Hospital Intervention to Reduce Overweight with Educational Reinforcement after Discharge: A Multicenter Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
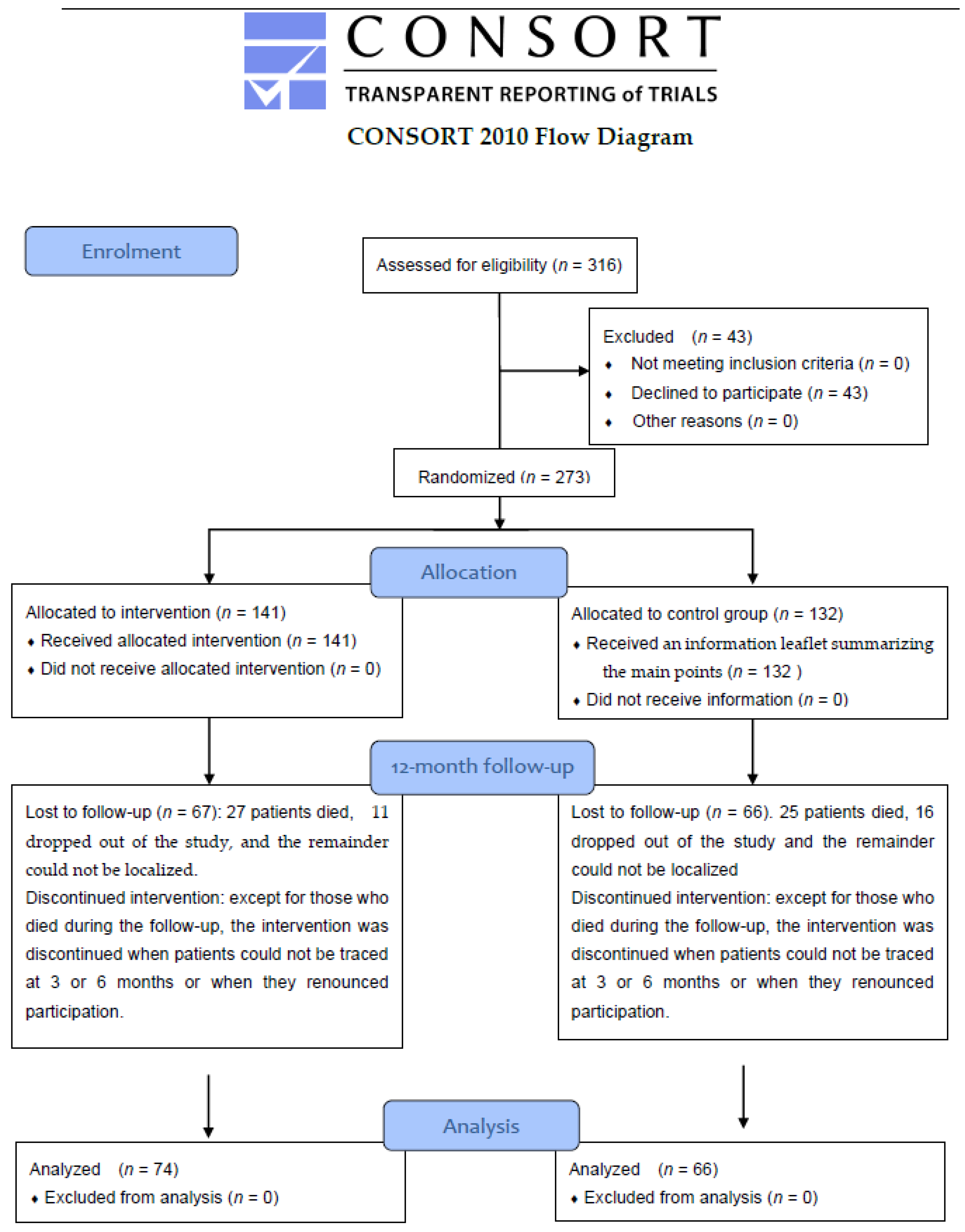
2.2. Sample
2.3. Evaluation Protocol and Study Variables
2.4. Measurement Instruments
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Comparison of Outcome Variables between Baseline (Two Days Post-Discharge) and Three Months
3.2. Comparison of Outcome Variables between Baseline and Six Months
3.3. Comparison of Outcome Variables between Baseline and 12 Months
3.4. Readmissions, Visits to Emergency Department, and Deaths during the Follow-up
3.5. Analysis Stratified by CCI
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 April 2022).
- Rodríguez Caro, A.; González López-Valcárcel, B. El trasfondo económico de las intervenciones sanitarias en la prevención de la obesidad. Rev. Esp. Salud. Publica. 2009, 83, 25–41. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003; pp. 1–149. [Google Scholar]
- Ministerio de Sanidad, Consumo y Bienestar Social. Estrategia NAOS. Invertir la Tendencia de la Obesidad. Estrategia Para la Nutrición, Actividad Física y Prevención de la Obesidad. 2005. Available online: https://www.aesan.gob.es/AECOSAN/web/nutricion/seccion/estrategia_naos.htm (accessed on 3 May 2022).
- Ministerio de Sanidad, Consumo y Bienestar Social. Encuesta Nacional de Salud España 2017. 2018, pp. 1–12. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE2017_notatecnica.pdf (accessed on 28 April 2022).
- Organizacion Mundial de la Salud. Alimentacion Sana. Centro de Prensa. Notas Descriptivas. 2018. Available online: https://www.who.int/es/news-room/fact-sheets/detail/healthy-diet (accessed on 28 April 2022).
- Farooqi, I.S.; O’Rahilly, S. Genetics of obesity in humans. Endocr. Rev. 2006, 27, 710–718. [Google Scholar] [CrossRef]
- Lara, M.; Amigo, H. What kind of intervention has the best results to reduce the weight in overweighted or obese adults? Arch. Lat. Nutr. 2011, 61, 45–54. [Google Scholar]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of directcom parisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Herrera-Espiñeira, C.; Rodríguez del Águila, M.M.; Navarro Espigares, J.L.; Godoy Montijano, A.; García Priego, A.; Rodríguez, J.G. Reyes Sánchez, I. Effect of a telephone care program after hospital discharge from a trauma surgery unit. Gac. Sanit. 2011, 25, 133–138. [Google Scholar] [CrossRef][Green Version]
- Atienza, F.; Anguita, M.; Martinez-Alzamora, N.; Osca, J.; Ojeda, S.; Almenar, L.; Ridocci, F.; Vallés, F.; de Velasco, J.A.; PRICE Study Group. Multicenter randomized trial of a comprehensive hospital discharge and outpatient heart failure management program. Eur. J. Heart Fail. 2004, 6, 643–652. [Google Scholar] [CrossRef]
- Ortega, F.; Vellisco, A.; Márquez, E.; López-Campos, J.L.; Rodríguez, A.; de los Ángeles Sánchez, M.; Barrot, E.; Cejudo, P. Efectividad de un programa de orientación cognitiva con y sin tratamiento sustitutivo con nicotina en la cesación tabáquica en pacientes hospitalizados. Arch. Bronconeumol. 2011, 47, 3–9. [Google Scholar] [CrossRef]
- Ng, L.B.; Yang, Y.; Koh, G.C.-H. The effect of a brief low-intensity therapeutic lifestyle counselling intervention on weight loss among overweight in primary care: A pilot randomised controlled trial. Proc. Singap. Healthc. 2014, 23, 118–125. [Google Scholar] [CrossRef]
- Servicio Andaluz de la Salud. Planes Integrales y de Salud. Available online: https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/el-sas/planes-integrales-y-de-salud (accessed on 26 April 2022).
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatr. 2016, 21, 51–66. [Google Scholar] [CrossRef]
- Librero, J.; Peiró, S.; Ordiñana, R. Chronic Comorbidity and Outcomes of Hospital Care. J. Clin. Epidemiol. 1999, 52, 171–179. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Pardo, A.; Ruiz, M.; Jódar, E.; Garrido, J.; De Rosendo, J.M.; Usán, L.A. Desarrollo de un cuestionario para la valoración y cuantificación de los hábitos de vida relacionados con el sobrepeso y la obesidad. Nutr. Hosp. 2004, 19, 99–109. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Goñi, J.M.; Craig, B.M.; Oppe, M.; Ramallo-Fariña, Y.; Pinto-Prades, J.L.; Luo, N.; Rivero-Aria, O. Handling Data Quality Issues to Estimate the Spanish EQ-5D-5L Value Set Using a Hybrid Interval Regression Approach. Value Health 2018, 21, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Junta de Andalucía. Consejería de Salud. Campañas y Materiales de Promoción de Alimentación Equilibrada y Actividad Física. Available online: https://www.juntadeandalucia.es/organismos/saludyfamilias/areas/salud-vida/adulta/paginas/alimentacion-saludable-materiales.html (accessed on 26 April 2022).
- Ministerio de Sanidad, Consumo y Bienestar Social. eCIE10ES. Edición Electrónica de la CIE-10-ES Diagnósticos. 3a edición. 2020. Available online: https://eciemaps.mscbs.gob.es/ecieMaps/browser/index_10_mc.html (accessed on 25 October 2021).
- Arrebola Vivas, E.; Gómez-Candela, C.; Fernández Fernández, C.; Bermejo López, L.; Kohen, V.L. Eficacia de un programa para el tratamiento del sobrepeso y la obesidad no mórbida en atención primaria y su influencia en la modificación de estilos de vida. Nutr. Hosp. 2013, 28, 137–141. [Google Scholar]
- Nakata, Y.; Okada, M.; Hashimoto, K.; Harada, Y.; Sone, H.; Tanaka, K. Weight loss maintenance for 2 years after a 6-month randomised controlled trial comparing education-only and group-based support in Japanese adults. Obes. Facts. 2014, 7, 376–387. [Google Scholar] [CrossRef]
- Chambliss, H.O.; Huber, R.C.; Finley, C.E.; McDoniel, S.O.; Kitzman-Ulrich, H.; Wilkinson, W.J. Computerized self-monitoring and technology-assisted feedback for weight loss with and without an enhanced behavioral component. Patient Educ. Couns. 2011, 85, 375–382. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Patnode, C.D.; Webber, E.M.; Redmond, N.; Rushkin, M.; O’Connor, E.A. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults updated evidence report and systematic review for the US preventive services task force. JAMA J. Am. Med. Assoc. 2018, 320, 1172–1191. [Google Scholar] [CrossRef]
- Taylor, P.J.; Kolt, G.S.; Vandelanotte, C.; Caperchione, C.M.; Mummery, W.K.; George, E.S.; Karunanithi, M.; Noakes, M.J. A review of the nature and effectiveness of nutrition interventions in adult males: A guide for intervention strategies. Int. J. Behav. Nutr. Phys. Act. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Bellido, D.; Monereo, S.; Lecube, A.; Sánchez, E.; Tinahones, F.J.; Ganancia de peso durante el confinamiento por la COVID-19; encuesta de la Sociedad Española de Obesidad. Revista de la Sociedad Española de Cirugía de Obesidad y Metabólica y de la Sociedad Española para el Estudio de la Obesidad, 10. Available online: https://www.bmi-journal.com/index.php/bmi/article/view/739 (accessed on 26 April 2022).
- Leblanc, V.; Provencher, V.; Bégin, C.; Corneau, L.; Tremblay, A.; Lemieux, S. Impact of a Health-At-Every-Size intervention on changes in dietary intakes and eating patterns in premenopausal overweight women: Results of a randomized trial. Clin. Nutr. 2012, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Yennurajalingam, S.; Palmer, J.L.; Perez-Cruz, P.E.; Frisbee-Hume, S.; Allo, J.A.; Williams, J.L.; Cohen, M.Z. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: A randomized, placebo-controlled, phase II trial. J. Clin. Oncol. 2013, 31, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Núñez, A.; Bruera, E. ¿Es posible generar efecto placebo como parte de las intervenciones paliativas? Med. Paliativa 2016, 23, 21–31. [Google Scholar] [CrossRef]
- Colloca, L.; Barsky, A.J. Placebo and nocebo effects. New Engl. J. Med. 2020, 382, 554–561. [Google Scholar] [CrossRef]
- Panayotov, V.S. Studying a possible placebo effect of an imaginary low-calorie diet. Front. Psychiatry 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Gonzalez Saenz de Tejada, M.; Escobar, A.; Bilbao, A.; Herrera-Espiñeira, C.; García-Perez, L.; Aizpuru, F.; Sarasqueta, C. A prospective study of the association of patient expectations with changes in health-related quality of life outcomes, following total joint replacement. BMC Musculoskelet Disord. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Feng, Y.; Parkin, D.; Devlin, N.J. Assessing the performance of the EQ-VAS in the NHS PROMs programme. Qual. Life Res. 2014, 23, 977–989. [Google Scholar] [CrossRef]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar]
| Variable | Intervention n 141(51.6%) | Control n 132(48.4%) | p-Value |
|---|---|---|---|
| Age (median (P25–P75)) | 69 (53.5–80) | 71.5 (58–81.75) | 0.140 |
| Sex (men) | 75 (53.2%) | 73 (55.3%) | 0.819 |
| Schooling | |||
| None | 37 (26.4%) | 43 (33.6%) | 0.419 |
| Primary | 68 (48.6%) | 58 (45.3%) | |
| Secondary/university | 35 (25%) | 27 (21.1%) | |
| Work situation | |||
| Unemployed | 15 (10.6%) | 11 (8.7%) | 0.651 |
| Actively employed/student | 28 (19.9%) | 21 (16.7%) | |
| Retired/permanent disability | 98 (69.5%) | 97 (74.6%) | |
| Municipality of residence | |||
| ≤20,000 inhabitants | 78 (54.3%) | 72 (55.4%) | 1 |
| >20,000 inhabitants | 63 (44.7%) | 58 (44.6%) | |
| Cohabitation (lives alone) | 21 (15.1%) | 13 (10.2%) | 0.315 |
| Smoker (yes) | 14 (9.9%) | 18 (14%) | 0.405 |
| Practices religion-based diet (yes) | 17 (13.2%) | 18 (16.4%) | 0.610 |
| Takes anti-depression drugs (yes) | 15 (10.9%) | 15 (11.8%) | 0.962 |
| Takes anxiolytics drugs (yes) | 16 (11.5%) | 13 (10.2%) | 0.892 |
| Concerning problems (yes) | 39 (27.9%) | 43 (34.4%) | 0.309 |
| Previous obesity program | 20 (14.4%) | 25 (19.8%) | 0.309 |
| Days of stay (median (P25–P75)) | 8 (6–10) | 8 (5–10.25) | 0.831 |
| Charlson (median (P25–P75)) | 2 (1–4) | 3 (2–5) | <0.001 |
| BMI (median (P25–P75)) | 31.2 (28.7–35.2) | 30.9 (28.7–34.7) | 0.545 |
| Weight at discharge (median (P25–P75)) | 84 (75.5–99) | 84 (75.2–99) | 0.638 |
| SBP after discharge (median (P25–P75)) | 130 (120–138) | 130 (120–137) | 0.223 |
| DBP after discharge (median (P25–P75)) | 75 (70–80) | 70 (65–80) | 0.012 |
| Diagnosis Grouping (ICD 10) | Group | Total | ||
|---|---|---|---|---|
| Intervention | Control | |||
| G1 Certain infectious and parasitic diseases (A00–B99) | Count | 6 | 1 | 7 |
| within group % | 4.3% | 0.8% | 2.6% | |
| G2 Neoplasms (C00–D49) | Count | 2 | 6 | 8 |
| within group % | 1.4% | 4.5% | 2.9% | |
| G3 Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism (D50–D89) | Count | 4 | 0 | 4 |
| within group % | 2.8% | 0.0% | 1.5% | |
| G4 Endocrine, nutritional, and metabolic diseases (E00–E89) | Count | 4 | 1 | 5 |
| within group % | 2.8% | 0.8% | 1.8% | |
| G6 Other diseases of organs and body systems (G00–N99) | Count | 102 | 106 | 208 |
| within group % | 72.3% | 80.3% | 76.2% | |
| G9 Various groups (R00–Z99) | Count | 23 | 18 | 41 |
| within group % | 16.3% | 13.6% | 15.0% | |
| Total | Count | 141 | 132 | 273 |
| within group % | 100% | 100% | 100% | |
| Variable | n | Baseline Mean ± SD | 3 Months Mean ± SD | p-Value Time Effect | p-Value Group Effect | p-Value Time*Group Effect |
|---|---|---|---|---|---|---|
| Weight after discharge | ||||||
| Intervention | 88 | 87.62 ± 19.23 | 84.09 ± 16.59 | <0.001 | 0.617 | 0.170 |
| Control | 69 | 85.31 ± 18.65 | 83.54 ± 18.28 | |||
| SBP after discharge | ||||||
| Intervention | 62 | 133.72 ± 19.60 | 109.37 ± 37.71 | <0.001 | 0.187 | <0.001 |
| Control | 57 | 127.42 ± 15.93 | 124.68 ± 13.94 | |||
| DBP after discharge | ||||||
| Intervention | 62 | 73.43 ± 15.06 | 63.90 ± 22.05 | 0.009 | 0.066 | 0.042 |
| Control | 57 | 73.17 ± 12.07 | 71.93 ± 11.25 | |||
| EQ-5D-5L value | ||||||
| Intervention | 90 | 0.71 ± 0.27 | 0.68 ± 0.30 | 0.035 | 0.589 | 0.563 |
| Control | 70 | 0.71 ± 0.24 | 0.65 ± 0.26 | |||
| VAS score (EQ-5D-5L) | ||||||
| Intervention | 84 | 58.19 ± 18.47 | 62.74 ± 22.63 | 0.046 | 0.310 | 0.983 |
| Control | 69 | 55.65 ± 20.93 | 60.10 ± 21.18 | |||
| Pardo Questionnaire | ||||||
| CC dimension | ||||||
| Intervention | 83 | 2.21 ± 0.69 | 2.95 ± 0.68 | <0.001 | 0.174 | 0.212 |
| Control | 63 | 2.17 ± 0.60 | 2.73 ± 0.88 | |||
| PW dimension | ||||||
| Intervention | 87 | 3.72 ± 1.07 | 4.03 ± 0.97 | 0.399 | 0.376 | 0.030 |
| Control | 68 | 4.06 ± 1.04 | 3.93 ± 1.01 | |||
| PE dimension | ||||||
| Intervention | 87 | 1.77 ± 0.92 | 1.78 ± 0.89 | 0.121 | 0.177 | 0.161 |
| Control | 68 | 1.79 ± 0.96 | 2.09 ± 1.20 | |||
| HD dimension | ||||||
| Intervention | 82 | 3.48 ± 0.57 | 4.40 ± 1.10 | <0.001 | 0.881 | 0.017 |
| Control | 64 | 3.67 ± 0.59 | 4.19 ± 0.51 | |||
| AC dimension | ||||||
| Intervention | 88 | 3.45 ± 0.73 | 3.51 ± 0.79 | 0.133 | 0.406 | 0.021 |
| Control | 70 | 3.56 ± 0.76 | 3.26 ± 0.46 | |||
| Variable | n | Baseline Mean ± SD | 6 Months Mean ± SD | p-Value Time Effect | p-Value Group Effect | p-Value Time*Group Effect |
|---|---|---|---|---|---|---|
| Weight after discharge | ||||||
| Intervention | 85 | 89.93 ± 20.99 | 85.73 ± 19.09 | <0.001 | 0.095 | 0.361 |
| Control | 65 | 84.45 ± 14.76 | 81.74 ± 13.91 | |||
| SBP after discharge | ||||||
| Intervention | 58 | 128.55 ± 15.01 | 121.41 ± 17.17 | 0.012 | 0.886 | 0.294 |
| Control | 53 | 126.09 ± 14.14 | 123.11 ± 21.95 | |||
| DBP after discharge | ||||||
| Intervention | 57 | 75.18 ± 10.38 | 67.79 ± 19.25 | 0.012 | 0.607 | 0.164 |
| Control | 53 | 71.58 ± 9.85 | 69.42 ± 14.15 | |||
| EQ-5D-5L value | ||||||
| Intervention | 86 | 0.71 ± 0.28 | 0.62 ± 0.34 | 0.001 | 0.482 | 0.970 |
| Control | 68 | 0.74 ± 0.25 | 0.65 ± 0.28 | |||
| VAS score (EQ-5D-5L) | ||||||
| Intervention | 82 | 56.8 ± 20.01 | 63.32 ± 23.09 | 0.018 | 0.451 | 0.587 |
| Control | 68 | 55.96 ± 20.58 | 60.06 ± 21.55 | |||
| Pardo Questionnaire | ||||||
| CC dimension | ||||||
| Intervention | 81 | 2.22 ± 0.74 | 2.84 ± 0.71 | <0.001 | 0.931 | 0.827 |
| Control | 65 | 2.21 ± 0.62 | 2.86 ± 0.99 | |||
| PW dimension | ||||||
| Intervention | 83 | 3.81 ± 1.05 | 3.84 ± 1.03 | 0.374 | 0.068 | 0.545 |
| Control | 63 | 4 ± 1.08 | 4.15 ± 0.87 | |||
| PE dimension | ||||||
| Intervention | 82 | 1.72 ± 0.94 | 2.07 ± 1.1 | 0.006 | 0.622 | 0.601 |
| Control | 66 | 1.85 ± 1.05 | 2.09 ± 1.32 | |||
| HD dimension | ||||||
| Intervention | 76 | 3.48 ± 0.62 | 4.2 ± 0.55 | <0.001 | 0.101 | 0.755 |
| Control | 61 | 3.65 ± 0.62 | 4.33 ± 1.02 | |||
| AC dimension | ||||||
| Intervention | 83 | 3.42 ± 0.65 | 3.4 ± 0.63 | 0.065 | 0.970 | 0.106 |
| Control | 68 | 3.55 ± 0.79 | 3.28 ± 0.54 | |||
| Variable | n | Baseline Mean ± SD | 12 Months Mean ± SD | p-Value Time Effect | p-Value Group Effect | p-Value Time*Group Effect |
|---|---|---|---|---|---|---|
| Weight after discharge | ||||||
| Intervention | 73 | 87.87 ± 20.51 | 84.72 ± 17.58 | <0.001 | 0.314 | 0.742 |
| Control | 66 | 84.98 ± 20.31 | 81.25 ± 18.45 | |||
| SBP after discharge | ||||||
| Intervention | 47 | 127.43 ± 12.91 | 121.28 ± 15.23 | 0.003 | 0.607 | 0.552 |
| Control | 43 | 127.56 ± 11.77 | 123.42 ± 12.21 | |||
| DBP after discharge | ||||||
| Intervention | 46 | 74.96 ± 8.64 | 70.76 ± 10.79 | 0.058 | 0.794 | 0.141 |
| Control | 43 | 72.69 ± 8.64 | 72.16 ± 10.28 | |||
| EQ-5D-5L value | ||||||
| Intervention | 74 | 0.74 ± 0.28 | 0.61 ± 0.35 | <0.001 | 0.975 | 0.315 |
| Control | 66 | 0.71 ± 0.26 | 0.64 ± 0.33 | |||
| VAS score (EQ-5D-5L) | ||||||
| Intervention | 73 | 57.51 ± 19.25 | 59.30 ± 24.35 | 0.757 | 0.911 | 0.688 |
| Control | 64 | 58.2 ± 18.86 | 57.97 ± 25.54 | |||
| Pardo Questionnaire | ||||||
| CC dimension | ||||||
| Intervention | 66 | 2.3 ± 0.73 | 3.20 ± 0.95 | <0.001 | 0.415 | 0.699 |
| Control | 57 | 2.23 ± 0.63 | 3.07 ± 0.95 | |||
| PW dimension | ||||||
| Intervention | 72 | 3.94 ± 0.97 | 4.09 ± 0.87 | 0.151 | 0.05 | 0.941 |
| Control | 61 | 4.17 ± 0.92 | 4.32 ± 0.82 | |||
| PE dimension | ||||||
| Intervention | 71 | 1.8 ± 0.97 | 1.87 ± 1.32 | 0.735 | 0.703 | 0.830 |
| Control | 63 | 1.89 ± 1.07 | 1.91 ± 1.45 | |||
| HD dimension | ||||||
| Intervention | 68 | 3.41 ± 0.63 | 4.15 ± 0.66 | <0.001 | 0.03 | 0.626 |
| Control | 59 | 3.68 ± 0.618 | 4.35 ± 0.41 | |||
| AC dimension | ||||||
| Intervention | 72 | 3.37 ± 0.712 | 3.29 ± 0.41 | 0.501 | 0.432 | 0.213 |
| Control | 64 | 3.61 ± 0.79 | 3.27 ± 0.48 | |||
| Variable | 0–3 Months | 3–6 Months | 6–12 Months | |||
|---|---|---|---|---|---|---|
| Readmissions | ||||||
| n | Readmissions | n | Readmissions | n | Readmissions | |
| Intervention | 125 | 32 (25.6%) | 113 | 12 (10.6) | 104 | 18 (17.3%) |
| Control | 111 | 25 (22.5%) | 96 | 10 (10.4%) | 92 | 20 (21.7%) |
| p 0–3 m = 0.690; p 3–6 m = 1; p 6–12 m = 0.547 | ||||||
| Visits to the emergency department | ||||||
| n | Visits to the emergency department | n | Visits to the emergency department | n | Visits to the emergency department | |
| Intervention | 125 | 47 (37.6%) | 113 | 29 (25.7%) | 104 | 26 (25%) |
| Control | 111 | 38 (34.2%) | 96 | 27 (27.6%) | 92 | 26 (28.3%) |
| p 0–3 m = 0.688; p 3–6 m = 0.878; p 6–12 m = 0.723 | ||||||
| Death | ||||||
| n | Deaths | n | Deaths | n | Deaths | |
| Intervention | 125 | 9 (7.4%) | 113 | 9 (8%) | 104 | 9 (8.7%) |
| Control | 111 | 16 (14.5%) | 96 | 4 (4.2%) | 92 | 5 (5.4%) |
| p 0–3 m = 0.079; p 0–6 m = 0.257; p 6–12 m = 0.382 | ||||||
| Variable | n | Baseline Mean ± SD | 12 Months Mean± SD | p-Value Time Effect | p-Value Group Effect | p-Value Time*Group Effect |
|---|---|---|---|---|---|---|
| Weight after discharge | ||||||
| Intervention | 27 | 83.75 ± 16.80 | 83.63 ± 17.04 | 0.069 | 0.220 | 0.0893 |
| Control | 48 | 80.43 ± 18.83 | 76.95 ± 15.95 | |||
| SBP after discharge | ||||||
| Intervention | 19 | 130.63 ± 15.55 | 121.05 ± 15.60 | 0.001 | 0.826 | 0.407 |
| Control | 34 | 128.03 ± 12.98 | 122.26 ± 11.67 | |||
| DBP after discharge | ||||||
| Intervention | 19 | 75.74 ± 7.30 | 70.53 ± 12.35 | 0.046 | 0.427 | 0.121 |
| Control | 34 | 71.65 ± 8.69 | 70.97 ± 9.22 | |||
| EQ-5D-5L value | ||||||
| Intervention | 27 | 0.61 ± 0.31 | 0.43 ± 0.34 | 0.001 | 0.098 | 0.267 |
| Control | 48 | 0.67 ± 0.25 | 0.58 ± 0.32 | |||
| VAS score (EQ-5D-5L) | ||||||
| Intervention | 26 | 52.43 ± 17.28 | 56.04 ± 24.26 | 0.514 | 0.341 | 0.667 |
| Control | 47 | 57.55 ± 18.62 | 58.30 ± 22.58 | |||
| Pardo Questionnaire | ||||||
| CC dimension | ||||||
| Intervention | 25 | 2.42 ± 0.57 | 3.53 ± 0.92 | <0.001 | 0.086 | 0.163 |
| Control | 44 | 2.28 ± 0.67 | 3.07 ± 0.98 | |||
| PW dimension | ||||||
| Intervention | 26 | 4.10 ± 1.05 | 4.29 ± 0.81 | 0.431 | 0.447 | 0.615 |
| Control | 47 | 4.29 ± 0.83 | 4.33 ± 0.79 | |||
| PE dimension | ||||||
| Intervention | 25 | 1.73 ± 0.90 | 1.57 ± 1.22 | 0.644 | 0.307 | 0.968 |
| Control | 46 | 1.99 ± 1.12 | 1.82 ± 1.42 | |||
| HD dimension | ||||||
| Intervention | 25 | 3.81 ± 0.47 | 4.26 ± 0.58 | <0.001 | 0.711 | 0.313 |
| Control | 43 | 3.75 ± 0.59 | 4.39 ± 0.40 | |||
| AC dimension | ||||||
| Intervention | 26 | 3.40 ± 0.87 | 3.17 ± 0.37 | 0.005 | 0.352 | 0.519 |
| Control | 47 | 3.59 ± 0.79 | 3.22 ± 0.45 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Espiñeira, C.; Martínez-Cirre, M.d.C.; López-Morales, M.; Lozano-Sánchez, A.; Rodríguez-Ruíz, A.; Salmerón-López, L.E.; Gómez-Crespo, M.I.; Expósito-Ruíz, M. Hospital Intervention to Reduce Overweight with Educational Reinforcement after Discharge: A Multicenter Randomized Clinical Trial. Nutrients 2022, 14, 2499. https://doi.org/10.3390/nu14122499
Herrera-Espiñeira C, Martínez-Cirre MdC, López-Morales M, Lozano-Sánchez A, Rodríguez-Ruíz A, Salmerón-López LE, Gómez-Crespo MI, Expósito-Ruíz M. Hospital Intervention to Reduce Overweight with Educational Reinforcement after Discharge: A Multicenter Randomized Clinical Trial. Nutrients. 2022; 14(12):2499. https://doi.org/10.3390/nu14122499
Chicago/Turabian StyleHerrera-Espiñeira, Carmen, María del Carmen Martínez-Cirre, Manuel López-Morales, Antonia Lozano-Sánchez, Antonia Rodríguez-Ruíz, Laura Esther Salmerón-López, María Isabel Gómez-Crespo, and Manuela Expósito-Ruíz. 2022. "Hospital Intervention to Reduce Overweight with Educational Reinforcement after Discharge: A Multicenter Randomized Clinical Trial" Nutrients 14, no. 12: 2499. https://doi.org/10.3390/nu14122499
APA StyleHerrera-Espiñeira, C., Martínez-Cirre, M. d. C., López-Morales, M., Lozano-Sánchez, A., Rodríguez-Ruíz, A., Salmerón-López, L. E., Gómez-Crespo, M. I., & Expósito-Ruíz, M. (2022). Hospital Intervention to Reduce Overweight with Educational Reinforcement after Discharge: A Multicenter Randomized Clinical Trial. Nutrients, 14(12), 2499. https://doi.org/10.3390/nu14122499





