Racial and Ethnic Differences in Eating Duration and Meal Timing: Findings from NHANES 2011–2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Measures
2.2. Statistical Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, A.; Aparicio, F.H.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, F.A.; Chamberlain, F.A.; Kissela, B.M.; Knutson, F.K.; Lee, C.D. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 2021, e00. [Google Scholar]
- Centers for Disease Control and Prevention. Underlying Cause of Death, 1999–2018; CDC WONDER Online Database; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018; p. 2020. [Google Scholar]
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020. Available online: https://stacks.cdc.gov/view/cdc/58669 (accessed on 5 April 2022).
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangaraj, V.R.; Siddula, A.; Burgess, H.J.; Pannain, S.; Knutson, K.L. Association between timing of energy intake and insulin sensitivity: A cross-sectional study. Nutrients 2020, 12, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, K.; Varady, K.A. Current research: Effect of time restricted eating on weight and cardiometabolic health. J. Physiol. 2020, 600, 1313–1326. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Panda, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Ahrén, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh-Taskar, P.; Nicklas, T.A.; Radcliffe, J.D.; O’Neil, C.E.; Liu, Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2013, 16, 2073–2082. [Google Scholar] [PubMed] [Green Version]
- Bi, H.; Gan, Y.; Yang, C.; Chen, Y.; Tong, X.; Lu, Z. Breakfast skipping and the risk of type 2 diabetes: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 3013–3019. [Google Scholar] [CrossRef]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef]
- Horikawa, C.; Kodama, S.; Yachi, Y.; Heianza, Y.; Hirasawa, R.; Ibe, Y.; Saito, K.; Shimano, H.; Yamada, N.; Sone, H. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: A meta-analysis. Prev. Med. 2011, 53, 260–267. [Google Scholar] [CrossRef]
- Purslow, L.R.; Sandhu, M.S.; Forouhi, N.; Young, E.H.; Luben, R.N.; Welch, A.A.; Khaw, K.T.; Bingham, S.A.; Wareham, N.J. Energy intake at breakfast and weight change: Prospective study of 6764 middle-aged men and women. Am. J. Epidemiol. 2008, 167, 188–192. [Google Scholar] [CrossRef]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 2014, 27, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Satia, J.A. Diet-related disparities: Understanding the problem and accelerating solutions. J. Am. Diet Assoc. 2009, 109, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Galvez, M.P.; Morland, K.; Raines, C.; Kobil, J.; Siskind, J.; Godbold, J.; Brenner, B. Race and food store availability in an inner-city neighbourhood. Public Health Nutr. 2008, 11, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansom, G.; Hannibal, B. Disparate access to nutritional food; place, race and equity in the United States. BMC Nutr. 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Compte, M.; Burrola-Méndez, S.; Lozano-Marrufo, A.; Ferré-Eguiluz, I.; Flores, D.; Gaitán-Rossi, P.; Teruel, G.; Pérez-Escamilla, R. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Youssef, G.; Procter-Gray, E.; Olendzki, B.; Cornish, T.; Hayes, R.; Churchill, L.; Kane, K.; Brown, K.; Magee, M.F. Racial differences in eating patterns and food purchasing behaviors among urban older women. J. Nutr. Health Aging 2017, 21, 1190–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, A.R.; Botoseneanu, A.; Markwardt, S.; Nagel, C.L.; Newsom, J.T.; Dorr, D.A.; Allore, H.G. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS ONE 2019, 14, e0218462. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.; Penha, J.; Mbowe, O.; Taira, D.A. Peer Reviewed: Prevalence of Single and Multiple Leading Causes of Death by Race/Ethnicity among US Adults Aged 60 to 79 Years. Prev. Chronic Dis. 2017, 14, E101. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention; National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Available online: http://www.cdc.gov/nchs/nhanes.htm (accessed on 11 February 2022).
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes. Res. 1998, 6 (Suppl. 2), 51S–209S.
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gómez-Abellán, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- Okada, C.; Imano, H.; Muraki, I.; Yamada, K.; Iso, H. The association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. J. Obes. 2019, 2019, 2439571. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, A.K.; Graubard, B.I. Clock Time of First Eating Episode and Prospective Risk of All-Cause Mortality in US Adults. J. Nutr. 2021, 152, 217–226. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Pickel, L.; Sung, H.K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Minors, D.; Atkinson, G.; Benton, D. Chronobiology and meal times: Internal and external factors. Br. J. Nutr. 1997, 77 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef] [Green Version]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Sánchez-Moreno, C.; Smith, C.E.; Lee, Y.C.; Nicolás, F.; Ordovás, J.M. Ghrelin, sleep reduction and evening preference: Relationships to CLOCK 3111 T/C SNP and weight loss. PLoS ONE 2011, 6, e17435. [Google Scholar] [CrossRef] [Green Version]
- Lund, J.; Arendt, J.; Hampton, S.M.; English, J.; Morgan, L.M. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J. Endocrinol. 2001, 171, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.I.; Molina, T.A.; Dziepak, M.E.; Smith, M.R. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans). Chronobiol. Int. 2012, 29, 1072–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastman, C.I.; Suh, C.; Tomaka, V.A.; Crowley, S.J. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci. Rep. 2015, 5, 8381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeballos, E.; Todd, J.E. The effects of skipping a meal on daily energy intake and diet quality. Public Health Nutr. 2020, 23, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Rehm, C.D.; Vieux, F. Breakfast in the United States: Food and Nutrient Intakes in Relation to Diet Quality in National Health and Examination Survey 2011–2014. A Study from the International Breakfast Research Initiative. Nutrients 2018, 10, 1200. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.A.; Cheng, P.; FarrHenderson, M.; Knutson, K. Understanding the determinants of circadian health disparities and cardiovascular disease. Chronobiol. Int. 2021, 1–8. [Google Scholar] [CrossRef]
- Statistics, U. Labor Force Characteristics by Race and Ethnicity. 2014. Available online: https://digital.library.unt.edu/ark:/67531/metadc949130/ (accessed on 12 April 2022).
- Ogata, H.; Kayaba, M.; Tanaka, Y.; Yajima, K.; Iwayama, K.; Ando, A.; Park, I.; Kiyono, K.; Omi, N.; Satoh, M.; et al. Effect of skipping breakfast for 6 days on energy metabolism and diurnal rhythm of blood glucose in young healthy Japanese males. Am. J. Clin. Nutr. 2019, 110, 41–52. [Google Scholar] [CrossRef]
- Rong, S.; Snetselaar, L.G.; Xu, G.; Sun, Y.; Liu, B.; Wallace, R.B.; Bao, W. Association of Skipping Breakfast With Cardiovascular and All-Cause Mortality. J. Am. Coll. Cardiol. 2019, 73, 2025–2032. [Google Scholar] [CrossRef]
- Fagt, S.; Matthiessen, J.; Thyregod, C.; Kørup, K.; Biltoft-Jensen, A. Breakfast in Denmark. Prevalence of Consumption, Intake of Foods, Nutrients and Dietary Quality. A Study from the International Breakfast Research Initiative. Nutrients 2018, 10, 1085. [Google Scholar] [CrossRef] [Green Version]
- Barr, S.I.; Vatanparast, H.; Smith, J. Correction: Breakfast in Canada: Prevalence of Consumption, Contribution to Nutrient and Food Group Intakes, and Variability across Tertiles of Daily Diet Quality. A Study from the International Breakfast Research Initiative. Nutrients 2018, 10, 985. [Google Scholar] [CrossRef] [Green Version]
- Gaal, S.; Kerr, M.A.; Ward, M.; McNulty, H.; Livingstone, M.B. Breakfast Consumption in the UK: Patterns, Nutrient Intake and Diet Quality. A Study from the International Breakfast Research Initiative Group. Nutrients 2018, 10, 999. [Google Scholar] [CrossRef] [Green Version]
- Altura, B.T.; Wilimzig, C.; Trnovec, T.; Nyulassy, S.; Altura, B.M. Comparative effects of a Mg-enriched diet and different orally administered magnesium oxide preparations on ionized Mg, Mg metabolism and electrolytes in serum of human volunteers. J. Am. Coll. Nutr. 1994, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Lieu, S.J.; Curhan, G.C.; Schernhammer, E.S.; Forman, J.P. Rotating night shift work and disparate hypertension risk in African-Americans. J. Hypertens. 2012, 30, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Block, G. Human dietary assessment: Methods and issues. Prev. Med. 1989, 18, 653–660. [Google Scholar] [CrossRef]
- Reid, K.J.; Baron, K.G.; Zee, P.C. Meal timing influences daily caloric intake in healthy adults. Nutr. Res. 2014, 34, 930–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s dietary intake data system. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Rob, P.M.; Niederstadt, C.; Finck, C.; Kreft, B.; Dibbelt, L.; Steinhoff, J. Dialysate magnesium, magnesium handling and clinical considerations in chronic hemodialysis patients. Trace Elem. Electrocytes 1999, 16, 124–130. [Google Scholar]
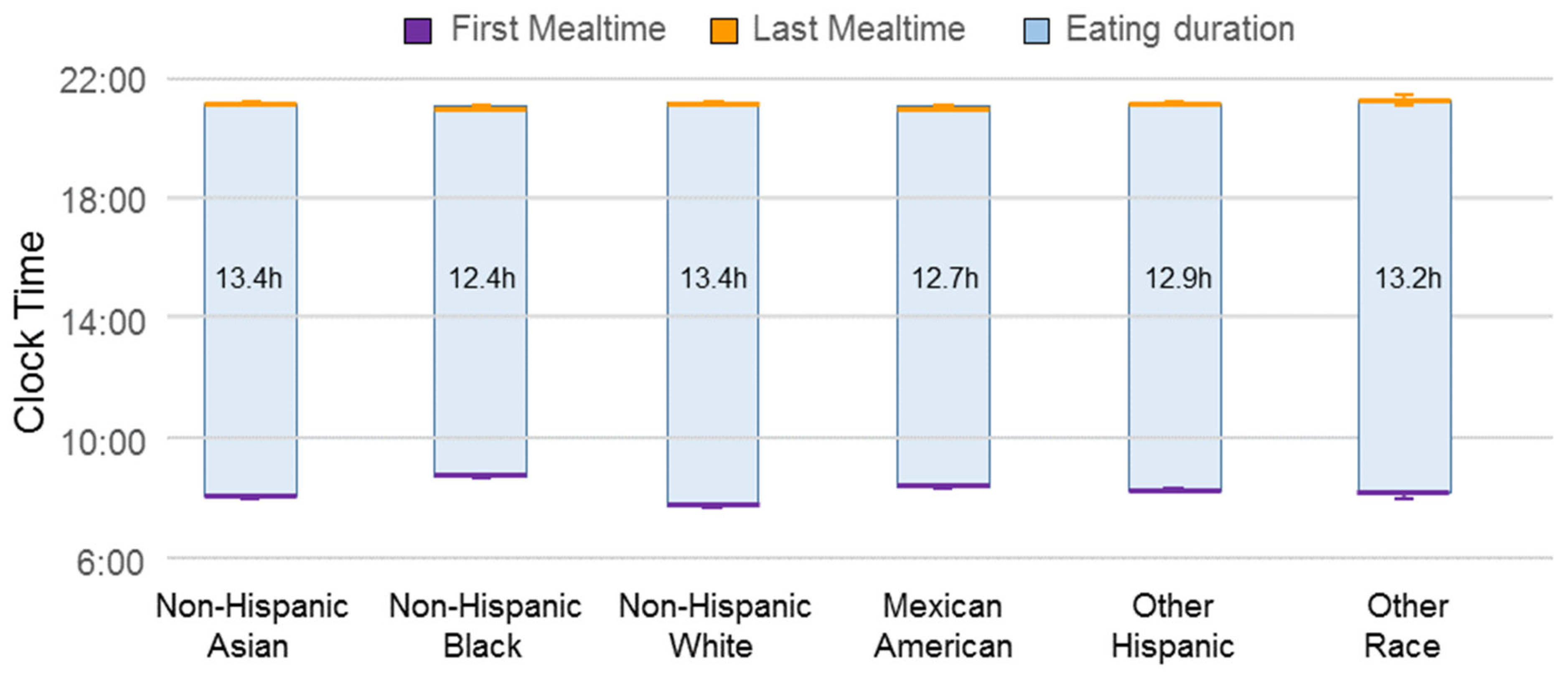
| Variable | n | Frequency | Weighted % |
|---|---|---|---|
| Gender Male Female | 13,084 | 6289 6795 | 47 53 |
| Race and Ethnicity Mexican American Non-Hispanic Asian Non-Hispanic Black Non-Hispanic White Other Hispanic Other Race | 13,084 | 1328 1447 3127 5501 1195 486 | 6.3 4.4 11.1 69.7 5.2 3.3 |
| Age 20–39 40–59 60+ | 13,084 | 4316 4460 4308 | 33.3 38 28.7 |
| Education 9th–11th grade High School and GED Some College College and above | 13,084 | 1750 3155 4408 3771 | 9.5 22.5 33.7 34.3 |
| Marital Status Married Widowed Divorced Separated Never married Living with partner | 13,084 | 6668 907 1494 407 2559 1044 | 56.1 5.3 10.6 2.2 18.1 7.7 |
| BMI Underweight Normal weight Overweight Obese | 13,084 | 208 3497 4121 5136 | 1.5 26.9 32.4 39.2 |
| n | Mean | Range | |
| Mean first meal (hrs.) | 13,084 | 7.91 | 5–22.50 |
| Mean last meal (hrs.) | 13,084 | 21.13 | 10.0–28.96 |
| Mean eating duration (hrs.) | 13,084 | 13.22 | 0–23.87 |
| Mean total energy (kcal) | 13,084 | 2083.54 | 96.50–10025 |
| First Meal (h) | Last Meal (h) | Eating Duration (h) | ||||
|---|---|---|---|---|---|---|
| Variable | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI |
| Race and Ethnicity White (Ref) Mexican American Non-Hispanic Asian Non-Hispanic Black Other Hispanic Other Race/Biracial | 0.39 (0.07) 0.25 (0.07) 0.76 (0.05) 0.34 (0.07) 0.24 (0.11) | 0.26–0.53 a 0.12–0.39 b 0.65–0.86 a 0.20–0.48 a 0.03–0.45 c | −0.21 (0.09) 0.41(0.09) −0.05 (0.08) −0.03 (0.08) 0.04 (0.17) | -0.39–0.03 c 0.23–0.58 a −0.21–0.10 −0.16–0.15 −0.30–0.39 | −0.60 (0.11) 0.15 (0.12) −0.81 (0.10) −0.34 (0.11) −0.20 (0.21) | −0.82–−0.38 a −0.08–0.39 −1.01–−0.61 a −0.56–−0.13 b −0.62–0.23 |
| Gender Male (Ref) Female | −0.05 (0.04) | −0.13–0.04 | −0.02 (0.06) a | −0.15–−0.10 | 0.02 (0.07) | −0.12–−0.16 |
| Age, years 20–39 (Ref) 40–59 60+ | −0.55 (0.05) −0.48 (0.05) | −0.65–−0.45 a −0.59–−0.36 a | 0.13 (0.07) 0.07 (0.09) | −0.003–0.27 −0.11–0.25 | 0.68 (0.09) 0.55 (0.10) | 0.50–0.86 a 0.35–0.75 a |
| Educational status College and above (Ref) Some College High School and GED 9th–11th grade | 0.02 (0.04) 0.10 (0.05) 0.30 (0.07) | −0.06–0.11 −0.04–0.21 0.17–0.44 a | 0.02 (0.07) −0.03 (0.09) −0.01 (0.09) | −0.12–−0.16 −0.21–0.15 −0.20–−0.17 | −0.01 (0.09) −0.13 (0.11) −0.33 (0.11) | −0.18–0.16 −0.35–−0.09 −0.32–−0.11 b |
| Marital Status Married (Ref) Widowed Divorced Separated Never married Living with a partner | −0.005 (0.06) 0.13 (0.07) 0.45 (0.13) 0.57 (0.05) 0.40 (0.06) | −0.12–0.13 −0.002–0.26 0.18–0.72 b 0.47–0.67 a 0.26–0.53 a | 0.09 (0.14) 0.22 (0.12) 0.20 (0.21) 0.25 (0.08) 0.34 (0.11) | −0.20–0.37 −0.01–0.45 −0.22–0.61 0.09–0.41 b 0.11–0.56 b | 0.08 (0.17) 0.09 (0.12) −0.26 (0.22) −0.32 (0.10) −0.06 (0.14) | −0.26–0.42 −0.15–0.33 −0.70–0.19 −0.51–−0.13 b −0.34–0.21 |
| BMI (kg/m2) Normal weight (Ref) Underweight Overweight Obese | 0.42 (0.16) −0.02 (0.05) 0.03 (0.05) | 0.10–0.74 b −0.11–0.08 −0.07–0.13 | 0.24 (0.27) −0.05 (0.07) −0.03 (0.07) | −0.31–0.78 −0.18–0.09 −0.17–0.12 | −0.18 (0.32) −0.03 (0.08) −0.06 (0.09) | −0.83–0.47 −0.19–0.14 −0.24–0.13 |
| R2 | 0.10 | 0.04 | 0.07 | |||
| First Meal (h) | Last Meal (h) | Eating Duration (h) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI | |
| One weekend One weekday | Race and Ethnicity White (Ref) Mexican American Non-Hispanic Asian Non-Hispanic Black Other Hispanic Other Race/Biracial | 0.28 (0.09) 0.22 (0.10) 0.71 (0.08) 0.27 (0.09) −0.03 (0.15) | 0.10–0.45 b 0.20–0.42 b 0.54–0.88 a 0.08–0.46 b −0.34–0.28 | −0.23 (0.09) 0.46 (0.12) −0.06 (0.09) 0.12 (0.11) 0.02 (0.19) | −0.43–0.03 c 0.21–0.71 b −0.25–0.12 −0.10–0.35 −0.36–0.40 | −0.51 (0.14) 0.24 (0.17) −0.77 (0.13) −0.15 (0.15) 0.05 (0.29) | −0.78–−0.24 a −0.10–0.58 −1.03–−0.51 a −0.45–−0.16 −0.54–0.64 |
| Two Weekdays | Race and Ethnicity White (Ref) Mexican American Non-Hispanic Asian Non-Hispanic Black Other Hispanic Other Race/Biracial | 0.54 (0.09) 0.29 (0.07) 0.84 (0.07) 0.43 (0.10) 0.57 (0.11) | 0.35–0.73 a 0.14–0.44 a 0.70–0.97 a 0.23–0.63 a 0.25–0.89 a | −0.19 (0.16) 0.32 (0.14) −0.07 (0.11) −0.18 (0.13) 0.04 (0.27) | −0.51–0.13 0.04–0.60 b −0.28–0.14 −0.44–0.08 −0.50–0.58 | −0.73 (0.19) 0.03 (0.13) −0.91 (0.13) −0.61 (0.15) −0.54 (0.26) | 1.10–−0.35 a −0.26–0.31 −1.16–−0.65 a −0.92–−0.30 a −1.06–−0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansu Baidoo, V.Y.; Zee, P.C.; Knutson, K.L. Racial and Ethnic Differences in Eating Duration and Meal Timing: Findings from NHANES 2011–2018. Nutrients 2022, 14, 2428. https://doi.org/10.3390/nu14122428
Ansu Baidoo VY, Zee PC, Knutson KL. Racial and Ethnic Differences in Eating Duration and Meal Timing: Findings from NHANES 2011–2018. Nutrients. 2022; 14(12):2428. https://doi.org/10.3390/nu14122428
Chicago/Turabian StyleAnsu Baidoo, Velarie Y., Phyllis C. Zee, and Kristen L. Knutson. 2022. "Racial and Ethnic Differences in Eating Duration and Meal Timing: Findings from NHANES 2011–2018" Nutrients 14, no. 12: 2428. https://doi.org/10.3390/nu14122428






