 110, 724–732. [Google Scholar] [CrossRef]
- Hinz, M.; Krappmann, D.; Eichten, A.; Heder, A.; Scheidereit, C.; Strauss, M. NF-κB Function in Growth Control: Regulation of Cyclin D1 Expression and G0/G1-to-S-Phase Transition. Mol. Cell. Biol. 1999, 19, 2690–2698. [Google Scholar] [CrossRef] [Green Version]
- Vizza, D.; Lupinacci, S.; Toteda, G.; Puoci, G.; Parisi, O.I.; De Bartolo, A.; Lofaro, D.; Scrivano, L.; Bonofiglio, R.; La Russa, A.; et al. An Olive Leaf Extract Rich in Polyphenols Promotes Apoptosis in Cervical Cancer Cells by Upregulating p21Cip/WAF1 Gene Expression. Nutr. Cancer 2019, 71, 320–333. [Google Scholar] [CrossRef]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic Anti-Breast-Cancer Effects of Combined Treatment with Oleuropein and Doxorubicin In Vivo. Altern. Ther. Health Med. 2019, 25, 17–24. [Google Scholar]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Tang, F.R. Oleuropein induces apoptosis via abrogating NFkappaB activation cascade in estrogen receptor-negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elamin, M.H.; Daghetani, M.H.; Omer, S.A.; Al-Olayan, E.M.; Virk, P.; Mohammed, O.B. Oleuropein Induces Anti-metastatic Effects in Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 13, 4555–4559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chovanec, M.; Abu Zaid, M.; Hanna, N.; El-Kouri, N.; Einhorn, L.H.; Albany, C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann. Oncol. 2017, 28, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An updated review on the potential antineoplastic actions of oleuropein. Phytother. Res. 2022, 36, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has antibreast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, X.; Du, L. P38 MAP kinase is involved in oleuropein-induced apoptosis in A549 cells by a mitochondrial apoptotic cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein inhibits STAT3, activates the apoptotic machinery, and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Santibanez, J.F.; Obradović, H.; Kukolj, T.; Krstić, J. Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev. Dyn. 2018, 247, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Teveroni, E.; Di Nicuolo, F.; Bianchetti, G.; Epstein, A.L.; Grande, G.; Maulucci, G.; De Spirito, M.; Pontecorvi, A.; Milardi, D.; Mancini, F. Nuclear Localization of PTTG1 Promotes Migration and Invasion of Seminoma Tumor through Activation of MMP-2. Cancers 2021, 13, 212. [Google Scholar] [CrossRef]
- Cui, L.; Ren, T.; Zhao, H.; Chen, S.; Zheng, M.; Gao, X.; Feng, D.; Yang, L.; Jin, X.; Zhuo, R. Suppression of PTTG1 inhibits cell angiogenesis, migration and invasion in glioma cells. Med. Oncol. 2020, 37, 73. [Google Scholar] [CrossRef]
- Perri, A.; Lofaro, D.; Izzo, G.; Aquino, B.; Bitonti, M.; Ciambrone, G.; La Vignera, S.; Pozza, C.; Gianfrilli, D.; Aversa, A. The Risky Health Behaviours of Male Adolescents in the Southern Italian Region: Implications for Sexual and Reproductive Disease. J. Clin. Med. 2019, 8, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cell Lines | IC50 Value (μM) | 95% Confidence Interval |
|---|---|---|
| TCAM-2 | 50 | 62.25–108.5 |
| SEM-1 | 140 | 169.5–32.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossio, S.; Perri, A.; Malivindi, R.; Giordano, F.; Rago, V.; Mirabelli, M.; Salatino, A.; Brunetti, A.; Greco, E.A.; Aversa, A. Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients 2022, 14, 2323. https://doi.org/10.3390/nu14112323
Bossio S, Perri A, Malivindi R, Giordano F, Rago V, Mirabelli M, Salatino A, Brunetti A, Greco EA, Aversa A. Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients. 2022; 14(11):2323. https://doi.org/10.3390/nu14112323
Chicago/Turabian StyleBossio, Sabrina, Anna Perri, Rocco Malivindi, Francesca Giordano, Vittoria Rago, Maria Mirabelli, Alessandro Salatino, Antonio Brunetti, Emanuela Alessandra Greco, and Antonio Aversa. 2022. "Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells" Nutrients 14, no. 11: 2323. https://doi.org/10.3390/nu14112323
APA StyleBossio, S., Perri, A., Malivindi, R., Giordano, F., Rago, V., Mirabelli, M., Salatino, A., Brunetti, A., Greco, E. A., & Aversa, A. (2022). Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients, 14(11), 2323. https://doi.org/10.3390/nu14112323












