The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders
Abstract
1. Introduction
2. Advanced Glycation End-Products (AGEs): Endogenous and Dietary Sources
3. Dietary AGE Pharmacokinetics
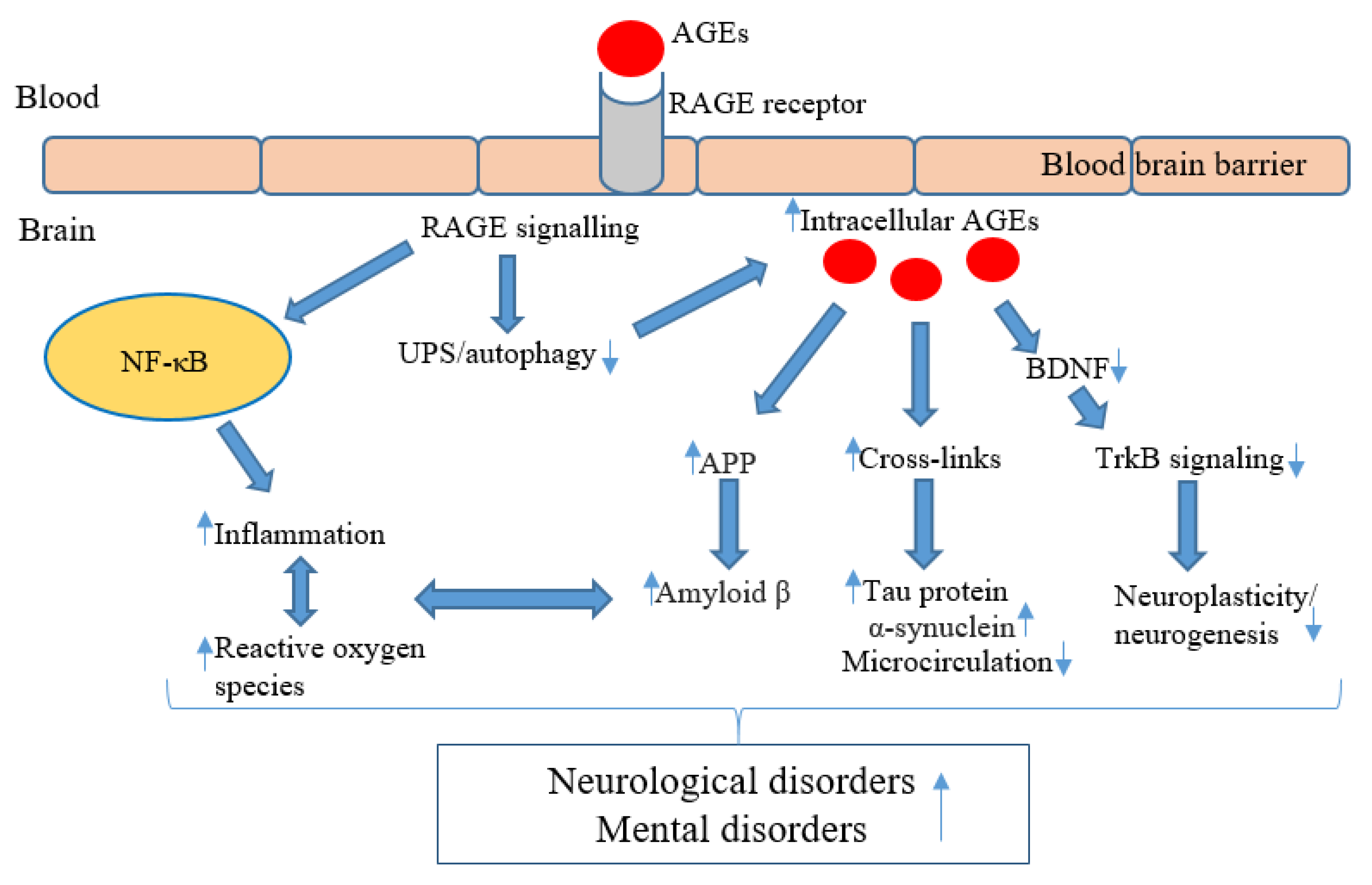
4. AGEs in Neurocognitive and Mental Disorders: Mechanisms of Action
5. AGEs and Neurocognitive Disorders
6. AGEs and Mental Health Disorders
6.1. Schizophrenia
6.2. Depression/Affective Disorders
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hampel, H.; Lista, S. The rising global tide of cognitive impairment. Nat. Rev. Neurol. 2016, 12, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Richards, D. Prevalence and clinical course of depression: A review. Clin. Psychol. Rev. 2011, 31, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, N.M.; McKune, A.J.; Panagiotakos, D.B.; Georgousopoulou, E.N.; Thomas, J.; Mellor, D.D.; Naumovski, N. Evaluation of dietary and lifestyle changes as modifiers of S100β levels in Alzheimer’s disease. Nutr. Neurosci. 2019, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 2895. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R. Dietary sugars and endogenous formation of advanced glycation endproducts: Emerging mechanisms of disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Goh, S.-Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Steenbeke, M.; Speeckaert, R.; Desmedt, S.; Glorieux, G.; Delanghe, J.R.; Speeckaert, M.M. The Role of Advanced Glycation End Products and Its Soluble Receptor in Kidney Diseases. Int. J. Mol. Sci. 2022, 23, 3439. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced glycation end products: Building on the concept of the “common soil” in metabolic disease. Endocrinology 2020, 161, bqz006. [Google Scholar] [CrossRef]
- Sharifi-Zahabi, E.; Sharafabad, F.H.; Abdollahzad, H.; Malekahmadi, M.; Rad, N.B. Circulating Advanced Glycation End Products and Their Soluble Receptors in Relation to All-Cause and Cardiovascular Mortality: A Systematic Review and Meta-analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 2157–2171. [Google Scholar] [CrossRef]
- Nass, N.; Bartling, B.; Santos, A.N.; Scheubel, R.; Börgermann, J.; Silber, R.; Simm, A. Advanced glycation end products, diabetes and ageing. Z. Gerontol. Geriatr. 2007, 40, 349–356. [Google Scholar] [CrossRef]
- Coker, L.H.; Wagenknecht, L.E. Advanced glycation end products, diabetes, and the brain. Neurology 2011, 77, 1326. [Google Scholar] [CrossRef]
- Lotan, R.; Ganmore, I.; Livny, A.; Itzhaki, N.; Waserman, M.; Shelly, S.; Zacharia, M.; Moshier, E.; Uribarri, J.; Beisswenger, P.; et al. Effect of Advanced Glycation End Products on Cognition in Older Adults with Type 2 Diabetes: Results from a Pilot Clinical Trial. J. Alzheimer’s Dis. 2021, 82, 1785–1795. [Google Scholar] [CrossRef]
- Di Pino, A.; Currenti, W.; Urbano, F.; Scicali, R.; Piro, S.; Purrello, F.; Rabuazzo, A.M. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 978–984. [Google Scholar] [CrossRef]
- Gauci, S.; Young, L.M.; White, D.J.; Reddan, J.M.; Lassemillante, A.C.; Meyer, D.; Pipingas, A.; Scholey, A. Diet May Moderate the Relationship between Arterial Stiffness and Cognitive Performance in Older Adults. J. Alzheimer’s Dis. 2022, 85, 815–828. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Hori, O.; Brett, J.; Yan, S.D.; Wautier, J.L.; Stern, D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. 1994, 14, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Du Yan, S.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The role of dietary advanced glycation end products in metabolic dysfunction. Mol. Nutr. Food Res. 2021, 65, 1900934. [Google Scholar] [CrossRef]
- Bongarzone, S.; Savickas, V.; Luzi, F.; Gee, A.D. Targeting the receptor for advanced glycation endproducts (RAGE): A medicinal chemistry perspective. J. Med. Chem. 2017, 60, 7213–7232. [Google Scholar] [CrossRef]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Soluble RAGE: Therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem. Pharmacol. 2010, 79, 1379–1386. [Google Scholar] [CrossRef]
- Pinkas, A.; Aschner, M. Advanced glycation end-products and their receptors: Related pathologies, recent therapeutic strategies, and a potential model for future neurodegeneration studies. Chem. Res. Toxicol. 2016, 29, 707–714. [Google Scholar] [CrossRef]
- Torreggiani, M.; Liu, H.; Wu, J.; Zheng, F.; Cai, W.; Striker, G.; Vlassara, H. Advanced glycation end product receptor-1 transgenic mice are resistant to inflammation, oxidative stress, and post-injury intimal hyperplasia. Am. J. Pathol. 2009, 175, 1722–1732. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Prasad, K.; Mishra, M. AGE-RAGE Stress, Stressors, and Antistressors in Health and Disease. Int. J. Angiol. 2018, 27, 1–12. [Google Scholar] [CrossRef]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced Glycation End Products (AGEs) May Be a Striking Link between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Scheijen, J.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Chen, G.; Smith, J.S. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015, 168, 190–195. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Sebeková, K.; Krajcoviová-Kudlácková, M.; Schinzel, R.; Faist, V.; Klvanová, J.; Heidland, A. Plasma levels of advanced glycation end products in healthy, long-term vegetarians and subjects on a western mixed diet. Eur. J. Nutr. 2001, 40, 275–281. [Google Scholar] [CrossRef]
- DeChristopher, L.R. Perspective: The Paradox in Dietary Advanced Glycation End Products Research—The Source of the Serum and Urinary Advanced Glycation End Products Is the Intestines, Not the Food. Adv. Nutr. 2017, 8, 679–683. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Fatahi, S.; Sharifi-Zahabi, E.; Santos, H.O.; Tripathi, N.; Lari, A.; Pourrajab, B.; Kord-Varkaneh, H.; Găman, M.-A.; Shidfar, F. The Impact of Low Advanced Glycation End Products Diet on Metabolic Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 766–776. [Google Scholar] [CrossRef]
- Di Pino, A.; Currenti, W.; Urbano, F.; Mantegna, C.; Purrazzo, G.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Low advanced glycation end product diet improves the lipid and inflammatory profiles of prediabetic subjects. J. Clin. Lipidol. 2016, 10, 1098–1108. [Google Scholar] [CrossRef]
- Goudarzi, R.; Sedaghat, M.; Hedayati, M.; Hekmatdoost, A.; Sohrab, G. Low advanced Glycation end product diet improves the central obesity, insulin resistance and inflammatory profiles in Iranian patients with metabolic syndrome: A randomized clinical trial. J. Diabetes Metab. Disord. 2020, 19, 1129–1138. [Google Scholar] [CrossRef]
- Baye, E.; de Courten, M.P.; Walker, K.; Ranasinha, S.; Earnest, A.; Forbes, J.M.; de Courten, B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: A randomised crossover trial. Sci. Rep. 2017, 7, 4123. [Google Scholar] [CrossRef]
- Van Dongen, K.C.; Kappetein, L.; Estruch, I.M.; Belzer, C.; Beekmann, K.; Rietjens, I.M. Differences in kinetics and dynamics of endogenous versus exogenous advanced glycation end products (AGEs) and their precursors. Food Chem. Toxicol. 2022, 164, 112987. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Fu, L. Dietary advanced glycation end-products: Perspectives linking food processing with health implications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2559–2587. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M.T. Dietary advanced glycation end products: Digestion, metabolism and modulation of gut microbial ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M. Bacterial enzymes that can deglycate glucose-and fructose-modified lysine. Biochem. J. 2005, 392 Pt 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; De Waard, P.; Plugge, C.M.; De Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef] [PubMed]
- Wiame, E.; Duquenne, A.; Delpierre, G.; Van Schaftingen, E. Identification of enzymes acting on α-glycated amino acids in Bacillus subtilis. FEBS Lett. 2004, 577, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Wiame, E.; Delpierre, G.; Collard, F.; Van Schaftingen, E. Identification of a pathway for the utilization of the Amadori product fructoselysine in Escherichia coli. J. Biol. Chem. 2002, 277, 42523–42529. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Troise, A.D.; Fogliano, V.; De Vos, W.M. Anaerobic degradation of N-ε-Carboxymethyllysine, a major glycation end-product, by human intestinal bacteria. J. Agric. Food Chem. 2019, 67, 6594–6602. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Tessier, F.J.; Niquet-Leridon, C.; Seiquer, I.; Pilar Navarro, M. Study of the urinary and faecal excretion of N ε-carboxymethyllysine in young human volunteers. Amino Acids 2012, 43, 595–602. [Google Scholar] [CrossRef]
- Tessier, F.J.; Niquet-Léridon, C.; Jacolot, P.; Jouquand, C.; Genin, M.; Schmidt, A.M.; Grossin, N.; Boulanger, E. Quantitative assessment of organ distribution of dietary protein-bound 13C-labeled Nɛ-carboxymethyllysine after a chronic oral exposure in mice. Mol. Nutr. Food Res. 2016, 60, 2446–2456. [Google Scholar] [CrossRef]
- Saito, A.; Takeda, T.; Sato, K.; Hama, H.; Tanuma, A.; Kaseda, R.; Suzuki, Y.; Gejyo, F. Significance of proximal tubular metabolism of advanced glycation end products in kidney diseases. Ann. N. Y. Acad. Sci. 2005, 1043, 637–643. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef]
- Chen, J.; Mooldijk, S.S.; Licher, S.; Waqas, K.; Ikram, M.K.; Uitterlinden, A.G.; Zillikens, M.C.; Ikram, M.A. Assessment of advanced glycation end products and receptors and the risk of dementia. JAMA Netw. Open 2021, 4, e2033012. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamagishi, S.-I. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2008, 14, 973–978. [Google Scholar] [CrossRef]
- Sasaki, N.; Fukatsu, R.; Tsuzuki, K.; Hayashi, Y.; Yoshida, T.; Fujii, N.; Koike, T.; Wakayama, I.; Yanagihara, R.; Garruto, R.; et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 1998, 153, 1149–1155. [Google Scholar] [CrossRef]
- Batkulwar, K.; Godbole, R.; Banarjee, R.; Kassaar, O.; Williams, R.J.; Kulkarni, M.J. Advanced glycation end products modulate amyloidogenic APP processing and tau phosphorylation: A mechanistic link between glycation and the development of Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 988–1000. [Google Scholar] [CrossRef]
- Fang, F.; Yu, Q.; Arancio, O.; Chen, D.; Gore, S.S.; Yan, S.S.; Yan, S.F. RAGE mediates Aβ accumulation in a mouse model of Alzheimer’s disease via modulation of β-and γ-secretase activity. Hum. Mol. Genet. 2018, 27, 1002–1014. [Google Scholar] [CrossRef]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-β and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar] [CrossRef]
- Sadrolashrafi, K.; Craft, S.; Decourt, B.; Adem, A.; Wilson, J.R.; Miller, J.; Sabbagh, M.N. Is diabetes associated with increased pathological burden in Alzheimer’s disease? Alzheimers Dement. 2021, 13, e12248. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Fang, F.; Lue, L.-F.; Yan, S.; Xu, H.; Luddy, J.S.; Chen, D.; Walker, D.G.; Stern, D.M.; Yan, S.; Schmidt, A.M. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010, 24, 1043–1055. [Google Scholar] [CrossRef]
- Münch, G.; Mayer, S.; Michaelis, J.; Hipkiss, A.R.; Riederer, P.; Müller, R.; Neumann, A.; Schinzel, R.; Cunningham, A.M. Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of β-amyloid peptide. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1997, 1360, 17–29. [Google Scholar] [CrossRef]
- Padmaraju, V.; Bhaskar, J.J.; Prasada Rao, U.J.; Salimath, P.V.; Rao, K. Role of advanced glycation on aggregation and DNA binding properties of α-synuclein. J. Alzheimer’s Dis. 2011, 24, 211–221. [Google Scholar] [CrossRef]
- Münch, G.; Lüth, H.; Wong, A.; Arendt, T.; Hirsch, E.; Ravid, R.A.; Riederer, P. Crosslinking of α-synuclein by advanced glycation endproducts—An early pathophysiological step in Lewy body formation? J. Chem. Neuroanat. 2000, 20, 253–257. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary advanced glycation endproducts induce an inflammatory response in human macrophages in vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef]
- Younessi, P.; Yoonessi, A. Advanced glycation end-products and their receptor-mediated roles: Inflammation and oxidative stress. Iran. J. Med. Sci. 2011, 36, 154. [Google Scholar]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, F.; Wang, J.; Liu, C.; Zhou, Y.; Xu, Z.; Zhang, C.; Sun, B.; Guan, Y. Pathological mechanisms linking diabetes mellitus and Alzheimer’s disease: The receptor for advanced glycation end products (RAGE). Front. Aging Neurosci. 2020, 12, 217. [Google Scholar] [CrossRef]
- Videira, P.A.; Castro-Caldas, M. Linking glycation and glycosylation with inflammation and mitochondrial dysfunction in Parkinson’s disease. Front. Neurosci. 2018, 12, 381. [Google Scholar] [CrossRef]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Swatton, J.; Ryan, M.; Huffaker, S.; Huang, J.-J.; Griffin, J.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry 2004, 9, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A. Mechanistically linking age-related diseases and dietary carbohydrate via autophagy and the ubiquitin proteolytic systems. Autophagy 2012, 8, 1404–1406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haddad, M.; Hervé, V.; Ben Khedher, M.R.; Rabanel, J.-M.; Ramassamy, C. Glutathione: An Old and Small Molecule with Great Functions and New Applications in the Brain and in Alzheimer’s Disease. Antioxid. Redox Signal. 2021, 35, 270–292. [Google Scholar] [CrossRef]
- Ko, S.-Y.; Ko, H.-A.; Chu, K.-H.; Shieh, T.-M.; Chi, T.-C.; Chen, H.-I.; Chang, W.-C.; Chang, S.-S. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef]
- Lubitz, I.; Ricny, J.; Atrakchi-Baranes, D.; Shemesh, C.; Kravitz, E.; Liraz-Zaltsman, S.; Maksin-Matveev, A.; Cooper, I.; Leibowitz, A.; Uribarri, J.; et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Aβ deposition in an Alzheimer mouse model. Aging Cell 2016, 15, 309–316. [Google Scholar] [CrossRef]
- Li, X.-H.; Lv, B.-L.; Xie, J.-Z.; Liu, J.; Zhou, X.-W.; Wang, J.-Z. AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol. Aging 2012, 33, 1400–1410. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.; Shi, C.; Chen, Y.; Yu, L.; Li, J.; Li, W.; Wei, Y.; He, R. Ribosylation-derived advanced glycation end products induce tau hyperphosphorylation through brain-derived neurotrophic factor reduction. J. Alzheimer’s Dis. 2019, 71, 291–305. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- de Vos, L.C.; Lefrandt, J.D.; Dullaart, R.P.; Zeebregts, C.J.; Smit, A.J. Advanced glycation end products: An emerging biomarker for adverse outcome in patients with peripheral artery disease. Atherosclerosis 2016, 254, 291–299. [Google Scholar] [CrossRef]
- McNulty, M.; Mahmud, A.; Feely, J. Advanced glycation end-products and arterial stiffness in hypertension. Am. J. Hypertens. 2007, 20, 242–247. [Google Scholar] [CrossRef]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2014, 3, 94–108. [Google Scholar] [CrossRef]
- Seldenrijk, A.; van Hout, H.P.; van Marwijk, H.W.; de Groot, E.; Gort, J.; Rustemeijer, C.; Diamant, M.; Penninx, B.W. Depression, anxiety, and arterial stiffness. Biol. Psychiatry 2011, 69, 795–803. [Google Scholar] [CrossRef]
- van Dooren, F.E.; Schram, M.T.; Schalkwijk, C.G.; Stehouwer, C.D.; Henry, R.M.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.; Koster, A.; Sep, S.J. Associations of low grade inflammation and endothelial dysfunction with depression—The Maastricht Study. Brain Behav. Immun. 2016, 56, 390–396. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Amad, A.; Arai, M.; Miyashita, M.; Lalau, J.-D.; Loas, G.; Itokawa, M. Advanced glycation end products and schizophrenia: A systematic review. J. Psychiatr. Res. 2015, 66, 112–117. [Google Scholar] [CrossRef]
- Tikellis, C.; Thomas, M.C.; Harcourt, B.E.; Coughlan, M.T.; Pete, J.; Bialkowski, K.; Tan, A.; Bierhaus, A.; Cooper, M.E.; Forbes, J.M. Cardiac inflammation associated with a Western diet is mediated via activation of RAGE by AGEs. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E323–E330. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, C.; Zhou, Y.; Qi, J.; Zhang, C.; Sun, B.; Wang, J.; Guan, Y. Progress of RAGE molecular imaging in Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 227. [Google Scholar] [CrossRef]
- Shaikh, S.; Nicholson, L.F. Advanced glycation end products induce in vitro cross-linking of α-synuclein and accelerate the process of intracellular inclusion body formation. J. Neurosci. Res. 2008, 86, 2071–2082. [Google Scholar] [CrossRef]
- Mørch, R.H.; Dieset, I.; Færden, A.; Reponen, E.J.; Hope, S.; Hoseth, E.Z.; Gardsjord, E.S.; Aas, M.; Iversen, T.; Joa, I. Inflammatory markers are altered in severe mental disorders independent of comorbid cardiometabolic disease risk factors. Psychol. Med. 2019, 49, 1749–1757. [Google Scholar] [CrossRef]
- Kouvari, M.; D’Cunha, N.M.; Travica, N.; Sergi, D.; Zec, M.; Marx, W.; Naumovski, N. Metabolic Syndrome, Cognitive Impairment and the Role of Diet: A Narrative Review. Nutrients 2022, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.-B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Bădescu, S.; Tătaru, C.; Kobylinska, L.; Georgescu, E.; Zahiu, D.; Zăgrean, A.; Zăgrean, L. The association between diabetes mellitus and depression. J. Med. Life 2016, 9, 120. [Google Scholar] [PubMed]
- Yaffe, K.; Lindquist, K.; Schwartz, A.V.; Vitartas, C.; Vittinghoff, E.; Satterfield, S.; Simonsick, E.M.; Launer, L.; Rosano, C.; Cauley, J.A.; et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011, 77, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.; Gella, A.; Fernàndez-Busquets, X.; Unzeta, M.; Durany, N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol. Dis. 2010, 37, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.D.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef]
- D’Cunha, N.; McKune, N.; Georgousopoulou, E.; Roach, P.D.; Mellor, D.D.; Thomas, J.; Kellett, J.; Naumovski, N. The effect of plant extracts on S100B levels in animal pre-clinical trials: A systematic review. J. Nutr. Intermed. Metab. 2017, 8, 106. [Google Scholar] [CrossRef]
- Taguchi, K.; Fukami, K.; Elias, B.C.; Brooks, C.R. Dysbiosis-Related Advanced Glycation Endproducts and Trimethylamine N-Oxide in Chronic Kidney Disease. Toxins 2021, 13, 361. [Google Scholar] [CrossRef]
- Seiquer, I.; Rubio, L.A.; Peinado, M.J.; Delgado-Andrade, C.; Navarro, M.P. Maillard reaction products modulate gut microbiota composition in adolescents. Mol. Nutr. Food Res. 2014, 58, 1552–1560. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, X.; Zhao, J.; Zhang, Y.; Hu, J.; Wang, J.; Li, J. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol. Nutr. Food Res. 2017, 61, 1700118. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.-A.M. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the microbiota–gut–brain Axis: Sowing the seeds of good mental health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Adams, J.N.; Martelle, S.E.; Raffield, L.M.; Freedman, B.I.; Langefeld, C.D.; Hsu, F.-C.; Maldjian, J.A.; Williamson, J.D.; Hugenschmidt, C.E.; Carr, J.J.; et al. Analysis of advanced glycation end products in the DHS Mind Study. J. Diabetes Complicat. 2016, 30, 262–268. [Google Scholar] [CrossRef]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; van der Schans, C.; Hobbelen, H. Association between advanced glycation end-products and functional performance in Alzheimer’s disease and mixed dementia. Int. Psychogeriatr. 2017, 29, 1525–1534. [Google Scholar] [CrossRef]
- Spauwen, P.J.J.; van Eupen, M.G.A.; Köhler, S.; Stehouwer, C.D.A.; Verhey, F.R.J.; van der Kallen, C.J.H.; Sep, S.J.S.; Koster, A.; Schaper, N.C.; Dagnelie, P.C.; et al. Associations of Advanced Glycation End-Products with Cognitive Functions in Individuals With and Without Type 2 Diabetes: The Maastricht Study. J. Clin. Endocrinol. Metab. 2015, 100, 951–960. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimer’s Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef]
- West, R.K.; Moshier, E.; Lubitz, I.; Schmeidler, J.; Godbold, J.; Cai, W.; Uribarri, J.; Vlassara, H.; Silverman, J.M.; Beeri, M.S. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech. Ageing Dev. 2014, 140, 10–12. [Google Scholar] [CrossRef]
- Chou, P.-S.; Wu, M.-N.; Yang, C.-C.; Shen, C.-T.; Yang, Y.-H. Effect of Advanced Glycation End Products on the Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 191–197. [Google Scholar] [CrossRef]
- Akhter, F.; Chen, D.; Akhter, A.; Sosunov, A.A.; Chen, A.; McKhann, G.M.; Yan, S.F.; Yan, S.S. High Dietary Advanced Glycation End Products Impair Mitochondrial and Cognitive Function. J. Alzheimer’s Dis. 2020, 76, 165–178. [Google Scholar] [CrossRef]
- Zheng, F.; Yan, L.; Yang, Z.; Zhong, B.; Xie, W. HbA1c, diabetes and cognitive decline: The English Longitudinal Study of Ageing. Diabetologia 2018, 61, 839–848. [Google Scholar] [CrossRef]
- Kerti, L.; Witte, A.V.; Winkler, A.; Grittner, U.; Rujescu, D.; Flöel, A. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 2013, 81, 1746. [Google Scholar] [CrossRef]
- Kalousová, M.; Kuběna, A.A.; Benáková, H.; Dusilová-Sulková, S.; Tesař, V.; Zima, T. EN-RAGE (extracellular newly identified receptor for advanced glycation end-products binding protein) and mortality of long-term hemodialysis patients: Aprospective observational cohort study. Clin. Biochem. 2012, 45, 556–560. [Google Scholar] [CrossRef]
- Sharma, A.; Weber, D.; Raupbach, J.; Dakal, T.C.; Fließbach, K.; Ramirez, A.; Grune, T.; Wüllner, U. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson’s and Alzheimer’s disease. Redox Biol. 2020, 34, 101546. [Google Scholar] [CrossRef]
- Sternberg, Z.; Ostrow, P.; Vaughan, M.; Chichelli, T.; Munschauer, F. AGE-RAGE in multiple sclerosis brain. Immunol. Investig. 2011, 40, 197–205. [Google Scholar] [CrossRef]
- Sternberg, Z.; Weinstock-Guttman, B.; Hojnacki, D.; Zamboni, P.; Zivadinov, R.; Chadha, K.; Lieberman, A.; Kazim, L.; Drake, A.; Rocco, P. Soluble receptor for advanced glycation end products in multiple sclerosis: A potential marker of disease severity. Mult. Scler. J. 2008, 14, 759–763. [Google Scholar] [CrossRef]
- Castellani, R.; Smith, M.; Richey, G.; Perry, G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996, 737, 195–200. [Google Scholar] [CrossRef]
- Miyashita, M.; Yamasaki, S.; Ando, S.; Suzuki, K.; Toriumi, K.; Horiuchi, Y.; Yoshikawa, A.; Imai, A.; Nagase, Y.; Miyano, Y. Fingertip advanced glycation end products and psychotic symptoms among adolescents. NPJ Schizophr. 2021, 7, 37. [Google Scholar] [CrossRef]
- Kobori, A.; Miyashita, M.; Miyano, Y.; Suzuki, K.; Toriumi, K.; Niizato, K.; Oshima, K.; Imai, A.; Nagase, Y.; Yoshikawa, A. Advanced glycation end products and cognitive impairment in schizophrenia. PLoS ONE 2021, 16, e0251283. [Google Scholar] [CrossRef]
- Hagen, J.M.; Sutterland, A.L.; Koeter, M.W.; Lutter, R.; Cohen, D.; de Haan, L. Advanced glycation end products in recent-onset psychosis indicate early onset of cardiovascular risk. J. Clin. Psychiatry 2017, 78, 1395–1401. [Google Scholar] [CrossRef]
- Hagen, J.M.; Sutterland, A.L.; Edrisy, S.; Tan, H.L.; de Haan, L. Accumulation rate of advanced glycation end products in recent onset psychosis: A longitudinal study. Psychiatry Res. 2020, 291, 113192. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, S.; Ghuloum, S.; Mahfoud, Z.; Yehya, A.; Mook-Kanamori, D.; Mook-Kanamori, M.; Suhre, K.; Abdulhakam, A.; Al-Mujalli, A.; Hani, Y. Advanced glycation end products among patients maintained on antipsychotics. Int. Clin. Psychopharmacol. 2017, 32, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, B.N.; Hagen, J.M.; Sutterland, A.L.; Schrantee, A.; de Haan, L. Association between Advanced Glycation End products and brain volumes in recent onset psychosis. Schizophr. Res. 2020, 224, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierski, J.; Miler, P.; Pawlak, A.; Jerczyńska, H.; Woźniak, J.; Frankowska, E.; Brzezińska, A.; Nowakowska, K.; Woźniak, K.; Krejca, M. Oxidative stress and soluble receptor for advanced glycation end-products play a role in the pathophysiology of delirium after cardiac surgery. Sci. Rep. 2021, 11, 23656. [Google Scholar] [CrossRef]
- Ha, C.H.; Kim, S.; Chung, J.; An, S.H.; Park, S.; Choi, D.; Kwon, K. Inhibitory effect of soluble RAGE in disturbed flow-induced atherogenesis. Int. J. Mol. Med. 2013, 32, 373–380. [Google Scholar] [CrossRef]
- van Dooren, F.E.; Pouwer, F.; Schalkwijk, C.G.; Sep, S.J.; Stehouwer, C.D.; Henry, R.M.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.; Koster, A. Advanced glycation end product (age) accumulation in the skin is associated with depression: The maastricht study. Depress. Anxiety 2017, 34, 59–67. [Google Scholar] [CrossRef]
- Hagen, J.M.; Sutterland, A.L.; Schirmbeck, F.; Cohn, D.M.; Lok, A.; Tan, H.L.; Zwinderman, A.H.; de Haan, L. Association between skin autofluorescence of advanced glycation end products and affective disorders in the lifelines cohort study. J. Affect. Disord. 2020, 275, 230–237. [Google Scholar] [CrossRef]
- Hagen, J.M.; Sutterland, A.L.; Schirmbeck, F.; Cohn, D.M.; Lok, A.; Tan, H.L.; Zwinderman, A.H.; de Haan, L. Skin autofluorescence of advanced glycation end products and course of affective disorders in the lifelines cohort study, a prospective investigation. J. Affect. Disord. 2020, 276, 424–432. [Google Scholar] [CrossRef]
- Eriksson, M.D.; Eriksson, J.G.; Kautiainen, H.; Salonen, M.K.; Mikkola, T.M.; Kajantie, E.; Wasenius, N.; von Bonsdorff, M.; Laine, M.K. Advanced glycation end products measured by skin autofluorescence are associated with melancholic depressive symptoms—Findings from Helsinki Birth Cohort Study. J. Psychosom. Res. 2021, 145, 110488. [Google Scholar] [CrossRef]
- Hagen, J.M.; Sutterland, A.L.; Liefers, T.; Schirmbeck, F.; Cohn, D.M.; Lok, A.; Tan, H.L.; Zwinderman, A.H.; de Haan, L. Skin autofluorescence of advanced glycation end products and mortality in affective disorders in the lifelines cohort study: A mediation analysis. J. Affect. Disord. 2021, 282, 1082–1089. [Google Scholar] [CrossRef]
- Emanuele, E.; Martinelli, V.; Carlin, M.V.; Fugazza, E.; Barale, F.; Politi, P. Serum levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with different psychiatric disorders. Neurosci. Lett. 2011, 487, 99–102. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Wang, T.; Liang, J.; Lin, W.; Li, L.; Wen, J.; Lin, L.; Huang, H. Association between serum endogenous secretory receptor for advanced glycation end products and risk of type 2 diabetes mellitus with combined depression in the Chinese population. Diabetes Technol. Ther. 2012, 14, 936–942. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Tsoporis, J.N.; Salpeas, V.; Bei, E.; Alevizos, B.; Anagnostara, C.; Izhar, S.; Proteau, G.; Rizos, E.; Hatziagelaki, E. Peripheral blood lymphocytes from patients with bipolar disorder demonstrate apoptosis and differential regulation of advanced glycation end products and S100B. Clin. Chem. Lab. Med. 2014, 52, 999–1007. [Google Scholar] [CrossRef]
- Yamashita, H.; Fukushima, E.; Shimomura, K.; Hirose, H.; Nakayama, K.; Orimo, N.; Mao, W.; Katsuta, N.; Nishimon, S.; Ohnuma, T. Use of skin advanced glycation end product levels measured using a simple noninvasive method as a biological marker for the diagnosis of neuropsychiatric diseases. Int. J. Methods Psychiatr. Res. 2020, 29, e1824. [Google Scholar] [CrossRef]
- Mook-Kanamori, M.J.; Selim, M.M.E.-D.; Takiddin, A.H.; Al-Homsi, H.; Al-Mahmoud, K.A.; Al-Obaidli, A.; Zirie, M.A.; Rowe, J.; Gherbi, W.S.; Chidiac, O.M. Ethnic and gender differences in advanced glycation end products measured by skin auto-fluorescence. Dermatoendocrinology 2013, 5, 325–330. [Google Scholar] [CrossRef]
- Perry, B.I.; Upthegrove, R.; Kappelmann, N.; Jones, P.B.; Burgess, S.; Khandaker, G.M. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: A bi-directional two-sample mendelian randomization study. Brain Behav. Immun. 2021, 97, 176–185. [Google Scholar] [CrossRef]
- Hayley, S.; Hakim, A.M.; Albert, P.R. Depression, dementia and immune dysregulation. Brain 2021, 144, 746–760. [Google Scholar] [CrossRef]
- Leonard, B.E.; Wegener, G. Inflammation, insulin resistance and neuroprogression in depression. Acta Neuropsychiatr. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- DeGroot, J.; Verzijl, N.; Wenting-Van Wijk, M.J.; Jacobs, K.M.; Van El, B.; Van Roermund, P.M.; Bank, R.A.; Bijlsma, J.W.; TeKoppele, J.M.; Lafeber, F.P. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004, 50, 1207–1215. [Google Scholar] [CrossRef]
- Ebert, H.; Lacruz, M.E.; Kluttig, A.; Simm, A.; Greiser, K.H.; Tiller, D.; Kartschmit, N.; Mikolajczyk, R. Advanced glycation end products and their ratio to soluble receptor are associated with limitations in physical functioning only in women: Results from the CARLA cohort. BMC Geriatr. 2019, 19, 299. [Google Scholar] [CrossRef]
- Mulder, D.J.; Water, T.V.D.; Lutgers, H.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: An overview of current clinical studies, evidence, and limitations. Diabetes Technol. Ther. 2006, 8, 523–535. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.; Links, T.; Jager, J.; Alderson, N.; Thorpe, S.; Baynes, J.; Gans, R.; Smit, A. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- Clarke, R.E.; Dordevic, A.L.; Tan, S.M.; Ryan, L.; Coughlan, M.T. Dietary advanced glycation end products and risk factors for chronic disease: A systematic review of randomised controlled trials. Nutrients 2016, 8, 125. [Google Scholar] [CrossRef]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Wu, C.-H.; Yen, G.-C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Srey, C.; Hull, G.L.; Connolly, L.; Elliott, C.T.; del Castillo, M.D.; Ames, J.M. Effect of inhibitor compounds on N ε-(carboxymethyl) lysine (CML) and N ε-(carboxyethyl) lysine (CEL) formation in model foods. J. Agric. Food Chem. 2010, 58, 12036–12041. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.A.; Khalifah, R.G.; Hudson, B.G. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: Comparison with aminoguanidine. Biochem. Biophys. Res. Commun. 1996, 220, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kihm, L.P.; Müller-Krebs, S.; Klein, J.; Ehrlich, G.; Mertes, L.; Gross, M.-L.; Adaikalakoteswari, A.; Thornalley, P.J.; Hammes, H.-P.; Nawroth, P.P. Benfotiamine protects against peritoneal and kidney damage in peritoneal dialysis. J. Am. Soc. Nephrol. 2011, 22, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, J.; Mahomoodally, F.M.; Ahmed, N.; Subratty, H.A. Natural inhibitors of advanced glycation end-products. Nutr. Food Sci. 2012, 42, 397–404. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—A review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Mattei, J.; Bigornia, S.J.; Sotos-Prieto, M.; Scott, T.; Gao, X.; Tucker, K.L. The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care 2019, 42, 1372–1379. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef]

| Study | Study Design | Sample Size | Age (Mean ± SD) Years | Participant Sex (Male) | AGE Measure | Results |
|---|---|---|---|---|---|---|
| Adams et al., 2017 | Cross-sectional | 816 | 66.0 ± 9.9 | 46.6% | Serum AGE | Higher AGEs were associated with poorer digit symbol substitution test performance and decreased grey matter volume. |
| Chen et al., 2021 | Longitudinal | 3889 | 72.5 ± 8.9 | 43.8% | Skin AGE, Plasma EN-RAGE & S-RAGE | At baseline, higher EN-RAGE associated with higher prevalence of dementia, whereas higher S-RAGE associated with a lower prevalence. After 12.4 years on average, only EN-RAGE was associated with dementia prevalence. |
| Chou et al., 2019 | Longitudinal | 25 | 79.0 ± 5.8 | 12% | Plasma AGE | Higher AGEs were associated with a decline in the CDR after a 48.6 ± 2.1 month follow-up in people with AD and T2DM. |
| Drenth et al., 2017 | Longitudinal | 144 | 80.7 ± 7.7 | 43.7% | Skin AGE | Functional ability was associated with AGE levels and dementia progression over one year. |
| Lotan et al., 2021 | Randomized control trial | 75 | Intervention: 71.9 ± 4.29 Control: 71.42 ± 3.99 | Intervention: 77.1% Control: 72.5% | Serum AGE | Reduced dietary AGE intake and standard dietary advice improved cognitive performance in people with T2DM. More improvement was observed in people with MCI in the intervention group. |
| Study | Study Design | Mental Disorder | Sample Size | Age (Mean ± SD) | Participant Sex | AGE Measure | Results |
|---|---|---|---|---|---|---|---|
| Chen et al., 2012 | Cross-sectional | Depression | 71 | 57.39 ± 9.80 | 37% male | esRAGE | Inverse correlation between esRAGE levels and depression in those diagnosed with T2DM. |
| Emanuele et al., 2011 | Cross-sectional | Schizophrenia, depression, | 148 | 48.4 ± 11.6 | 40% male | sRAGE | Significantly lower serum sRAGE levels amongst patients with major depression in comparison to a control group. |
| Errikson et al. 2021 | Cross-sectional | Depression | 815 | 76 | 43.8% male | Skin AGEs | The highest AGEs levels were found in those with melancholic depressive symptoms, followed by non-melancholic symptoms |
| Hammoudeh et al., 2017 | Cross-sectional | Bipolar disorder (41%), schizophrenia, depression | 48 | 35.8 ± 10.1 | Na | Skin AGEs | No differences between higher AGEs levels among patients on antipsychotics compared with the controls. |
| Hagen et al., 2017 | Case-control | Recent onset psychosis | 532 | Na | Na | Skin AGEs | Patients with a recent onset of psychosis had increased AGEs levels compared to healthy controls. |
| Hagen et al., 2020 | Prospective | Recent onset psychosis | 238 | 26.6 | 78.8% male | Skin AGEs | Increased AGE-accumulation rate was shown in recent onset psychosis compared to healthy controls |
| Hagen et al., 2020 | Cross-sectional | Depression, dysthymia, GAD, panic disorder, social phobia | 81,041 | 44.1 ± 12.3 | 41.7% male | Skin AGEs | The strongest association between AGEs and affective disorders was observed for major depressive disorder, after controlling for sociodemographic, cardio metabolic factors, and somatic morbidities. |
| Hagen et al., 2020 | Prospective | Depression, dysthymia, GAD, panic disorder, social phobia | 43,267 | 42.2 ± 10.4 | 41.8% | Skin AGEs | Elevated AGEs significantly raised the odds of incident affective disorders, most prominently for major depressive disorder. Incidence was reduced after adjusting for socioeconomic status. |
| Hagen et al., 2021 | Prospective | Depression, dysthymia, GAD, panic disorder, social phobia | 81,041 | Na | Na | Skin AGEs | In major depression, mortality was most largely mediated by AGEs. |
| Kabori et al., 2021 | Cross-sectional | Schizophrenia | 58 | 46.8 ± 11.4 | 71% male | Plasma | Processing speed was associated with AGEs. |
| Kaźmierski et al., 2021 | Prospective | Post-operative delirium | 177 | 67 | 78% male | sRAGE | Both pre- and post-operative sRAGE levels were increased in patients who developed delirium compared to non-delirium patients |
| Miyashita et al., 2021 | Prospective | Psychosis | 282 | 13.4 ± 0.6 | 55.3% male | Skin AGEs | Fingertip AGEs potentially predicted the trajectory of psychotic symptoms among drug-naive adolescents over 12 months. |
| Moutsatsou et al., 2014 | Cross-sectional | Bipolar disorder | 45 | 44.6 ± 3.9 | 40% male | Leukocyte AGEs | Lower lymphocyte AGE concentrations were displayed in bipolar patients compared to healthy controls. |
| van Dooren et al., 2017 | Cross-sectional | Depression | 862 | 59.8 ± 8.5 | 55% male | Skin and plasma AGEs | Higher skin AGEs were associated with depressive symptoms and depressive disorder. Pentosidine was associated with somatic symptoms only. |
| Yamashita et al., 2020 | Cross-sectional | Depression, schizophrenia | 87 | 55.3 ± 7.8 | 33% male | Skin AGEs | A mental disorder diagnosis did not significantly affect the skin AGEs in comparison to a healthy control group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Cunha, N.M.; Sergi, D.; Lane, M.M.; Naumovski, N.; Gamage, E.; Rajendran, A.; Kouvari, M.; Gauci, S.; Dissanayka, T.; Marx, W.; et al. The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders. Nutrients 2022, 14, 2421. https://doi.org/10.3390/nu14122421
D’Cunha NM, Sergi D, Lane MM, Naumovski N, Gamage E, Rajendran A, Kouvari M, Gauci S, Dissanayka T, Marx W, et al. The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders. Nutrients. 2022; 14(12):2421. https://doi.org/10.3390/nu14122421
Chicago/Turabian StyleD’Cunha, Nathan M., Domenico Sergi, Melissa M. Lane, Nenad Naumovski, Elizabeth Gamage, Anushri Rajendran, Matina Kouvari, Sarah Gauci, Thusharika Dissanayka, Wolfgang Marx, and et al. 2022. "The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders" Nutrients 14, no. 12: 2421. https://doi.org/10.3390/nu14122421
APA StyleD’Cunha, N. M., Sergi, D., Lane, M. M., Naumovski, N., Gamage, E., Rajendran, A., Kouvari, M., Gauci, S., Dissanayka, T., Marx, W., & Travica, N. (2022). The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders. Nutrients, 14(12), 2421. https://doi.org/10.3390/nu14122421









