Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
- For intervention factors, the treatment group received probiotics in any form (i.e., tablet, powder, capsule, soft gel, or fortified food forms), species, strains, dose, or treatment regimen, while the control group received a placebo;
- The primary outcomes included stool consistency and changes in the number of fecal colonies. The secondary outcomes were abdominal pain, bloating, QoL, and adverse events.
- Animal trials;
- Duplicate studies;
- Studies with incomplete data (e.g., abstracts, conference proceedings, or research protocols, among others). The authors were contacted when data were not available.
2.2. Literature Search
2.3. Literature Screening and Data Extraction
- Basic information on the included RCTs, including the year of publication, country, and author(s);
- The main characteristics of participants, including the sample size, age, gender, and the diagnostic criteria;
- Details of the interventions implemented (e.g., probiotic strains, dose, duration, form) and the use of placebo;
- Qualitative and quantitative analysis results of outcome indicators (e.g., the report of different symptoms and the use of scales).
2.4. Assessment of Risk of Bias
2.5. Data Synthesis and Statistical Analysis
2.6. Certainty Assessment
3. Results
3.1. Selection Results for the Included Studies
3.2. Characteristics of the Included Studies
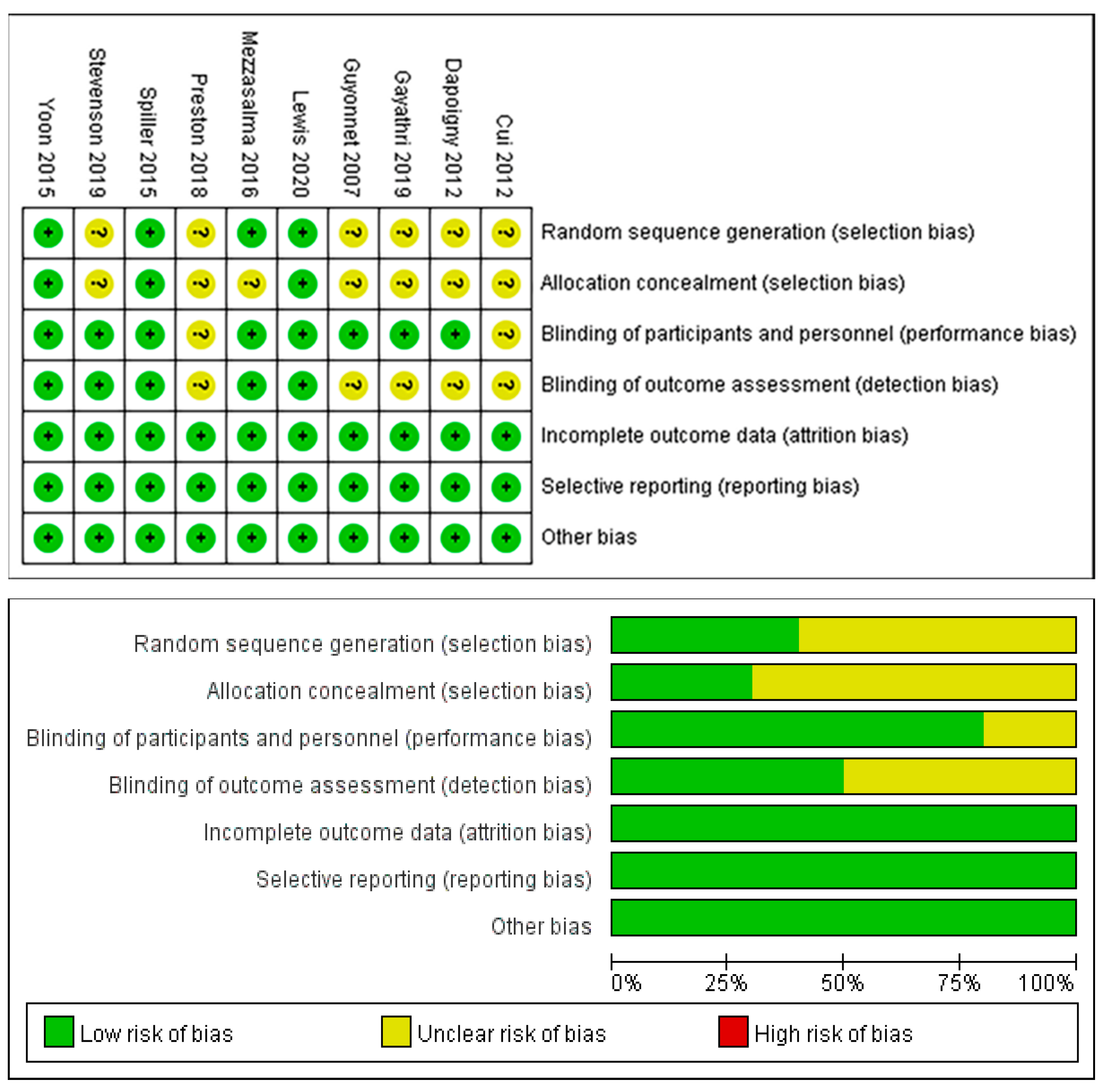
3.3. Risk of Bias of the Included Studies
3.4. Meta-Analysis
3.4.1. Stool Consistency Scores
3.4.2. Fecal Bifidobacterium and Lactobacillus Counts
3.4.3. Abdominal Pain Scores
3.4.4. Bloating Scores
3.4.5. QoL Scores
3.4.6. Adverse Events
3.5. Publication Bias
3.6. Certainty Assessment
4. Discussion
4.1. Summary of the Main Results
4.2. Assessment of Evidence Quality
4.3. Strengths and Limitations
4.4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Definition & Facts for Irritable Bowel Syndrome National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/digestive-diseases/irritable-bowel-syndrome/definition-facts (accessed on 15 January 2022).
- Quigley, E.M.; Fried, M.; Gwee, K.A.; Khalif, I.; Hungin, A.P.S.; Lindberg, G.; Abbas, Z.; Fernandez, L.B.; Bhatia, S.J.; Schmulson, M.; et al. World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A Global Perspective Update September 2015. J. Clin. Gastroenterol. 2016, 50, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar]
- Lovell, R.M.; Ford, A.C. Effect of gender on prevalence of irritable bowel syndrome in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Ballou, S.; McMahon, C.; Lee, H.N.; Katon, J.; Shin, A.; Rangan, V.; Singh, P.; Nee, J.; Camilleri, M.; Lembo, A.; et al. Effects of Irritable Bowel Syndrome on Daily Activities Vary Among Subtypes Based on Results From the IBS in America Survey. Clin. Gastroenterol. Hepatol. 2019, 17, 2471–2478.e2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsari, A.; Ceccarelli, A.; Dubini, F.; Fesce, E.; Poli, G. The fecal microbial population in the irritable bowel syndrome. Microbiologica 1982, 5, 185–194. [Google Scholar]
- Wen, Y.; Li, J.; Long, Q.; Yue, C.C.; He, B.; Tang, X.G. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: A systematic review and meta-analysis based on seventeen randomized controlled trials. Int. J. Surg. 2020, 79, 111–119. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef]
- Kerckhoffs, A.P.; Samsom, M.; van der Rest, M.E.; de Vogel, J.; Knol, J.; Ben-Amor, K.; Akkermans, L.M.A. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J. Gastroenterol. 2009, 15, 2887–2892. [Google Scholar] [CrossRef]
- Ji, M.; Huang, H.; Lan, X. Correlation between Intestinal Microflora in Irritable Bowel Syndrome and Severity. Dis. Markers 2022, 2022, 1031844. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Q.; Jang, B.; Wang, H.; Song, J.; Ye, F. The changes and significances of intestinal microflora in patients with irritable bowel syndrome. Acta Univ. Med. Anhui 2012, 47, 86–88. [Google Scholar]
- Jang, B.; Wang, Q.; Hu, L. Therapeutic efficacy of probiotics and analysis of intestinal flora in patients with irritable bowel syndrome. Chin. J. Clin. Healthc. 2012, 15, 132–134. [Google Scholar]
- Lee, K.J.; Tack, J. Altered intestinal microbiota in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.L. Review article: Evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment. Pharmacol. Ther. 2014, 39, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.R.; Kong, C.F.; Qu, X.K.; Deng, C.; Lou, Y.N.; Jia, L.Q. Efficacy and safety of probiotics in irritable bowel syndrome: A systematic review and meta-analysis. Saudi J. Gastroenterol. 2020, 26, 66–77. [Google Scholar]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef] [Green Version]
- Hulisz, D. The burden of illness of irritable bowel syndrome: Current challenges and hope for the future. J. Manag. Care Pharm. JMCP 2004, 10, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanghellini, V.; et al. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Talley, N.J.; Schoenfeld, P.S.; Quigley, E.M.; Moayyedi, P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: Systematic review and meta-analysis. Gut 2009, 58, 367–378. [Google Scholar] [CrossRef]
- Olafsdottir, L.B.; Gudjonsson, H.; Jonsdottir, H.H.; Jonsson, J.S.; Bjornsson, E.; Thjodleifsson, B. Irritable bowel syndrome: Physicians’ awareness and patients’ experience. World J. Gastroenterol. 2012, 18, 3715–3720. [Google Scholar] [CrossRef]
- Guerin, A.; Carson, R.T.; Lewis, B.; Yin, D.; Kaminsky, M.; Wu, E. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: A retrospective analysis of a Medicaid population. J. Med. Econ. 2014, 17, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H. Probiotics and Prebiotics. Gastroenterol. Hepatol. 2014, 10, 680–681. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyland, N.P.; Quigley, E.M.; Brint, E. Microbiota-host interactions in irritable bowel syndrome: Epithelial barrier, immune regulation and brain-gut interactions. World J. Gastroenterol. 2014, 20, 8859–8866. [Google Scholar]
- Ortiz-Lucas, M.; Tobías, A.; Saz, P.; Sebastián, J.J. Effect of probiotic species on irritable bowel syndrome symptoms: A bring up to date meta-analysis. Rev. Esp. Enferm. Dig. 2013, 105, 19–36. [Google Scholar] [CrossRef] [Green Version]
- Whelan, K. Probiotics and prebiotics in the management of irritable bowel syndrome: A review of recent clinical trials and systematic reviews. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 581–587. [Google Scholar] [CrossRef]
- Angelakis, E.; Merhej, V.; Raoult, D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect. Dis. 2013, 13, 889–899. [Google Scholar] [CrossRef]
- Nikfar, S.; Rahimi, R.; Rahimi, F.; Derakhshani, S.; Abdollahi, M. Efficacy of probiotics in irritable bowel syndrome: A meta-analysis of randomized, controlled trials. Dis. Colon Rectum 2008, 51, 1775–1780. [Google Scholar] [CrossRef]
- McFarland, L.V.; Dublin, S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J. Gastroenterol. 2008, 14, 2650–2661. [Google Scholar] [CrossRef]
- Brenner, D.M.; Moeller, M.J.; Chey, W.D.; Schoenfeld, P.S. The utility of probiotics in the treatment of irritable bowel syndrome: A systematic review. Am. J. Gastroenterol. 2009, 104, 1033–1049. [Google Scholar] [CrossRef]
- Hoveyda, N.; Heneghan, C.; Mahtani, K.R.; Perera, R.; Roberts, N.; Glasziou, P. A systematic review and meta-analysis: Probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hungin, A.P.S.; Mitchell, C.R.; Whorwell, P.; Mulligan, C.; Cole, O.; Agréus, L.; Fracasso, P.; Lionis, C.; Mendive, J.; Philippart de Foy, J.M.; et al. Systematic review: Probiotics in the management of lower gastrointestinal symptoms—An updated evidence-based international consensus. Aliment. Pharmacol. Ther. 2018, 47, 1054–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, D.; Gibb, R.D.; Quigley, E.M. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013, 4, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Lyra, A.; Hillilä, M.; Huttunen, T.; Männikkö, S.; Taalikka, M.; Tennilä, J.; Tarpila, A.; Lahtinen, S.; Ouwehand, A.C.; Veijola, L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J. Gastroenterol. 2016, 22, 10631–10642. [Google Scholar] [CrossRef]
- Sisson, G.; Ayis, S.; Sherwood, R.A.; Bjarnason, I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome—A 12 week double-blind study. Aliment. Pharmacol. Ther. 2014, 40, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.G.; Dotevall, G.; Drossman, D.A.; Heaton, K.W.; Kruis, W.J.G.I. Irritable bowel syndrome: Guidelines for the diagnosis. Gastroenterol. Int. 1989, 2, 92–95. [Google Scholar]
- Hammer, J.; Talley, N.J. Diagnostic criteria for the irritable bowel syndrome. Am. J. Med. 1999, 107, 5S–11S. [Google Scholar] [CrossRef]
- Drossman, D.A. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu-xia, L.; Ya, Z.; Yao-long, C.; Ke-hu, Y.; Zong-jiu, Z. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy 2015, 119, 503–510. [Google Scholar] [CrossRef]
- Cochrane. RevMan 5. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 10 April 2021).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balshem, H.; Helfanda, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE Guidelines:3. Rating the Quality of Evidence. Chin. J. Evid.-Based Med. 2011, 11, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, Y.S.; Lee, D.H.; Seo, J.G.; Shin, C.M.; Kim, N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2015, 57, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Preston, K.; Krumian, R.; Hattner, J.; de Montigny, D.; Stewart, M.; Gaddam, S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: A double-blind, randomised, placebo-controlled study. Benef. Microbes 2018, 9, 697–706. [Google Scholar] [CrossRef]
- Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Piano, A.; Bhardwaj, R.; Tompkins, T.A.; Evans, M. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 1159. [Google Scholar] [CrossRef] [Green Version]
- Dapoigny, M.; Piche, T.; Ducrotte, P.; Lunaud, B.; Cardot, J.M.; Bernalier-Donadille, A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: A randomized, double-blind study. World J. Gastroenterol. 2012, 18, 2067–2075. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. BioMed Res. Int. 2016, 2016, 4740907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyonnet, D.; Chassany, O.; Ducrotté, P.; Picard, C.; Mouret, M.; Mercier, C.-H.; Matuchansky, C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment. Pharmacol. Ther. 2007, 26, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.; Blaauw, R.; Fredericks, E.; Visser, J.; Roux, S. Probiotic effect and dietary correlations on faecal microbiota profiles in irritable bowel syndrome. S. Afr. J. Clin. Nutr. 2021, 34, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Hu, Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int. J. Clin. Exp. Med. 2012, 5, 238–244. [Google Scholar] [PubMed]
- Spiller, R.; Pélerin, F.; Cayzeele Decherf, A.; Maudet, C.; Housez, B.; Cazaubiel, M.; Jüsten, P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2016, 4, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayathri, R.; Aruna, T.; Malar, S.; Shilpa, B.; Dhanasekar, K.R. Efficacy of Saccharomyces cerevisiae CNCM I-3856 as an add-on therapy for irritable bowel syndrome. Int. J. Colorectal Dis. 2020, 35, 139–145. [Google Scholar] [CrossRef]
- Akehurst, R.; Kaltenthaler, E. Treatment of irritable bowel syndrome: A review of randomised controlled trials. Gut 2001, 48, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, W.E. Patient subgroups in irritable bowel syndrome that can be defined by symptom evaluation and physical examination. Am. J. Med. 1999, 107, 33s–40s. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Akl, E.A.; Tokalić, R.; Marušić, A.; Qaseem, A.; Falck-Ytter, Y.; Lee, M.S.; Siedler, M.; Barber, S.L.; et al. The reporting checklist for public versions of guidelines: RIGHT-PVG. Implement. Sci. IS 2021, 16, 10. [Google Scholar] [CrossRef]
- Yao, L.; Sun, R.; Chen, Y.-L.; Wang, Q.; Wei, D.; Wang, X.; Yang, K. The quality of evidence in Chinese meta-analyses needs to be improved. J. Clin. Epidemiol. 2016, 74, 73–79. [Google Scholar] [CrossRef]







| Author (Year) | Country | Sample Size (I/C) | Age (I/C) | Gender (M/F) | IBS-C Sample Size (I/C) | Diagnostic Criteria | Probiotics Genus, Strain, and Species | Dosage and Form | Type of Control | Duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoon 2015 [49] | Korea | 81 (39/42) | - | - | 15 (9/6) | Rome II | B. bifidum, B. lactis, B. longum, L. acidophilus, L. rhamnosus, Strept. thermophilus | 5 × 109 viable cells in a lyophilized powder; 2 capsules/day | Placebo powder | 4 weeks | IBS symptom relief, stool form and frequency |
| Spiller 2015 [57] | France | 379 (192/187) | 43.1 ± 15.5 45.4 ± 14.1 | 18/162 | 180 (82/98) | Rome III | S. cerevisiae I-3856 | 8 × 109 cfu/g; 2 capsules/day | Calcium phosphate and maltodextrin | 12 weeks | Abdominal pain, bloating, flatulence, difficulty with defecation, adverse events |
| Preston 2018 [50] | USA | 113 (76/37) | - | - | 40 * | Rome III | L. acidophilus CL1285, L. casei LBC80R, L. rhamnosus CLR2 | 50 × 109 cfu; 2 capsules/day | Placebo capsule (inert ingredients) | 12 weeks | Abdominal pain, distention, QoL, stool consistency and frequency, adverse events |
| Gayathri 2019 [58] | India | 100 (52/48) | - | - | 24 (12/12) | Rome III | S. cerevisiae CNCM I-3856 | 2 × 109 cfu; 2 capsules/day | Placebo capsule | 8 weeks | Abdominal pain, stool consistency, adverse events |
| Lewis 2020 [51] | Canada | 285 (190/95) | - | - | 28 (15/13) | Rome III | B. longum; L. paracasei | 10 × 109 cfu; 1 capsule/day | Placebo capsule (potato starch and magnesium stearate) | 8 weeks | Stool consistency, adverse events |
| Dapoigny 2012 [52] | France | 50 (25/25) | - | - | 11 (4/7) | Rome III | L. casei rhamnosus | 6 × 108 cfu; 3 capsules/day | Placebo capsule | 4 weeks | IBS severity |
| Mezzasalma 2016 [53] | Italy | 150 (100/50) Δ | I1:36.0 ± 11.9 I2:38 ± 12.1 C:38.1 ± 13.5 | - | 150 (100/50) | Rome III | F1: L. acidophilus, L. reuteri; F2: L. plantarum, L. rhamnosus, B. animalis subsp. Lactis | 5 × 109 cfu; 1 capsule/day | Placebo capsule | 60 day | IBS-related symptom, stool consistency and frequency, QoL |
| Guyonnet 2007 [54] | France | 267 (135/132) Δ | 49.4 ± 11.4 49.2 ± 11.4 | 68/199 | 267 (135/132) | Rome II | B. animalis DN-; Strept. thermophilus; L. bulgaricus | 1.25 × 1010 cfu/pot, 1.2 × 109 cfu/pot; 2 pots of fermented milk/day | Heat-treated yoghurt (nonliving bacteria) | 6 weeks | Abdominal pain, QoL, bloating, adverse events |
| Stevenson 2021 [55] | South Africa | 52 (35/17) | 51.5 ± 9.9 49.4 ± 13.9 | - | 24 (16/8) | Rome II | L. plantarum 299V | 5 × 109 cfu, 1 capsule/day | Placebo capsule (micro-crystalline cellulose powder) | 8 weeks | Fecal counts of Bifidobacterium and Lactobacillus |
| Cui 2012 [56] | China | 60 (37/23) | - | - | 18 (11/7) | Rome II | B. longum, L. acidophilus | 6 capsules/day | Placebo capsule | 4 weeks | Fecal counts of Bifidobacterium and Lactobacillus |
| Estimates of Effects, Confidence Intervals, and Certainty of the Evidence for Probiotics in IBS-C Patients | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: IBS-C Patients Interventions: Probiotics Comparator: Placebo Setting: Hospital or No Hospital | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | No. of Patients (Studies) | Certainty Of Evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with Probiotics | |||||
| Abdominal pain | - | SMD 0.28 lower (0.60 lower to 0.05 higher) | - | 488 (4 RCTs) | ⨁⨁◯◯ Low a | There was no difference in abdominal pain in IBS-C patients treated with probiotics compared with placebo. The evidence is uncertain because randomization, allocation concealment, and blinding were inadequately reported in most of the trials; some heterogeneity (I2 was 53%). |
| Stool consistency | - | MD 0.72 higher (0.18 higher to 1.26 higher) | - | 71 (3 RCTs) | ⨁⨁◯◯ Low b | Probiotics could improve stool consistency scores of IBS-C patients despite study limitations (lacked sufficient details on random sequence generation and allocation concealment), and sample sizes were small (imprecision). |
| Quality of life | - | SMD 3.92 lower (8.09 lower to 0.25 higher) | - | 487 (3 RCTs) | ⨁◯◯◯ Very Low c | There was no difference in QoL in IBS-C patients treated with probiotics compared with placebo. The evidence is very uncertain because randomization and allocation concealment were inadequately reported; blinding was unclear; significant heterogeneity was found (I2 was 99%); and wide confidence intervals existed (lack of precision). |
| Bloating | - | SMD 0.14 lower (0.46 lower to 0.18 higher) | - | 447 (2 RCTs) | ⨁⨁◯◯ Low a | There was no difference in bloating in IBS-C patients treated with probiotics compared with placebo. The evidence is uncertain because there was no adequate explanation for random sequence generation, allocation concealment, or blinding; significant heterogeneity(I2 was 64%). |
| The number of Bifidobacteria in feces | - | MD 1.75 higher (1.51 higher to 2.00 higher) | - | 38 (2 RCTs) | ⨁⨁◯◯ Low b | Probiotics significantly increased the number of fecal Bifidobacteria despite study limitations (lacked sufficient details on random sequence generation and allocation concealment), and sample sizes were small(imprecision). |
| The number of Lactobacilli in feces | - | MD 1.69 higher (1.48 higher to 1.89 higher) | - | 36 (2 RCTs) | ⨁⨁◯◯ Low b | Probiotics significantly increased the number of fecal Lactobacilli despite study limitations (lacked sufficient details on random sequence generation and allocation concealment), and sample sizes were small(imprecision). |
| Adverse events | 50 per 1000 | 76 per 1000 (43 to128) | OR 1.57 (0.87 to 2.82) | 859 (4 RCTs) | ⨁⨁◯◯ Low b | There was no difference in adverse events in IBS-C patients treated with probiotics compared with placebo. The evidence is uncertain due to study limitations (randomization, allocation concealment, and blinding were inadequately reported), and sample sizes were small (imprecision) in most trials. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; E, F.-F.; Guo, K.-L.; Li, Y.-F.; Zhao, H.-L.; Wang, Y.; Chen, N.; Nian, T.; Yang, C.-Q.; Yang, K.-H.; et al. Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Nutrients 2022, 14, 2482. https://doi.org/10.3390/nu14122482
Shang X, E F-F, Guo K-L, Li Y-F, Zhao H-L, Wang Y, Chen N, Nian T, Yang C-Q, Yang K-H, et al. Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Nutrients. 2022; 14(12):2482. https://doi.org/10.3390/nu14122482
Chicago/Turabian StyleShang, Xue, Fen-Fen E, Kang-Le Guo, Yan-Fei Li, Hong-Lin Zhao, Yan Wang, Nan Chen, Tao Nian, Chao-Qun Yang, Ke-Hu Yang, and et al. 2022. "Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials" Nutrients 14, no. 12: 2482. https://doi.org/10.3390/nu14122482
APA StyleShang, X., E, F.-F., Guo, K.-L., Li, Y.-F., Zhao, H.-L., Wang, Y., Chen, N., Nian, T., Yang, C.-Q., Yang, K.-H., & Li, X.-X. (2022). Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Nutrients, 14(12), 2482. https://doi.org/10.3390/nu14122482