Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- A structured interview;
- Anthropometric measurements;
- Serum biochemical analysis.
2.2. Determination of Biochemical Parameters
2.3. Analysis of Serum Element Concentrations
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. The Concentrations of the Tested Elements at the Surgery and during Chemotherapy
3.3. The Concentrations of the Tested Elements by the Stage of Therapy and the Type of Cancer
3.4. The Correlations between the Concentrations of the Tested Elements and the Stage of Therapy
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GLOBOCAN 2020, Cancer Incidence and Mortality Worldwide in 2020. International Agency of Research on Cancer. WHO. 2020. Available online: https://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=968#WOMEN (accessed on 1 January 2020).
- Krajowy Rejestr Nowotworów. Available online: https://epid.coi.waw.pl/krn/ (accessed on 1 January 2020).
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogliano, V.J. International Agency for Research on Cancer (IARC). Toxicol. Pathol. 2016, 34, 405–406. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Basta, A.; Bidziński, M.; Bieńkiewicz, A.; Blecharz, P.; Bodnar, L.; Jach, R.; Knapp, P.; Kojs, Z.; Kotarski, J.; Markowska, J.; et al. Zalecenia Polskiego Towarzystwa Ginekologii Onkologicznej dotyczące diagnostyki i leczenia raka jajnika. Curr. Gynecol. Oncol. 2017, 15, 5–23. [Google Scholar] [CrossRef]
- Zirpoli, G.R.; Brennan, P.M.; Hong, C.C.; McCann, S.E.; Ciupak, G.; Davis, W.; Unger, J.M.; Budd, G.T.; Hershman, D.L.; Moore, H.C.; et al. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast. Cancer Res. Treat. 2013, 137, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in Oncological Intervention. Nutrients 2016, 2, 163. [Google Scholar] [CrossRef] [Green Version]
- Ströhle, A.; Zänker, K.; Hahn, A. Nutrition in oncology: The case of micronutrients (review). Oncol. Rep. 2010, 24, 815–828. [Google Scholar] [CrossRef] [Green Version]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef]
- Muecke, R.; Micke, O.; Schomburg, L.; Glatzel, M.; Reichl, B.; Kisters, K.; Schaefer, U.; Eich, H.T.; Fakhrian, K.; Adamietz, I.A.; et al. German Working Group Trace Elements and Electrolytes in Oncology-AKTE. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: Follow-up analysis of the survival data 6 years after cessation of randomization. Integr. Cancer Ther. 2014, 13, 463–467. [Google Scholar]
- Jaakkola, K.; Lähteenmäki, P.; Laakso, J.; Harju, E.; Tykkä, H.; Mahlberg, K. Treatment with antioxidant and other nutrients in combination with chemotherapy and irradiation in patients with small-cell lung cancer. Anticancer Res. 1992, 12, 599–606. [Google Scholar]
- Jatoi, A.; Williams, B.A.; Marks, R.; Nichols, F.C.; Aubry, M.C.; Wampfler, J.; Yang, P. Exploring vitamin and mineral supplementation and purported clinical effects in patients with small cell lung cancer: Results from the Mayo Clinic lung cancer cohort. Nutr. Cancer. 2005, 51, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Williams, B.; Nichols, F.; Aubry, M.C.; Wampfler, J.; Yang, P. Is voluntary vitamin and mineral supplementation associated with better outcome in non-small cell lung cancer patients? Results from the Mayo Clinic lung cancer cohort. Lung Cancer 2005, 49, 77–84. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J. Clin. 2005, 55, 319–321. [Google Scholar] [CrossRef]
- Block, K.; Koch, A.; Mead, M.; Newman, R.A.; Gyllenhaal, C. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2009, 101, 124–125. [Google Scholar] [CrossRef] [Green Version]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2015, 15, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Lappano, R.; Malaguarnera, R.; Belfiore, A.; Maggiolini, M. Recent advances on the stimulatory effects of metals in breast cancer. Molecular and Cellular Endocrinology. Mol. Cell. Endocrinol. 2017, 5, 49–56. [Google Scholar] [CrossRef]
- Caglayan, A.; Katlan, D.C.; Tuncer, Z.S.; Yüce, K. Evaluation of trace elements associated with antioxidant enzymes in blood of primary epithelial ovarian cancer patients. J. Trace Elem. Med. Biol. 2019, 52, 254–262. [Google Scholar] [CrossRef]
- Gurzau, E.; Neagu, C.; Gurzau, A. Essential metals—case study on iron. Ecotoxicol. Environ. Saf. 2003, 56, 190–200. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Czeczot, H.; Majewska, M. Kadm—zagrożenie i skutki zdrowotne. Toksykologia 2010, 66, 243–250. [Google Scholar]
- Liao, Y.; Cao, H.; Xia, B.; Xiao, Q.; Liu, P.; Hu, G.; Zhang, C. Changes in Trace Element Contents and Morphology in Bones of Duck Exposed to Molybdenum or/and Cadmium. Biol. Trace Elem. Res. 2017, 175, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.; Fowler, B.; Nordberg, M. Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Brodziak-Dopierala, B.; Kwapuliński, J.; Sobczyk, K.; Kowol, J. The occurrence of nickel and other elements in tissues of the hip joint. Ecotoxicol. Environ. Saf. 2011, 74, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Krzysik, M.; Biernat, J.; Grajeta, M. Wpływ wybranych składników odżywczych pożywienia na funkcjonowanie układu odpornościowego Cz. II. Immunomodulacyjne działanie witamin i pierwiastków śladowych na organizm człowieka. Adv. Clin. Exp. Med. 2007, 16, 123–133. [Google Scholar]
- Da Silva, A.; Barrocas, P.; Jacob, S.; Moreira, J. Dietary intake and health effects of selected toxic elements. Braz. J. Plant Physiol. 2005, 17, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roychoudhury, S.; Nath, S.; Massanyi, P.; Stawarz, R.; Kacaniova, M.; Kolesarova, A. Copper-induced changes in reproductive functions: In vivo and in vitro effects. Physiol. Res. 2016, 65, 11–22. [Google Scholar] [CrossRef]
- Atakul, T.; Altinkaya, S.O.; Abas, B.I.; Yenisev, C. Serum Copper and Zinc Levels in Patients with Endometrial Cancer. Biol. Trace Elem. Res. 2019, 195, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Rzymski, P.; Tomczyk, K.; Kozak, L.; Poniedziałek, B. Metal accumulation in the human uterus varies by pathology and smoking status. Fertil. Steril. 2016, 105, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Qdaisat, A.; Soliman, P.T.; Ramondetta, L.; Lopez, G.; Narayanan, S.; Zhou, S.; Cohen, L.; Bruera, E.; Yeung, S.J. Hypomagnesemia and Survival in Patients with Ovarian Cancer Who Received Chemotherapy with Carboplatin. Oncologist 2019, 24, e312–e317. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, S.; Maier, J.A.M. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, 92–100. [Google Scholar] [CrossRef]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin Nephrotoxicity: A Review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Watanabe, S.; Ohtsubo, A.; Shoji, S.; Ishikawa, D.; Tanaka, T.; Nozaki, K.; Kondo, R.; Okajima, M.; Miura, S.; et al. Nephrotoxicity of cis-platin combination chemotherapy in thoracic malig-nancy patients with CKD risk factors. BMC Cancer 2016, 16, 222. [Google Scholar] [CrossRef] [Green Version]
- Rademaker-Lakhai, J.M.; Crul, M.; Zuur, L.; Baas, P.; Beijnen, J.H.; Simis, Y.J.; van Zandwijk, N.; Schellens, J.H. Relationship between cisplatin administration and the development of ototoxicity. J. Clin. Oncol. 2006, 24, 918–924. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Cisplatin ototoxicity and protection: Clinical and experimental studies. Tohoku J. Exp. Med. 2009, 219, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Theunissen, E.A.; Zuur, C.L.; Bosma, S.C.; Lopez-Yurda, M.; Hauptmann, M.; van der Baan, S.; de Boer, J.P.; van der Molen, L.; Rasch, C.R.; Dreschler, W.A.; et al. Long-term hearing loss after chemoradiation in patients with head and neck cancer. Laryngoscope 2014, 124, 2720–2725. [Google Scholar] [CrossRef]
- Travis, L.B.; Fossa, S.D.; Sesso, H.D.; Frisina, R.D.; Herrmann, D.N.; Beard, C.J.; Feldman, D.R.; Pagliaro, L.C.; Miller, R.C.; Vaughn, D.J.; et al. Platinum Study Group, Chemotherapy-induced peripheral neurotoxicity and ototoxicity: New paradigms for translational genomics. J. Natl. Cancer Inst. 2014, 106, dju044. [Google Scholar] [CrossRef] [Green Version]
- Sprauten, M.; Darrah, T.H.; Peterson, D.R.; Campbell, M.E.; Hannigan, R.E.; Cvancarova, M.; Beard, C.; Haugnes, H.S.; Fosså, S.D.; Oldenburg, J.; et al. Impact of long-term serum platinum concentra-tions on neuro- and ototoxicity in Cisplatin-treated sur-vivors of testicular cancer. J. Clin. Oncol. 2012, 30, 300–307. [Google Scholar] [CrossRef]
- Hodgkinson, E.; Neville-Webbe, H.L.; Coleman, R.E. Magnesium depletion in patients receiving cisplatin-based chemotherapy. Clin. Oncol. 2006, 18, 710–718. [Google Scholar] [CrossRef]
- Taguchi, T.; Nazneen, A.; Abid, M.R.; Razzaque, M.S. Cisplatin-associated nephrotoxicity and patho-logical events. Contrib. Nephrol. 2005, 148, 107–121. [Google Scholar] [CrossRef]
- Jian, J.; Yang, Q.; Dai, J.; Eckard, J.; Axelrod, D.; Smith, J.; Huang, X. Effects of iron deficiency and iron overload on angiogenesis and oxidative stress—A potential dual role for iron in breast cancer. Free Radic. Biol. Med. 2011, 50, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Huang, X. Does iron have a role in breast cancer? Lancet Oncol. 2008, 9, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.; Ogihara, T.; Hayashi, M.; Nakagawa, T.; Ishizaki, Y.; Kume, M.; Yano, I.; Niigata, R.; Hiraoka, J.; Yasui, H.; et al. Association between dexamethasone treatment and alterations in serum concentrations of trace metals. Pharmazie 2020, 75, 218–222. [Google Scholar] [CrossRef]
- Nakamura, T.; Takahashi, M.; Niigata, R.; Yamashita, K.; Kume, M.; Hirai, M.; Yasui, H. Changes in blood concentrations of trace metals in cancer patients receiving cisplatin-based chemotherapy. Biomed. Rep. 2016, 5, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Sartori, S.; Nielsen, I.; Masotti, M.; Malacarne, P. Early and late hyperferremia during cisplatin chemotherapy. J. Chemother. 1991, 3, 45–50. [Google Scholar] [CrossRef]
- Sartori, S.; Nielsen, I.; Malacarne, P. Variazioni del livello di sideremia durante chemioterapia con cisplatino. Risultati preliminari Changes in blood iron levels during cisplatin chemotherapy. Preliminary results. Medicina 1990, 10, 48–49. [Google Scholar]
- Pollera, C.F.; Ameglio, F.; Reina, S.; Nardi, M.; Abbolito, M.R.; Parracino, C. Changes in serum iron levels following very high-dose cisplatin. Cancer Chemother. Pharmacol. 1987, 19, 257–260. [Google Scholar] [CrossRef]
- Omoyajowo, K.; Asaolu, M.; Adenekan, O.; Ogidan, J.; Olaniyan, K.; Idowu, I.; Akande, J.; Babalola, O. Investigation of plasma electrolyte levels in selected uterine cancer patients. Sci. J. Res. Rev. 2017, 4, 63–69. [Google Scholar] [CrossRef]

| Variable | Level of Education | p | |||
| Cancer Type | Primary | Vocational | Secondary | Higher | |
| Ovarian cancer | 3 | 5 | 13 | 5 | 0.59 |
| Endometrial cancer | 1 | 8 | 11 | 4 | |
| Marital status | p | ||||
| Cancer type | Unmarried | Married | Widowed | Divorced | |
| Ovarian cancer | 3 | 10 | 11 | 2 | 0.49 |
| Endometrial cancer | 2 | 5 | 15 | 2 | |
| Employment status | p | ||||
| Cancer type | Employed | Unemployed | Sickness pension | Pension | |
| Ovarian cancer | 9 | 5 | 11 | 1 | 0.79 |
| Endometrial cancer | 6 | 4 | 12 | 2 | |
| Place of residence | p | ||||
| Cancer type | Village | City with a population under 10,000 | City with a population of 10,000–100,000 | City with a population of >100,000 | |
| Ovarian cancer | 4 | 4 | 9 | 9 | 0.48 |
| Endometrial cancer | 4 | 8 | 6 | 6 | |
| BMI | p | ||||
| Cancer type | Normal weight | Obese | Overweight | Underweight | |
| Ovarian cancer | 30 | 19 | 28 | 3 | 0.0032 |
| Endometrial cancer | 17 | 38 | 15 | 4 | |
| Variable | Ovarian Cancer | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery (n = 11) | 1st Cycle (n = 26) | 3rd Cycle (n = 24) | 6th Cycle (n = 20) | |||||||||||||
| M | Me | SD | 25–75 P | M | Me | SD | 25–75 P | M | Me | SD | 25–75 P | M | Me | SD | 25–75 P | |
| Ca | 122.2130 | 118.9650 | 15.4327 | 107.826–130.972 | 119.8880 | 115.6750 | 18.0889 | 107.096–126.459 | 132.8760 | 131.8890 | 24.1160 | 116.873–153.754 | 125.7940 | 120.3780 | 19.4164 | 113.917–144.401 |
| Cd | 0.0004 | 0.0003 | 0.0002 | 0.000330–0.000330 | 0.0004 | 0.0003 | 0.0002 | 0.000330–0.000330 | 0.0011 | 0.0003 | 0.0021 | 0.000330–0.000330 | 0.0011 | 0.0003 | 0.0022 | 0.000330–0.000330 |
| Cu | 1.7360 | 1.8380 | 0.4284 | 1.434–2.106 | 1.5610 | 1.4870 | 0.3109 | 1.383–1.738 | 1.4860 | 1.4450 | 0.4025 | 1.195–1.668 | 1.5660 | 1.5300 | 0.2756 | 1.402–1.679 |
| Fe | 1.1620 | 1.1000 | 0.6060 | 0.758–1.154 | 1.2270 | 1.1400 | 0.6332 | 0.816–1.528 | 2.2090 | 1.8170 | 1.1307 | 1.499–2.649 | 2.2000 | 1.9500 | 0.9382 | 1.437–2.967 |
| Mg | 24.5280 | 24.7090 | 5.1852 | 22.212–26.020 | 22.5440 | 21.4910 | 3.9238 | 20.437–25.036 | 24.0880 | 24.9970 | 3.5235 | 20.900–26.263 | 22.8180 | 22.9270 | 2.4318 | 21.280–25.038 |
| Na | 3918.0500 | 3996.5980 | 311.9501 | 3657.605–4102.884 | 3368.0200 | 3463.8950 | 376.2940 | 3166.725–3587.460 | 3617.3110 | 3720.3850 | 371.1486 | 3335.260–3913.682 | 3581.6050 | 3629.4930 | 338.6305 | 3341.971–3847.588 |
| Ni 2 | 0.0015 | 0.0008 | 0.0020 | 0.000770–0.000770 | 0.0029 | 0.0008 | 0.0050 | 0.000770–0.000770 | 0.0064 | 0.0008 | 0.0072 | 0.000770–0.0121 | 0.0043 | 0.0008 | 0.0061 | 0.000770–0.00732 |
| P | 369.4810 | 401.3790 | 149.9710 | 214.098–451.331 | 228.2620 | 183.8610 | 111.5522 | 162.129–229.317 | 246.4140 | 218.7890 | 115.5608 | 184.970–259.033 | 278.5980 | 209.3050 | 118.9908 | 193.992–398.847 |
| Sr | 0.0423 | 0.0291 | 0.0433 | 0.00972–0.0638 | 0.0542 | 0.0575 | 0.0318 | 0.0322–0.0776 | 0.0588 | 0.0597 | 0.0316 | 0.0385–0.0852 | 0.0488 | 0.0491 | 0.0185 | 0.0324–0.0610 |
| Zn | 2.3530 | 1.6960 | 1.3231 | 1.177–3.654 | 2.4600 | 2.0950 | 1.9281 | 1.203–2.677 | 2.4600 | 2.3950 | 1.1458 | 1.412–3.202 | 2.4240 | 2.0430 | 1.1056 | 1.552–3.330 |
| Variable | Endometrial cancer | |||||||||||||||
| Surgery (n = 10) | 1st cycle (n = 21) | 3rd cycle (n = 24) | 6th cycle (n = 18) | |||||||||||||
| Ca | 121.907 | 119.281 | 12.4636 | 115.115–124.657 | 123.221 | 120.833 | 16.454 | 115.850–128.818 | 121.806 | 113.47 | 28.4485 | 96.114–136.605 | 121.277 | 122.043 | 21.9249 | 106.810–136.223 |
| Cd | 0.00137 | 0.00033 | 0.0026 | 0.000330–0.000330 | 0.001850 | 0.00033 | 0.003668 | 0.000330–0.000330 | 0.000881 | 0.00033 | 0.002677 | 0.000330–0.000330 | 0.00045 | 0.00033 | 0.00051 | 0.000330–0.000330 |
| Cu | 1.421 | 1.327 | 0.3331 | 1.125–1.691 | 1.556 | 1.548 | 0.2484 | 1.375–1.699 | 1.488 | 1.486 | 0.2888 | 1.252–1.651 | 1.53 | 1.511 | 0.3519 | 1.245–1.621 |
| Fe | 1.175 | 1.215 | 0.6189 | 0.835–1.353 | 1.222 | 1.228 | 0.524 | 0.926–1.334 | 1.829 | 1.683 | 0.8677 | 1.322–2.291 | 1.987 | 1.98 | 0.8455 | 1.327–2.383 |
| Mg | 26.831 | 27.291 | 2.5907 | 26.580–28.243 | 22.072 | 22.054 | 4.5432 | 19.263–24.022 | 21.438 | 22.755 | 3.9275 | 18.905–23.472 | 22.082 | 22.271 | 3.4344 | 20.567–23.940 |
| Na | 3746.173 | 3837.134 | 393.5432 | 3629.755–3956.274 | 3372.38 | 3441.793 | 339.4302 | 3135.612–3678.553 | 3384.109 | 3405.028 | 462.3386 | 3031.447–3813.382 | 3560.183 | 3714.223 | 549.641 | 3091.267–3847.698 |
| Ni 2 | 0.00077 | 0.00077 | 0 | 0.000770–0.000770 | 0.00352 | 0.00077 | 0.005297 | 0.000770–0.00219 | 0.00335 | 0.00077 | 0.004522 | 0.000770–0.00403 | 0.00302 | 0.00077 | 0.004937 | 0.000770–0.00357 |
| P | 316.265 | 244.567 | 162.7694 | 189.481–510.812 | 195.062 | 197.239 | 49.8238 | 158.817–215.579 | 247.982 | 199.012 | 116.664 | 169.353–304.745 | 232.496 | 202.774 | 106.6331 | 173.238–218.308 |
| Sr | 0.0265 | 0.0138 | 0.03619 | 0.00341–0.0393 | 0.0646 | 0.0666 | 0.02715 | 0.0455–0.0798 | 0.0578 | 0.0518 | 0.03343 | 0.0342–0.0816 | 0.0561 | 0.0455 | 0.03688 | 0.0278–0.0750 |
| Zn | 2.797 | 2.404 | 1.5473 | 1.937–2.888 | 2.177 | 2.175 | 0.8629 | 1.539–2.414 | 2.368 | 2.126 | 1.2732 | 1.447–2.939 | 2.635 | 2.174 | 1.5041 | 1.478–3.116 |
| Stage of Therapy | Element | Ovarian Cancer | Endometrial Cancer | * | ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | M | Me | 25–75 P | Min | M | Me | 25–75 P | ||||
| Surgery | Ca | 4.658 | 4.799 | 4.779 | 4.680–4.875 | 4.668 | 4.799 | 4.781 | 4.746–4.826 | 0.267 | 0.135 |
| 1st cycle | 4.55 | 4.776 | 4.751 | 4.674–4.840 | 4.549 | 4.806 | 4.794 | 4.752–4.858 | |||
| 3rd cycle | 4.477 | 4.873 | 4.882 | 4.761–5.035 | 4.498 | 4.778 | 4.731 | 4.566–4.917 | |||
| 6th cycle | 4.556 | 4.824 | 4.791 | 4.735–4.973 | 4.433 | 4.783 | 4.804 | 4.671–4.914 | |||
| Surgery | Cd | −6.928 | −6.928 | −6.928 | −6.928–−6.928 | −5.98 | −5.374 | −5.374 | −5.980–−4.768 | 0.47 | 0.96 |
| 1st cycle | −6.978 | −6.789 | −6.789 | −6.978–−6.600 | −6.18 | −4.981 | −4.67 | −5.507–−4.454 | |||
| 3rd cycle | −5.209 | −5.035 | −5.033 | −5.165–−4.905 | −7.733 | −6.021 | −6.021 | −7.733–−4.309 | |||
| 6th cycle | −5.689 | −5.257 | −5.402 | −5.617–−4.861 | −5.994 | −5.994 | −5.994 | −5.994–−5.994 | |||
| Surgery | Cu | 0.0452 | 0.521 | 0.609 | 0.360–0.743 | 0.035 | 0.328 | 0.283 | 0.118–0.525 | 0.557 | 0.892 |
| 1st cycle | 0.0881 | 0.427 | 0.397 | 0.325–0.553 | 0.142 | 0.43 | 0.437 | 0.318–0.530 | |||
| 3rd cycle | 0.0452 | 0.367 | 0.368 | 0.178–0.512 | 0.0719 | 0.38 | 0.396 | 0.224–0.501 | |||
| 6th cycle | 0.158 | 0.435 | 0.425 | 0.338–0.518 | 0.0512 | 0.402 | 0.412 | 0.219–0.483 | |||
| Surgery | Fe | −0.633 | 0.0454 | 0.0949 | −0.278–0.143 | −1.06 | 0.0335 | 0.193 | −0.180–0.302 | 0.595 | <0.001 |
| 1st cycle | −1.124 | 0.0747 | 0.131 | −0.204–0.424 | −1.592 | 0.0959 | 0.205 | −0.0769–0.288 | |||
| 3rd cycle | −0.393 | 0.687 | 0.596 | 0.405–0.974 | −0.537 | 0.49 | 0.52 | 0.276–0.827 | |||
| 6th cycle | 0.13 | 0.705 | 0.661 | 0.363–1.088 | −0.284 | 0.597 | 0.683 | 0.283–0.868 | |||
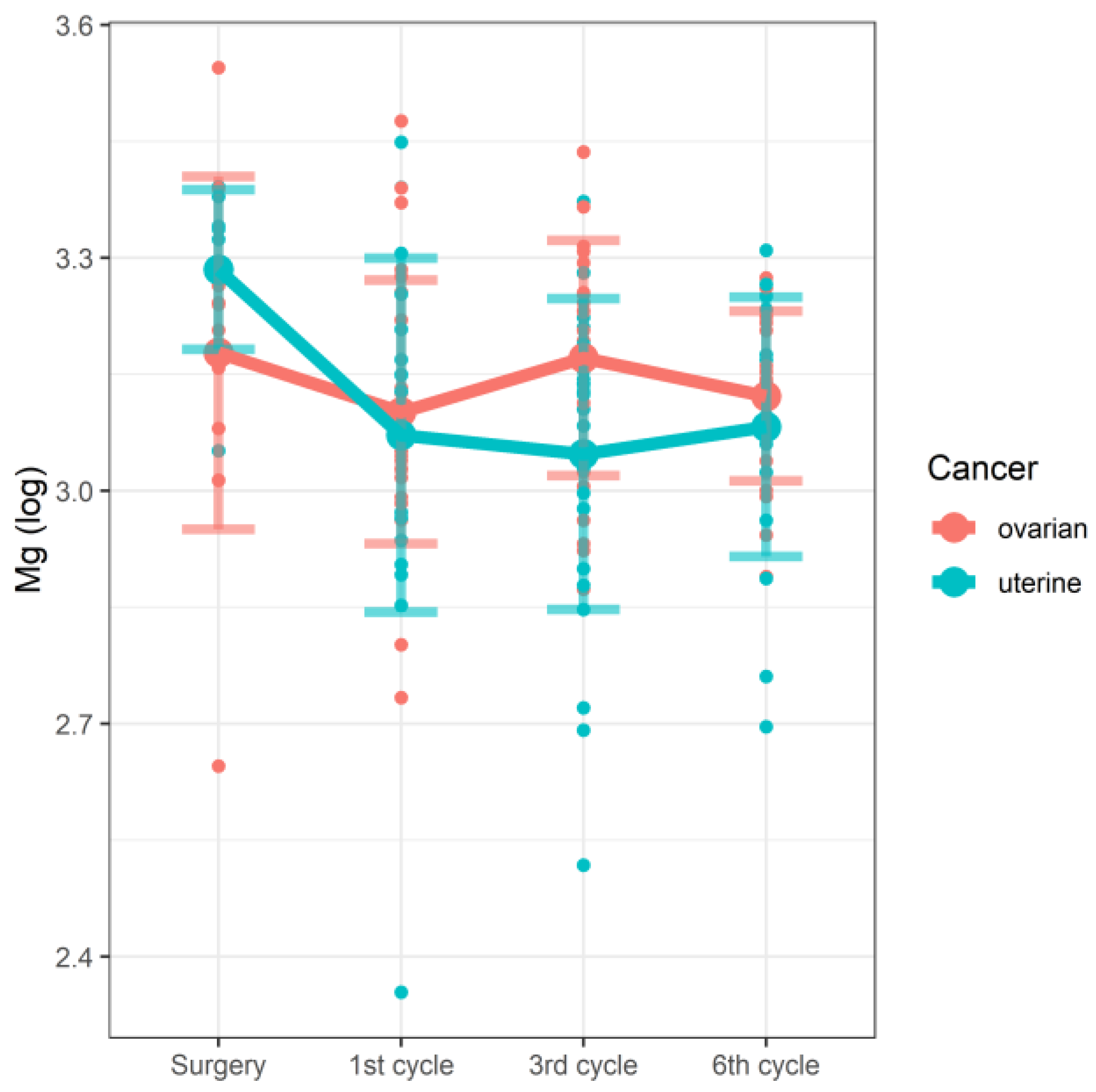
| Surgery | Mg | 2.645 | 3.178 | 3.207 | 3.100–3.259 | 3.052 | 3.285 | 3.306 | 3.280–3.341 | 0.748 | 0.038 |
| 1st cycle | 2.734 | 3.101 | 3.068 | 3.017–3.220 | 2.354 | 3.072 | 3.093 | 2.958–3.179 | |||
| 3rd cycle | 2.873 | 3.171 | 3.219 | 3.040–3.268 | 2.518 | 3.047 | 3.125 | 2.939–3.156 | |||
| 6th cycle | 2.89 | 3.122 | 3.132 | 3.058–3.220 | 2.696 | 3.082 | 3.103 | 3.024–3.176 | |||
| Surgery | Na | 8.159 | 8.271 | 8.293 | 8.205–8.319 | 7.94 | 8.223 | 8.252 | 8.197–8.283 | 0.187 | 0.014 |
| 1st cycle | 7.832 | 8.116 | 8.15 | 8.060–8.185 | 7.879 | 8.118 | 8.144 | 8.051–8.210 | |||
| 3rd cycle | 7.92 | 8.188 | 8.222 | 8.112–8.272 | 7.813 | 8.118 | 8.133 | 8.017–8.246 | |||
| 6th cycle | 7.989 | 8.179 | 8.197 | 8.114–8.255 | 7.895 | 8.166 | 8.22 | 8.036–8.255 | |||
| Surgery | Ni | −6.237 | −5.57 | −5.57 | −6.237–−4.904 | - | - | - | - | 0.069 | 0.037 |
| 1st cycle | −6.207 | −4.85 | −4.636 | −5.456–−4.225 | −5.042 | −4.44 | −4.369 | −4.573–−4.212 | |||
| 3rd cycle | −5.362 | −4.433 | −4.402 | −4.653–−4.218 | −6.768 | −5.155 | −4.913 | −5.766–−4.384 | |||
| 6th cycle | −5.16 | −4.464 | −4.453 | −4.722–−4.082 | −5.634 | −4.916 | −5.139 | −5.352–−4.457 | |||
| Surgery | P | 5.158 | 5.829 | 5.995 | 5.366–6.112 | 4.972 | 5.639 | 5.493 | 5.244–6.236 | 0.053 | 0.201 |
| 1st cycle | 4.977 | 5.347 | 5.214 | 5.088–5.435 | 4.739 | 5.245 | 5.284 | 5.067–5.373 | |||
| 3rd cycle | 4.853 | 5.431 | 5.388 | 5.220–5.555 | 4.676 | 5.421 | 5.293 | 5.131–5.718 | |||
| 6th cycle | 4.977 | 5.549 | 5.344 | 5.268–5.988 | 5.029 | 5.376 | 5.312 | 5.155–5.386 | |||
| Surgery | Sr | −5.137 | −3.296 | −3.372 | −3.781–−2.613 | −5.681 | −3.945 | −3.824 | −4.851–−3.202 | 0.639 | 0.07 |
| 1st cycle | −5.165 | −3.119 | −2.853 | −3.418–−2.543 | −4.004 | −2.851 | −2.71 | −3.094–−2.529 | |||
| 3rd cycle | −4.985 | −2.968 | −2.81 | −3.209–−2.440 | −6.26 | −3.018 | −2.828 | −3.350–−2.497 | |||
| 6th cycle | −3.777 | −3.088 | −3.015 | −3.430–−2.797 | −3.931 | −3.065 | −3.092 | −3.581–−2.590 | |||
| Surgery | Zn | −0.317 | 0.687 | 0.528 | 0.162–1.295 | 0.0947 | 0.908 | 0.877 | 0.661–1.060 | 0.436 | 0.637 |
| 1st cycle | −0.0352 | 0.729 | 0.739 | 0.185–0.985 | 0.223 | 0.715 | 0.777 | 0.431–0.881 | |||
| 3rd cycle | −0.114 | 0.788 | 0.873 | 0.341–1.164 | −0.216 | 0.739 | 0.754 | 0.364–1.078 | |||
| 6th cycle | −0.0358 | 0.79 | 0.714 | 0.440–1.199 | 0.205 | 0.84 | 0.776 | 0.391–1.136 | |||
| Stage of Therapy | Estimate | Standard Error | df | t | p |
|---|---|---|---|---|---|
| Mg | |||||
| Ovarian surgery | 3.19190 | 0.04811 | 153.85117 | 66.347 | <2 × 10−16 |
| Ovarian 1st cycle | −0.09045 | 0.05128 | 117.94225 | −1.764 | 0.0803 |
| Ovarian 3rd cycle | −0.02175 | 0.05244 | 120.95496 | −0.415 | 0.6790 |
| Ovarian 6th cycle | −0.07223 | 0.05482 | 124.49789 | −1.317 | 0.1901 |
| Endometrial surgery | 0.10228 | 0.06969 | 153.88577 | 1.468 | 0.1442 |
| Endometrial 1st cycle | −0.13273 | 0.07560 | 121.30789 | −1.756 | 0.0817 |
| Endometrial 3rd cycle | −0.22711 | 0.07552 | 122.42949 | −3.007 | 0.0032 |
| Endometrial 6th cycle | −0.14127 | 0.07881 | 123.15295 | −1.792 | 0.0755 |
| Cd | |||||
| Ovarian surgery | −6.9280 | 0.7184 | 18.0000 | −9.644 | 1.56 × 10−8 |
| Ovarian 1st cycle | 0.1386 | 0.8798 | 18.0000 | 0.158 | 0.8766 |
| Ovarian 3rd cycle | 1.32 | 0.8295 | 18.0000 | 2.282 | 0.0348 |
| Ovarian 6th cycle | 1.04 | 0.8295 | 18.0000 | 2.014 | 0.0592 |
| Endometrial surgery | 1,43 | 0.8798 | 18.0000 | 1.767 | 0.0942 |
| Endometrial 1st cycle | 0.2543 | 1.0775 | 18.0000 | 0.236 | 0.8161 |
| Endometrial 3rd cycle | −2.5407 | 1.0973 | 18.0000 | −2.315 | 0.0326 |
| Endometrial 6th cycle | −2.2912 | 1.92 | 18.0000 | −1.895 | 0.0743 |
| Element | Whole Group (N = 154) | Ovarian Cancer (n = 81) | Endometrial Cancer (n = 73) | |
|---|---|---|---|---|
| Ca | Correlation coefficient | 0.045 | 0.137 | −0.039 |
| p | 0.581 | 0.223 | 0.7407 | |
| Cd | Correlation coefficient | −0.039 | 0.1 | −0.182 |
| p | 0.6307 | 0.3725 | 0.1225 | |
| Cu | Correlation coefficient | −0.051 | −0.107 | −0.006 |
| p | 0.5294 | 0.3397 | 0.96 | |
| Fe | Correlation coefficient | 0.483 | 0.529 | 0.426 |
| p | <0.0001 | <0.0001 | 0.0002 | |
| Mg | Correlation coefficient | −0.14 | −0.027 | −0.261 |
| p | 0.0826 | 0.8143 | 0.0255 | |
| Na | Correlation coefficient | −0.037 | −0.032 | −0.041 |
| p | 0.6528 | 0.7789 | 0.7279 | |
| Ni 2 | Correlation coefficient | 0.153 | 0.157 | 0.154 |
| p | 0.059 | 0.1612 | 0.1934 | |
| P | Correlation coefficient | −0.02 | 0.004 | −0.032 |
| p | 0.8017 | 0.974 | 0.7898 | |
| Sr | Correlation coefficient | 0.086 | 0.057 | 0.126 |
| p | 0.2906 | 0.6106 | 0.2894 | |
| Zn | Correlation coefficient | 0.029 | 0.07 | −0.023 |
| p | 0.7235 | 0.5322 | 0.8468 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieder-Huszla, S.; Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Karakiewicz, B.; Bosiacki, M.; Chlubek, D.; Jurczak, A. Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study. Nutrients 2022, 14, 2368. https://doi.org/10.3390/nu14122368
Wieder-Huszla S, Chudecka-Głaz A, Cymbaluk-Płoska A, Karakiewicz B, Bosiacki M, Chlubek D, Jurczak A. Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study. Nutrients. 2022; 14(12):2368. https://doi.org/10.3390/nu14122368
Chicago/Turabian StyleWieder-Huszla, Sylwia, Anita Chudecka-Głaz, Aneta Cymbaluk-Płoska, Beata Karakiewicz, Mateusz Bosiacki, Dariusz Chlubek, and Anna Jurczak. 2022. "Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study" Nutrients 14, no. 12: 2368. https://doi.org/10.3390/nu14122368
APA StyleWieder-Huszla, S., Chudecka-Głaz, A., Cymbaluk-Płoska, A., Karakiewicz, B., Bosiacki, M., Chlubek, D., & Jurczak, A. (2022). Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study. Nutrients, 14(12), 2368. https://doi.org/10.3390/nu14122368







